Stem Cell Banking for Regenerative and Personalized Medicine
Abstract
:1. Background Introduction
2. Practical Stem Cell Sources
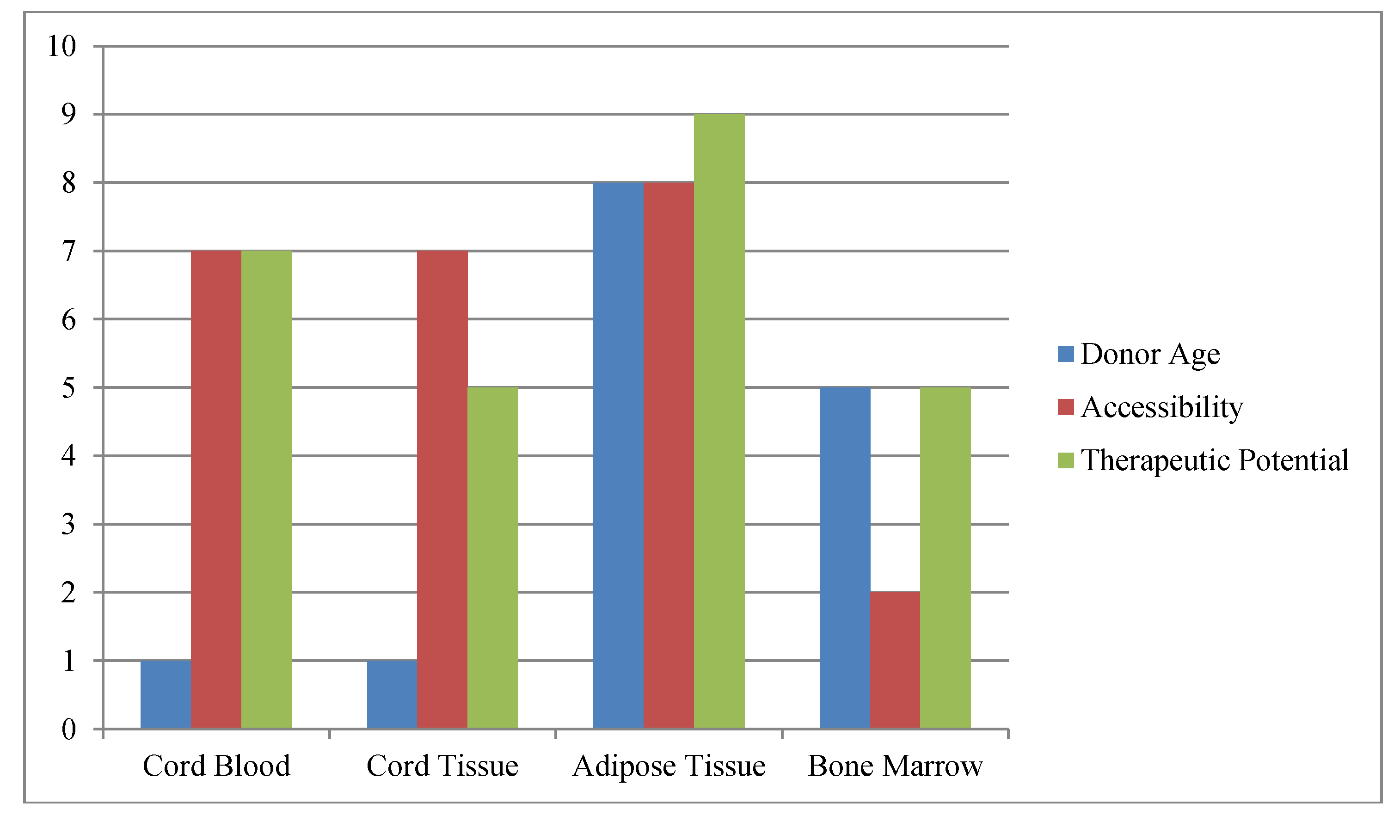
3. Stem Cell Banking Approaches
3.1. Cord Blood
3.2. Cord Tissue
- 1.5 mol/L DMSO in PBS with 20% autologous plasma & 0.1 mol/L sucrose,
- 1.0 mol/L DMSO in PBS with 20% autologous plasma & 0.1 mol/L sucrose,
- 0.5 mol/L DMSO in PBS with 20% autologous plasma & 0.1 mol/L sucrose, and
- 0.0 mol/L DMSO in PBS with 20% autologous plasma & 0.1 mol/L sucrose.
