Neuromodulation and Habituation: A Literature Review and Conceptional Analysis of Sustaining Therapeutic Efficacy and Mitigating Habituation
Abstract
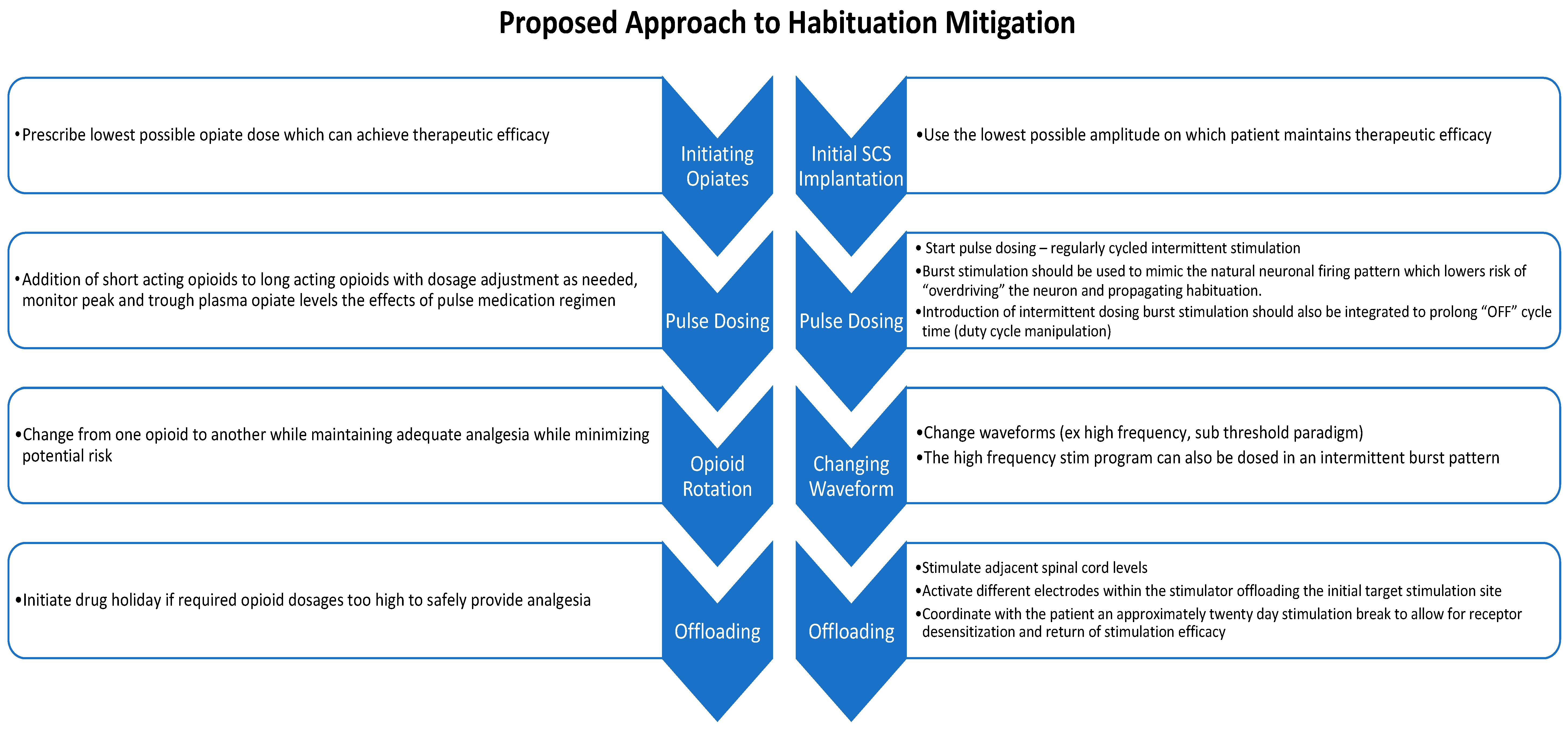
:1. Introduction
2. Understanding Spinal Cord Stimulation and Waveforms
2.1. Conventional/Tonic Stimulation
2.2. High-Frequency Stimulation
2.3. Burst Stimulation
2.4. Intermittent Dosing Burst Paradigm
3. Closed-Loop Spinal Cord Stimulation
4. Habituation and Spinal Cord Stimulator Explantation
5. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Nahin, R.L.; Feinberg, T.; Kapos, F.P.; Terman, G.W. Estimated Rates of Incident and Persistent Chronic Pain Among US Adults, 2019–2020. JAMA Netw. Open 2023, 6, e2313563. [Google Scholar] [CrossRef]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.J.; McGeary, D.D.; McGeary, C.A.; Lippe, B. Interdisciplinary chronic pain management: Past, present, and future. Am. Psychol. 2014, 69, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Dale, R.; Stacey, B. Multimodal treatment of chronic pain. Med. Clin. N. Am. 2016, 100, 55–64. [Google Scholar] [CrossRef]
- Vakkala, M.; Järvimäki, V.; Kautiainen, H.; Haanpää, M.; Alahuhta, S. Incidence and predictive factors of spinal cord stimulation treatment after lumbar spine surgery. J. Pain Res. 2017, 10, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth. Analg. 1967, 46, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for chronic pain. Lancet 2021, 397, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.V.; Nevitt, S.; Maden, M.; Meier, K.; Taylor, R.S.; Eldabe, S.; de Vos, C.C. Spinal cord stimulation for the management of painful diabetic neuropathy: A systematic review and meta-analysis of individual patient and aggregate data. Pain 2021, 162, 2635–2643. [Google Scholar] [CrossRef]
- Al-Kaisy, A.; Van Buyten, J.P.; Kapural, L.; Amirdelfan, K.; Gliner, B.; Caraway, D.; Subbaroyan, J.; Edgar, D.; Rotte, A. 10 kHz spinal cord stimulation for the treatment of non-surgical refractory back pain: Subanalysis of pooled data from two prospective studies. Anaesthesia 2020, 75, 775–784. [Google Scholar] [CrossRef]
- Eckermann, J.M.; Pilitsis, J.G.; Vannaboutathong, C.; Wagner, B.J.; Province-Azalde, R.; Bendel, M.A. Systematic literature review of spinal cord stimulation in patients with chronic back pain without prior spine surgery. Neuromodul. Technol. Neural Interface 2021, 25, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Vannemreddy, P.; Slavin, K.V. Spinal cord stimulation: Current applications for treatment of chronic pain. Anesth. Essays Res. 2011, 5, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.M.; Latif, U.; Sack, A.; Govindan, S.; Sanderson, M.; Vu, D.T.; Smith, G.; Sayed, D.; Khan, T. Advances in Spinal Cord Stimulation. Bioengineering 2023, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.P.; Eldabe, S.; Buchser, E.; Johanek, L.M.; Guan, Y.; Linderoth, B. Parameters of spinal cord stimulation and their role in electrical charge delivery: A review. Neuromodul. Technol. Neural Interface 2016, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Brill, S.; Defrin, R.; Aryeh, I.G.; Zusman, A.M.; Benyamini, Y. Short-and long-term effects of conventional spinal cord stimulation on chronic pain and health perceptions: A longitudinal controlled trial. Eur. J. Pain 2022, 26, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.M.; Veizi, E.; Hanes, M. Treatment-Limiting Complications of Percutaneous Spinal Cord Stimulator Implants: A Review of Eight Years of Experience from an Academic Center Database. Neuromodul. Technol. Neural Interface 2015, 18, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Brown, L.L.; Yearwood, T.L. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: The SENZA-RCT randomized controlled trial. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef]
- Provenzano, D.; Tate, J.; Gupta, M.; Yu, C.; Verrills, P.; Guirguis, M.; Harrison, N.; Smith, T.; Azalde, R.; Bradley, K. Pulse Dosing of 10-kHz Paresthesia-Independent Spinal Cord Stimulation Provides the Same Efficacy with Substantial Reduction of Device Recharge Time. Pain Med. 2022, 23, 152–163. [Google Scholar] [CrossRef]
- Reddy, R.D.; Moheimani, R.; Yu, G.G.; Chakravarthy, K.V. A Review of Clinical Data on Salvage Therapy in Spinal Cord Stimulation. Neuromodul. Technol. Neural Interface 2020, 23, 562–571. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Linderoth, B.; Meyerson, B.A. Spinal cord stimulation: Exploration of the physiological basis of a widely used therapy. J. Am. Soc. Anesthesiol. 2010, 113, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; North, R.; Taylor, R.; Sculpher, M.; van den Abeele, C.; Gehring, M.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; et al. Spinal cord stimulation vs. conventional medical management: A prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (PROCESS study). Neuromodul. Technol. Neural Interface 2005, 8, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.H. Spinal cord stimulation in pain management: A review. Korean J. Pain 2012, 25, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Barolat, G.; Massaro, F.; He, J.; Zeme, S.; Ketcik, B. Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J. Neurosurg. 1993, 78, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M. Anatomic considerations for spinal cord stimulation. Neuromodul. Technol. Neural Interface 2014, 17, 2–11. [Google Scholar] [CrossRef]
- Schultz, D.M.; Webster, L.; Kosek, P.; Dar, U.; Tan, Y.; Sun, M. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Physician 2012, 15, 1–12. [Google Scholar] [CrossRef]
- Barolat, G.; Oakley, J.C.; Law, J.D.; North, R.B.; Ketcik, B.; Sharan, A. Epidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable low back pain. Neuromodul. Technol. Neural Interface 2001, 4, 59–66. [Google Scholar] [CrossRef]
- Turner, J.A.; Loeser, J.D.; Bell, K.G. Spinal cord stimulation for chronic low back pain: A systematic literature synthesis. Neurosurgery 1995, 37, 1088–1095; discussion 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Aló, K.M.; Redko, V.; Charnov, J. Four Year Follow-up of Dual Electrode Spinal Cord Stimulation for Chronic Pain. Neuromodul. Technol. Neural Interface 2002, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Caylor, J.; Reddy, R.; Yin, S.; Cui, C.; Huang, M.; Huang, C.; Rao, R.; Baker, D.G.; Simmons, A.; Souza, D. Spinal cord stimulation in chronic pain: Evidence and theory for mechanisms of action. Bioelectron. Med. 2019, 5, 12. [Google Scholar] [CrossRef]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G. The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008, 63, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Yearwood, T.L.; Bundschu, R.; et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results from a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery 2016, 79, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisy, A.; Palmisani, S.; Smith, T.E.; Pang, D.; Lam, K.; Burgoyne, W.; Houghton, R.; Hudson, E.; Lucas, J. 10 kHz High-Frequency Spinal Cord Stimulation for Chronic Axial Low Back Pain in Patients with no History of Spinal Surgery: A Preliminary, Prospective, Open Label and Proof-of-Concept Study. Neuromodul. Technol. Neural Interface 2017, 20, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisy, A.; Van Buyten, J.