3.3. Adipose Tissue
4. Common Stem Cell Uses
4.1. Hematologic Settings: CB and CT
4.2. Neurological Settings
4.2.1. CB
4.2.2. CT and AT
4.3. Cardiovascular Settings
4.3.1. CB
4.3.2. AT
4.4. Orthopedic Applications
4.4.1. CB
4.4.2. CT
4.4.3. AT
5. Factors Impacting Stem Cell Banking and Use
5.1. Stem Cell Origin and Age
6. Optimal Treatment Windows
7. Regulatory Oversight
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Lee, C.C.; Ye, F.; Tarantal, A.F. Comparison of growth and differentiation of fetal and adult rhesus monkey mesenchymal stem cells. Stem Cells Dev. 2006, 15, 209–220. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Harris, D.T. Comparison of human adipose and cord tissue derived mesenchymal stem cells. Cytotherapy 2013, 15, 330–343. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef]
- Abumaree, M.; Al Jumah, M.; Pace, R.A.; Kalionis, B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012, 8, 375–392. [Google Scholar] [CrossRef]
- Amado, L.C.; Saliaris, A.P.; Schuleri, K.H.; St. John, M.; Xie, J.S.; Cattaneo, S.; Durand, D.J.; Fitton, T.; Kuang, J.Q.; Stewart, G.; et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA 2005, 102, 11474–11479. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Khan, M.; Mahmood, R.; Mohsin, S.; Akhtar, S.; Ali, F.; Khan, S.N.; Riazuddin, S. Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair -infarcted myocardium. J. Cell. Mol. Med. 2012, 16, 2518–2529. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Shen, L.H.; Liu, X.; Wang, X.; Zhang, J.; Pourabdollah-Nejad, D.S.; Zhang, C.; Zhang, L.; Jiang, H.; et al. Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PLoS One 2010, 5, e9027. [Google Scholar] [CrossRef]
- Taylor, S.E.; Smith, R.K.; Clegg, P.D. Mesenchymal stem cell therapy in equine musculoskeletal disease: Scientific fact or clinical fiction? Equine Vet. J. 2007, 39, 172–180. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Jin, Y.Q.; Liu, W.; Zhang, W.J.; Hong, T.H.; Zhou, G.; Baggett, L.S.; Mikos, A.G.; Cao, Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008, 9, 60. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Khan, M.; Mahmood, R.; Mehmood, A.; Khan, S.N.; Riazuddin, S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilitie. Cell Biol. Int. 2012, 36, 747–753. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; de Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multi-lineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Butler, M.G.; Menitove, J.E. Umbilical cord blood banking: An update. J. Assist. Reprod. Genet. 2011, 28, 669–676. [Google Scholar] [CrossRef]
- McGuckin, C.P.; Forraz, N.; Baradez, M.O.; Navran, S.; Zhao, J.; Urban, R.; Tilton, R.; Denner, L. Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif. 2005, 38, 245–255. [Google Scholar] [CrossRef]
- McGuckin, C.P.; Forraz, N.; Allouard, Q.; Pettengell, R. Umbilical cord blood stem cells can expand hematopoietic and neuroglial progenitors in vitro. Exp. Cell Res. 2004, 295, 350–359. [Google Scholar] [CrossRef]
- Rogers, I.; Yamanaka, N.; Bielecki, R.; Wong, C.J.; Chua, S.; Yuen, S.; Casper, R.F. Identification and analysis of in vitro cultured CD45-positive cells capable of multi-lineage differentiation. Exp. Cell Res. 2007, 313, 1839–1852. [Google Scholar] [CrossRef]