-P.; Smet, I.; Palmisani, S.; Pang, D.; Smith, T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014, 15, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisy, A.; Palmisani, S.; Smith, T.E.; Carganillo, R.; Houghton, R.; Pang, D.; Burgoyne, W.; Lam, K.; Lucas, J. Long-Term Improvements in Chronic Axial Low Back Pain Patients Without Previous Spinal Surgery: A Cohort Analysis of 10-kHz High-Frequency Spinal Cord Stimulation over 36 Months. Pain Med. 2018, 19, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S.; Plazier, M.; van der Loo, E.; Menovsky, T. Burst spinal cord stimulation: Toward paresthesia-free pain suppression. Neurosurgery 2010, 66, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Kirketeig, T.; Schultheis, C.; Zuidema, X.; Hunter, C.W.; Deer, T. Burst Spinal Cord Stimulation: A Clinical Review. Pain Med. 2019, 20 (Suppl. 1), S31–S40. [Google Scholar] [CrossRef] [PubMed]
- de Vos, C.C.; Bom, M.J.; Vanneste, S.; Lenders, M.W.; De Ridder, D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodul. Technol. Neural Interface 2014, 17, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Schu, S.; Slotty, P.J.; Bara, G.; von Knop, M.; Edgar, D.; Vesper, J. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodul. Technol. Neural Interface 2014, 17, 443–450. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S. Burst and Tonic Spinal Cord Stimulation: Different and Common Brain Mechanisms. Neuromodul. Technol. Neural Interface 2016, 19, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kinfe, T.M.; Pintea, B.; Link, C.; Roeske, S.; Güresir, E.; Güresir, Á.; Vatter, H. High frequency (10 kHz) or burst spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: Preliminary data from a prospective observational study. Neuromodul. Technol. Neural Interface 2016, 19, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Roeske, S.; Chaudhry, S.R.; Kinfe, T.M. Burst or high-frequency (10 kHz) spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: One year comparative data. Neuromodul. Technol. Neural Interface 2017, 20, 661–667. [Google Scholar] [CrossRef]
- De Ridder, D.; Plazier, M.; Kamerling, N.; Menovsky, T.; Vanneste, S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013, 80, 642–649.e1. [Google Scholar] [CrossRef] [PubMed]
- Yearwood, T.; De Ridder, D.; Yoo, H.B.; Falowski, S.; Venkatesan, L.; Ting To, W.; Vanneste, S. Comparison of Neural Activity in Chronic Pain Patients During Tonic and Burst Spinal Cord Stimulation Using Fluorodeoxyglucose Positron Emission Tomography. Neuromodul. Technol. Neural Interface 2020, 23, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Vesper, J.; Slotty, P.; Schu, S.; Poeggel-Kraemer, K.; Littges, H.; Van Looy, P.; Agnesi, F.; Venkatesan, L.; Van Havenbergh, T. Burst SCS microdosing is as efficacious as standard burst SCS in treating chronic back and leg pain: Results from a randomized controlled trial. Neuromodul. Technol. Neural Interface 2019, 22, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Patterson, D.G.; Baksh, J.; Pope, J.E.; Mehta, P.; Raza, A.; Agnesi, F.; Chakravarthy, K.V. Novel intermittent dosing burst paradigm in spinal cord stimulation. Neuromodul. Technol. Neural Interface 2021, 24, 566–573. [Google Scholar] [CrossRef]
- Ranger, M.R.B.; Irwin, G.J.; Bunbury, K.M.; Peutrell, J.M. Changing body position alters the location of the spinal cord within the vertebral canal: A magnetic resonance imaging study. Br. J. Anaesth. 2008, 101, 804–809. [Google Scholar] [CrossRef]