- Kucia, M.; Halasa, M.; Wysoczynski, M.; Baskiewicz-Masiuk, M.; Moldenhawer, S.; Zuba-Surma, E.; Czajka, R.; Wojakowski, W.; Machalinski, B.; Ratajczak, M.Z. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human umbilical cord blood-preliminary report. Leukemia 2007, 21, 297–303. [Google Scholar] [CrossRef]
- Harris, D.T.; He, X.; Badowski, M.; Nichols, J.C. Regenerative Medicine of the Eye: A Short Review. In Stem Cell Repair & Regeneration; Levicar, N., Habib, N.A., Dimarakis, I., Gordon, M.Y., Eds.; Imperial College Press: London, UK, 2008; Volume 3, pp. 211–225. [Google Scholar]
- Sunkomat, J.N.E.; Goldman, S.; Harris, D.T. Cord blood-derived MNCs delivered intracoronary contribute differently to vascularization compared to CD34+ cells in the rat model of acute ischemia. J. Mol. Cell. Cardiol. 2007, 42, S97. [Google Scholar]
- Perry, D. Patient’s voices: The powerful sound in the stem cell debate. Science 2000, 287, 1423. [Google Scholar] [CrossRef]
- Norambuena, G.A.; Khoury, M.; Jorgensen, C. Mesenchymal stem cells in osteoarticular pediatric diseases: An update. Pediatr. Res. 2012, 71, 452–458. [Google Scholar] [CrossRef]
- Pati, S.; Gerber, M.H.; Menge, T.D.; Wataha, K.A.; Zhao, Y.; Baumgartner, J.A.; Zhao, J.; Letourneau, P.A.; Huby, M.P.; Baer, L.A.; et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One 2011, 6, e25171. [Google Scholar] [CrossRef]
- See, E.Y.; Toh, S.L.; Goh, J.C. Multilineage potential of bone marrow-derived mesenchymal stem cell sheets: Implications for tissue engineering. Tissue Eng. Part A 2010, 6, 1421–1431. [Google Scholar]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011, 2, 269–291. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.; Tan, Z.; Ye, C.; Zhao, Q.; Chen, Y. Age-related BMAL1 change affects mouse bone marrow stromal cell proliferation and osteo-differentiation potential. Arch. Med. Sci. 2012, 8, 30–38. [Google Scholar]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Kitagawa, Y.K.M.; Toriyama, K.; Kamei, Y.; Torii, S. History of discovery of human adipose-derived stem cells and their clinical applications. J. Plast. Reconstr. Surg. 2006, 49, 1097–1104. [Google Scholar]
- Vieira, N.M.; Brandalise, V.; Zucconi, E.; Secco, M.; Strauss, B.E.; Zatz, M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010, 19, 279–289. [Google Scholar] [CrossRef]
- Rubinstein, P.; Rosenfield, R.E.; Adamson, J.W.; Stevens, C.E. Stored placental blood for unrelated bone marrow reconstitution. Blood 1993, 81, 1679–1690. [Google Scholar]
- Badowski, M.S.; Harris, D.T. Collection, processing, and banking of umbilical cord blood stem cells for transplantation and regenerative medicine. Somat. Stem Cells 2011, 879, 279–290. [Google Scholar]
- Papassavas, A.C.; Gioka, V.; Chatzistamatiou, T.; Kokkinos, T.; Anagnostakis, I.; Gecka, G.; Redoukas, I.; Paterakis, G.; Stavropoulos-Giokas, C. A strategy of splitting individual high volume cord blood units into two half subunits prior to processing increases the recovery of cells and facilitates ex vivo expansion of the infused hematopoietic progenitor cells in adults. Int. J. Lab. Hematol. 2008, 30, 124–132. [Google Scholar] [CrossRef]
- Harris, D.T.; McGaffey, A.P.; Schwarz, R.H. Comparing the mononuclear cell (MNC) recovery of AXP and Hespan. Obstet. Gynecol. 2007, 109, 93S. [Google Scholar]
- American Association of Blood Banks (AABB). Standards for Cellular Therapy Product Services, 5th ed.; AABB Press: Bethesda, MD, USA, 2012. [Google Scholar]