- Parker, J.L.; Karantonis, D.M.; Single, P.S.; Obradovic, M.; Cousins, M.J. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain 2012, 153, 593–601. [Google Scholar] [CrossRef]
- Russo, M.; Brooker, C.; Cousins, M.J.; Taylor, N.; Boesel, T.; Sullivan, R.; Holford, L.; Hanson, E.; Gmel, G.E.; Shariati, N.H.; et al. Sustained Long-Term Outcomes with Closed-Loop Spinal Cord Stimulation: 12-Month Results of the Prospective, Multicenter, Open-Label Avalon Study. Neurosurgery 2020, 87, E485–E495. [Google Scholar] [CrossRef]
- Deer, T.R.; Jain, S.; Hunter, C.; Chakravarthy, K. Neurostimulation for Intractable Chronic Pain. Brain Sci. 2019, 9, 23. [Google Scholar] [CrossRef]
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M.; et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): A double-blind, randomised, controlled trial. Lancet Neurol. 2020, 19, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Cousins, M.J.; Brooker, C.; Taylor, N.; Boesel, T.; Sullivan, R.; Poree, L.; Shariati, N.H.; Hanson, E.; Parker, J. Effective Relief of Pain and Associated Symptoms with Closed-Loop Spinal Cord Stimulation System: Preliminary Results of the Avalon Study. Neuromodul. Technol. Neural Interface 2018, 21, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M. Durability of clinical and quality-of-life outcomes of closed-loop spinal cord stimulation for chronic back and leg pain: A secondary analysis of the evoke randomized clinical trial. JAMA Neurol. 2022, 79, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Dupré, D.A.; Tomycz, N.; Whiting, D.; Oh, M. Spinal Cord Stimulator Explantation: Motives for Removal of Surgically Placed Paddle Systems. Pain Pract. 2018, 18, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Pope, J.E.; Falowski, S.M.; Pilitsis, J.G.; Hunter, C.W.; Burton, A.W.; Connolly, A.T.; Verrills, P. Clinical Longevity of 106,462 Rechargeable and Primary Cell Spinal Cord Stimulators: Real World Study in the Medicare Population. Neuromodul. Technol. Neural Interface 2023, 26, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, T.; Aner, M.; Sharma, S.; Ghosh, P.; Gill, J.S. Explantation of Percutaneous Spinal Cord Stimulator Devices: A Retrospective Descriptive Analysis of a Single-Center 15-Year Experience. Pain Med. 2019, 20, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Van Buyten, J.P.; Wille, F.; Smet, I.; Wensing, C.; Breel, J.; Karst, E.; Devos, M.; Pöggel-Krämer, K.; Vesper, J. Therapy-Related Explants After Spinal Cord Stimulation: Results of an International Retrospective Chart Review Study. Neuromodul. Technol. Neural Interface 2017, 20, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisy, A.; Royds, J.; Al-Kaisy, O.; Palmisani, S.; Pang, D.; Smith, T.; Padfield, N.; Harris, S.; Wesley, S.; Yearwood, T.L.; et al. Explant rates of electrical neuromodulation devices in 1177 patients in a single center over an 11-year period. Reg. Anesth. Pain Med. 2020, 45, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Hunter, G.; Demeria, D. Spinal cord stimulation in treatment of chronic benign pain: Challenges in treatment planning and present status, a 22-year experience. Neurosurgery 2006, 58, 481–496. [Google Scholar] [CrossRef]
- Hagedorn, J.M.; Layno-Moses, A.; Sanders, D.T.; Pak, D.J.; Bailey-Classen, A.; Sowder, T. Overview of HF10 spinal cord stimulation for the treatment of chronic pain and an introduction to the Senza Omnia™ system. Pain Manag. 2020, 10, 367–376. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Her, Y.F. Stimulation holiday rescues analgesia after habituation and loss of efficacy from 10-kilohertz dorsal column spinal cord stimulation. Reg. Anesth. Pain Med. 2022, 47, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Mekhail, N.; Kramer, J.; Poree, L.; Amirdelfan, K.; Grigsby, E.; Staats, P.; Burton, A.W.; Burgher, A.H.; Scowcroft, J.; et al. Therapy Habituation at 12 Months: Spinal Cord Stimulation Versus Dorsal Root Ganglion Stimulation for Complex Regional Pain Syndrome Type I and II. J. Pain 2020, 21, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.B.; Spiegel, M.A.; van Helmond, N.; Patel, K.V.; Yang, A.; Yousef, T.A.; Mandelberg, N.; Deer, T.; Mogilner, A.Y. Dorsal Root Ganglion Stimulation as a Salvage Therapy Following Failed Spinal Cord Stimulation. Neuromodul. Technol. Neural Interface 2022, 25, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Cahill, C.M.; Walwyn, W.; Taylor, A.M.W.; Pradhan, A.A.A.; Evans, C.J. Allostatic mechanisms of opioid tolerance beyond desensitization and downregulation. Trends Pharmacol. Sci. 2016, 37, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; McLellan, A.T. Opioid abuse in chronic pain-misconceptions and mitigation strategies. N. Engl. J. Med. 2016, 374, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Li, Y.-Q. The Downregulation of Opioid Receptors and Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 5981. [Google Scholar] [CrossRef] [PubMed]
- Glajchen, M. Chronic pain: Treatment barriers and strategies for clinical practice. J. Am. Board. Fam. Pract. 2001, 14, 211–218. [Google Scholar] [PubMed]
- Rauck, R.L.; Loudermilk, E.; Thomson, S.J.; Paz-Solis, J.F.; Bojrab, L.; Noles, J.; Vesper, J.; Atallah, J.; Roth, D.; Hegarty, J.; et al. Long-term safety of spinal cord stimulation systems in a prospective, global registry of patients with chronic pain. Pain Manag. 2023, 13, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Kapural, L.; Sayed, D.; Kim, B.; Harstroem, C.; Deering, J. Retrospective assessment of salvage to 10 kHz spinal cord stimulation (SCS) in patients who failed traditional SCS therapy: RESCUE study. J. Pain Res. 2020, 13, 2861–2867. [Google Scholar] [CrossRef]
- Andrade, P.; Heiden, P.; Visser-Vandewalle, V.; Matis, G. 1.2 kHz high-frequency stimulation as a rescue therapy in patients with chronic pain refractory to conventional spinal cord stimulation. Neuromodul. Technol. Neural Interface 2021, 24, 540–545. [Google Scholar] [CrossRef]
- Kumar, V.; Prusik, J.; Lin, Y.; Hwang, R.; Feustel, P.; Pilitsis, J.G. Efficacy of alternating conventional stimulation and high frequency stimulation in improving spinal cord stimulation outcomes: A pilot study. Neuromodul. Technol. Neural Interface 2018, 21, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Cordero Tous, N.; Sanchez Corral, C.; Ortiz Garcia, I.M.; Jover Vidal, A.; Galvez Mateos, R.; Olivares Granados, G. High-frequency spinal cord stimulation as rescue therapy for chronic pain patients with failure of conventional spinal cord stimulation. Eur. J. Pain 2021, 25, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Courtney, P.; Espinet, A.; Mitchell, B.; Russo, M.; Muir, A.; Verrills, P.; Davis, K. Improved pain relief with burst spinal cord stimulation for two weeks in patients using tonic stimulation: Results from a small clinical study. Neuromodul. Technol. Neural Interface 2015, 18, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Breitfeld, C.; Eikermann, M.; Kienbaum, P.; Peters, J. Opioid “holiday” following antagonist supported detoxification during general anesthesia improves opioid agonist response in a cancer patient with opioid addiction. Anesthesiology 2003, 98, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Howland, R.H. Medication holidays. J. Psychosoc. Nurs. Ment. Health Serv. 2009, 47, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Corona, T.; Rivera, C.; Otero, E.; Stopp, L. A longitudinal study of the effects of an L-dopa drug holiday on the course of Parkinson’s disease. Clin. Neuropharmacol. 