- Chow, R.; Nademanee, A.; Rosenthal, J.; Karanes, C.; Jaing, T.-H.; Graham, M.L.; Tsukahara, E.; Wang, B.; Gjertson, D.; Tan, P.; et al. Analysis of hematopoietic cell transplants using plasma-depleted cord blood products that are not red blood cell reduced. Biol. Blood Marrow Transplant. 2007, 13, 1346–1357. [Google Scholar] [CrossRef]
- Lindenmair, A.; Hatlapatka, T.; Kollwig, G.; Hennerbichler, S.; Gabriel, C.; Wolbank, S.; Redl, H.; Kasper, C. Mesenchymal stem or stromal cells from amnion and umbilical cord tissue and their potential for clinical applications. Cells 2012, 1, 1061–1088. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Parolini, O.; La Rocca, G.; Deng, L. Umbilical cord versus bone marrow-derived mesenchymal stromal cells. Stem Cells Dev. 2012, 21, 2900–2903. [Google Scholar] [CrossRef]
- Zhang, X.; Hirai, M.; Cantero, S.; Ciubotariu, R.; Dobrila, L.; Hirsh, A.; Igura, K.; Satoh, H.; Yokomi, I.; Nishimura, T.; et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J. Cell. Biochem. 2011, 112, 1206–1218. [Google Scholar] [CrossRef]
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. Adult bone marrow is a rich source of human mesenchymal stem cells but umbilical cord and mobilized blood adult blood are not. Br. J. Haematol. 2003, 121, 368–374. [Google Scholar] [CrossRef]
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef]
- Musina, R.A.; Bekchanova, E.S.; Belyavskii, A.V.; Grinenko, T.S.; Sukhikh, G.T. Umbilical cord blood mesenchymal stem cells. Bull. Exp. Biol. Med. 2007, 143, 127–131. [Google Scholar] [CrossRef]
- Secco, M.; Zucconi, E.; Vieira, N.M.; Fogaça, L.L.; Cerqueira, A.; Carvalho, M.D.; Jazedje, T.; Okamoto, O.K.; Muotri, A.R.; Zatz, M. Multipotent stem cells from umbilical cord: Cord is richer than blood. Stem Cells 2008, 26, 146–150. [Google Scholar] [CrossRef]
- Henry, G.; William, P.L.; Bannister, L.H. Grays Anatomy, 38th ed.; ELBS Churchill Livingstone: London, UK, 1995. [Google Scholar]
- Bongso, A.; Fong, C.-Y. The therapeutic potential, challenges and future clinical directions of stem cells from Wharton’s jelly of the human umbilical cord. Stem Cell Rev. Rep. 2013, 9, 226–240. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Gauglitz, G.G.; Phan, T.T.; Herndon, D.N.; Kita, K. Umbilical cord lining membrane and Wharton’s jelly-derived mesenchymal stem cells: The similarities and differences. Open Tissue Eng. Regen. Med. J. 2011, 4, 21–27. [Google Scholar] [CrossRef]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef]
- Seshareddy, K.; Troyer, D.; Weiss, M.L. Methods to isolate mesenchymal-like cells from Wharton’s jelly of umbilical cord. Methods Cell Biol. 2008, 86, 101–119. [Google Scholar] [CrossRef]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s Jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef]
- Fong, C.Y.; Richards, M.; Manasi, N.; Biswas, A.; Bongso, A. Comparative growth behavior and characterization of stem cells from human Wharton’s jelly. Reprod. Biomed. Online 2007, 15, 708–718. [Google Scholar] [CrossRef]
- Fong, C.Y.; Gauthaman, K.; Bongso, A. Reproductive Stem Cells of Embryonic Origin: Comparative Properties and Potential Benefits of Human Embryonic Stem Cells and Wharton’s Jelly Stem Cells. In Stem Cells in Human Reproduction, 2nd ed.; Simon, C., Pellicer, A., Eds.; Informa Healthcare: New York, NY, USA, 2009; pp. 136–149. [Google Scholar]
- Fong, C.Y.; Subramanian, A.; Biswas, A.; Gauthaman, K.; Srikanth, P.; Hande, M.; Bongso, A. Derivation efficiency, cell proliferation, frozen-thaw survival, ‘stemness’ properties, and differentiation of human Wharton’s jelly stem cells: Their potential for concurrent banking with cord blood for regenerative medicine purpose. Reprod. Biomed. Online 2010, 21, 391–401. [Google Scholar] [CrossRef]
- Angelucci, S.; Marchisio, M.; Giuseppe, F.D.; Pierdomenico, L.; Sulpizio, M.; Eleuterio, E. Proteome analysis of human Wharton’s jelly cells during in vitro expansion. Proteome Sci. 2010, 8, 18–25. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z.; Liu, H.W.; Chiou, S.H.; Chu, T.Y. Human umbilical cord mesenchymal stem cells support non-tumorigenic expansion of human embryonic stem cells. Cell Transplant. 2012, 21, 1515–1527. [Google Scholar] [CrossRef]
- Kikuchi-Taura, A.; Taguchi, A.; Kanda, T.; Inoue, T.; Kasahara, Y.; Hirose, H.; Sato, I.; Matsuyama, T.; Nakagomi, T.; Yamahara, K.; et al. Human umbilical cord provides a significant source of unexpanded mesenchymal stromal cells. Cytotherapy 2012, 14, 441–450. [Google Scholar] [CrossRef]
- Lu, L.L.; Liu, Y.J.; Yang, S.G.; Zhao, Q.J.; Wang, X.; Gong, W.; Han, Z.B.; Xu, Z.S.; Lu, Y.X.; Liu, D.; et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006, 91, 1017–1026. [Google Scholar]
- Schugar, R.C.; Chirieleison, S.M.; Wescoe, K.E.; Schmidt, B.T.; Askew, Y.; Nance, J.J.; Evron, J.M.; Peault, B.; Deasy, B.M. High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue. J. Biomed. Biotechnol. 2009. [Google Scholar] [CrossRef]
- Capelli, C.; Gotti, E.; Morigi, M.; Rota, C.; Weng, L.; Dazzi, F.; Spinelli, O.; Cazzaniga, G.; Trezzi, R.; Gianatti, A.; et al. Minimally manipulated whole human umbilical cord is a rich source of clinical grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy 2011, 13, 786–801. [Google Scholar] [CrossRef]
- Bosch, J.; Houben, A.P.; Radke, T.F.; Stapelkamp, D.; Bünemann, E.; Balan, P.; Buchheiser, A.; Liedtke, S.; Kögler, G. Distinct differentiation potential of ‘MSC’ derived from cord blood and umbilical cord: Are cord-derived cells true mesenchymal stromal cells? Stem Cells Dev. 2011, 21, 1977–1988. [Google Scholar]
- Kita, K.; Gauglitz, G.G.; Phan, T.T.; Herndon, D.N.; Jeschke, M.G. Isolation and characterization of mesenchymal stem cells from the sub amniotic human umbilical cord lining membrane. Stem Cells Dev. 2010, 19, 491–502. [Google Scholar] [CrossRef]
- Sarugaser, R.; Lickorish, D.; Baksh, D.; Hosseini, M.M.; Davies, J.E. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells 2005, 23, 220–229. [Google Scholar] [CrossRef]
- Romanov, Y.A.; Svintsitskaya, V.A.; Smirnov, V.N. Searching for alternative sources of postnatal human mesenchymal stem cells: Candidate MSC-like cells from umbilical cord. Stem Cells 2003, 21, 105–110. [Google Scholar] [CrossRef]
- Fu, Y.S.; Shih, Y.T.; Cheng, Y.C.; Min, M.Y. Transformation of human umbilical mesenchymal cells into neurons in vitro. J. Biomed. Sci. 2004, 11, 652–660. [Google Scholar] [CrossRef]
- Tsagias, N.; Koliakos, I.; Karagiannis, V.; Eleftheriadou, M.; Koliakis, G.G. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus. Med. 2011, 21, 253–261. [Google Scholar] [CrossRef]
- Dominici, M.; le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Tao, Y.; Cheng, M.; Hu, J.; Xu, M.; Chen, H. Isolation and characterization of mesenchymal stem cells from whole human umbilical cord applying a single enzyme approach. Cell Biochem. Funct. 2012, 30, 643–649. [Google Scholar] [CrossRef]