1995, 18, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhalili, E.; Rogers, E.R.; Lempka, S.F. An optimization framework for targeted spinal cord stimulation. J. Neural Eng. 2023, 20, 056026. [Google Scholar] [CrossRef]
- Rigoard, P.; Ounajim, A.; Moens, M.; Goudman, L.; Roulaud, M.; Lorgeoux, B.; Baron, S.; Nivole, K.; Many, M.; Lampert, L.; et al. Should we Oppose or Combine Waveforms for Spinal Cord Stimulation in PSPS-T2 Patients? A Prospective Randomized Crossover Trial (MULTIWAVE Study). J. Pain 2023, 24, 2319–2339. [Google Scholar] [CrossRef]
| Stimulation Paradigm | Mechanism (Altered Parameter) | Benefit |
|---|---|---|
| High Frequency Stimulation | High frequency of stimulation ranging from 1–10 kHz with amplitudes below the sensory threshold | No parasthesia at target sites [30], greater decrease in mean back pain score compared to cSCS [17], sustained pain relief up to three years after implant [35] |
| Burst Stimulation | High frequency stimulation period followed by rest phase thought to mimic natural neuronal firing pattern [36] | Notable improvement in back, limb and general pain compared to cSCS [40], superior to HF–SCS in reducing leg pain [41,42], positive effects on mood [40,43,44] |
| Intermittent Dosing Burst Stimulation | Duty cycle alteration (amount of time burst stimulation is active and inactive) | No difference in pain relief or quality of life compared to burst [45], increasing time to habituation due to overall decrease in time nerve is stimulated [45], decreased energy consumption of system [45], increased customizability to patient preference [46] |
| Closed Loop System | Constant measure and response to changing electrophysiological and postural changes (ie more efficacious stimulation to target) [51] | Greater pain relief as compared to open-loop systems from three months to two years [48,50], improvement in patient quality of life, emotional functioning and reduciton in opiate use at two years [51,53] |
| Stimulation Holidays | Discontinue stimulation for a period of time before restarting, thought to reset receptor mediate pathways and re-establish sensitivity [74,75,76] | Approximatley 20 day holiday can lead to significant, sustained pain relief 6 months later [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, A.S.; Levasseur, B.; Gupta, M. Neuromodulation and Habituation: A Literature Review and Conceptional Analysis of Sustaining Therapeutic Efficacy and Mitigating Habituation. Biomedicines 2024, 12, 930. https://doi.org/10.3390/biomedicines12050930
Patil AS, Levasseur B, Gupta M. Neuromodulation and Habituation: A Literature Review and Conceptional Analysis of Sustaining Therapeutic Efficacy and Mitigating Habituation. Biomedicines. 2024; 12(5):930. https://doi.org/10.3390/biomedicines12050930
Chicago/Turabian StylePatil, Anand S., Brittni Levasseur, and Mayank Gupta. 2024. "Neuromodulation and Habituation: A Literature Review and Conceptional Analysis of Sustaining Therapeutic Efficacy and Mitigating Habituation" Biomedicines 12, no. 5: 930. https://doi.org/10.3390/biomedicines12050930