- Marmotti, A.; Mattia, S.; Bruzzone, M.; Buttiglieri, S.; Risso, A.; Bonasia, D.E.; Blonna, D.; Castoldi, F.; Rossi, R.; Zanini, C.; et al. Minced umbilical cord fragments as a source of cells for orthopaedic tissue engineering: An in vitro study. Stem Cells Int. 2012. [Google Scholar] [CrossRef]
- Nekanti, U.; Mohanty, L.; Venugopal, P.; Balasubramanian, S.; Totey, S.; Ta, M. Optimization and scale-up of Wharton’s jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2001, 5, 244–254. [Google Scholar]
- Pilz, G.A.; Ulrich, C.; Ruh, M.; Abele, H.; Schäfer, R.; Kluba, T.; Bühring, H.J.; Rolauffs, B.; Aicher, W.K. Human term placenta-derived mesenchymal stromal cells are less prone to osteogenic differentiation than bone marrow-derived mesenchymal stromal cells. Stem Cells Dev. 2011, 20, 635–646. [Google Scholar] [CrossRef]
- Girdlestone, J.; Limbani, V.A.; Cutler, A.J.; Navarrete, C.V. Efficient expansion of mesenchymal stromal cells from umbilical cord under low serum conditions. Cytotherapy 2009, 11, 738–748. [Google Scholar] [CrossRef]
- Xue, G.; He, M.; Zhao, J.; Chen, Y.; Tian, Y.; Zhao, B. Intravenous umbilical cord mesenchymal stem cell infusion for the treatment of combined malnutrition nonunion of the humerus and radial nerve injury. Regen. Med. 2011, 6, 733–741. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Cryopreservation of whole adipose tissue for future use in regenerative medicine. J. Surg. Res. 2013. [Google Scholar] [CrossRef]
- Wu, K.H.; Chan, C.K.; Tsai, C.; Chang, Y.H.; Sieber, M.; Chiu, T.H.; Ho, M.; Peng, C.T.; Wu, H.P.; Huang, J.L. Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation 2011, 91, 1412–1416. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Hua, B.; Wang, H.; Wang, J.; Han, Z.; Sun, L. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult. Scler. 2009, 15, 644–646. [Google Scholar] [CrossRef]
- Liang, J.; Gu, F.; Wang, H.; Hua, B.; Hou, Y.; Shi, S.; Lu, L.; Sun, L. Mesenchymal stem cell transplantation for diffuse alveolar hemorrhage in SLE. Nat. Rev. Rheumatol. 2010, 6, 486–489. [Google Scholar] [CrossRef] [Green Version]
- D’Ippolito, G.; Schiller, P.C.; Ricordi, C.; Roos, B.A.; Howard, G.A. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J. Bone Miner. Res. 1999, 14, 1115–1122. [Google Scholar] [CrossRef]
- Tokalov, S.V.; Gruner, S.; Schindler, S.; Wolf, G.; Baumann, M.; Abolmaali, N. Age-related changes in the frequency of mesenchymal stem cells in the bone marrow of rats. Stem Cells Dev. 2007, 16, 439–446. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Aging Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Bonyadi, M.; Waldman, S.D.; Liu, D.; Aubin, J.E.; Grynpas, M.D.; Stanford, S.L. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc. Natl. Acad. Sci. USA 2003, 100, 5840–5845. [Google Scholar] [CrossRef]
- Stolzing, A.; Scutt, A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell 2006, 5, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.M.; Dixon, K.; Beck, S.; Fabian, D.; Feldman, A.; Barry, F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002, 46, 704–713. [Google Scholar] [CrossRef]
- Zhang, H.; Fazel, S.; Tian, H.; Mickle, D.A.; Weisel, R.D.; Fujii, T.; Li, R.K. Increasing donor age adversely impacts beneficial effects of bone marrow but no smooth muscle myocardial cell therapy. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2089–H2096. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: http://ClinicalTrials.gov (accessed on 1 June 2013).
- Furfaro, M.E.K.; Gaballa, M.A. Do adult stem cells ameliorate the damaged myocardium? Is human cord blood a potential source of stem cells? Curr. Vasc. Pharmacol. 2007, 5, 27–44. [Google Scholar] [CrossRef]
- Chen, J.; Sanberg, P.R.; Li, Y.; Wang, L.; Lu, M.; Willing, A.E.; Sanchez-Ramos, J.; Chopp, M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001, 32, 2682–2688. [Google Scholar] [CrossRef]
- Willing, A.E.; Lixian, J.; Milliken, M.; Poulos, S.; Zigova, T.; Song, S.; Hart, C.; Sanchez-Ramos, J.; Sanberg, P.R. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J. Neurosci. Res. 2003, 73, 296–307. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Hadman, M.; Sanberg, C.D.; Sanberg, P.R. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 2004, 35, 2385–2389. [Google Scholar] [CrossRef]
- Newman, M.B.; Willing, A.E.; Manressa, J.J.; Sanberg, C.D.; Sanberg, P.R. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: Implications for brain repair. Exp. Neurol. 2006, 199, 201–208. [Google Scholar] [CrossRef]
- Vendrame, M.; Cassady, J.; Newcomb, J.J.; Butler, T.; Pennypacker, K.R.; Zigova, T.; Sanberg, C.D.; Sanberg, P.R.; Willing, A.E. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke 2004, 35, 2390–2395. [Google Scholar] [CrossRef]
- Xiao, J.; Nan, Z.; Motooka, Y.; Low, W.C. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev. 2005, 14, 722–733. [Google Scholar] [CrossRef]
- Newcomb, J.D.; Ajrno, C.T.; Sanberg, C.D.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant. 2006, 15, 213–223. [Google Scholar] [CrossRef]
- Vendrame, M.; Gemma, C.; Pennypacker, K.R.; Bickford, P.C.; Davis Sanberg, C.; Sanberg, P.R.; Willing, A.E. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp. Neurol. 2006, 199, 191–200. [Google Scholar] [CrossRef]
- Meier, C.; Middelanis, J.; Wasielewski, B.; Neuhoff, S.; Roth-Haerer, A.; Gantert, M.; Dinse, H.R.; Dermietzel, R.; Jensen, A. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr. Res. 2006, 59, 244–249. [Google Scholar] [CrossRef]
- Chen, S.H.; Chang, F.M.; Tsai, Y.C.; Huang, K.F.; Lin, C.L.; Lin, M.T. Infusion of human umbilical cord blood cells protect against cerebral ischemia and damage during heatstroke in the rat. Exp. Neurol. 2006, 199, 67–76. [Google Scholar]
- Vendrame, M.; Gemma, C.; de Mesquita, D.; Collier, L.; Bickford, P.C.; Sanberg, C.D.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005, 14, 595–604. [Google Scholar] [CrossRef]
- Nan, Z.; Grande, A.; Sanberg, C.D.; Sanberg, P.R.; Low, W.C. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann. N. Y. Acad. Sci. 2005, 1049, 84–96. [Google Scholar] [CrossRef]
- Nystedt, J.; Mäkinen, S.; Laine, J.; Jolkkonen, J. Human cord blood CD34+ cells and behavioral recovery following focal cerebral ischemia in rats. Acta Neurobiol. Exp. (Wars) 2006, 66, 293–300. [Google Scholar]
- Mäkinen, S.; Kekarainen, T.; Nystedt, J.; Liimatainen, T.; Huhtala, T.; Närvänen, A.; Laine, J.; Jolkkonen, J. Human umbilical cord blood cells do not improve sensorimotor or cognitive outcome following transient middle cerebral artery occlusion in rats. Brain Res. 2006, 1123, 207–215. [Google Scholar] [CrossRef]
- Chang, C.K.; Chang, C.P.; Chiu, W.T.; Lin, M.T. Prevention and repair of circulatory shock and cerebral ischemia/injury by various agents in experimental heatstroke. Curr. Med. Chem. 2006, 13, 3145–3154. [Google Scholar] [CrossRef]
- Bliss, T.; Guzman, R.; Daadi, M.; Steinberg, G.K. Cell transplantation therapy for stroke. Stroke 2007, 38, 817–826. [Google Scholar]
- Lu, D.; Sanberg, P.R.; Mahmood, A.; Li, Y.; Wang, L.; Sanchez-Ramos, J.; Chopp, M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002, 11, 275–281. [Google Scholar]
- Kurtzberg, J. Update on umbilical cord blood transplantation. Curr. Opin. Pediatr. 2009, 21, 22–29. [Google Scholar] [CrossRef]
- Kuh, S.U.; Cho, Y.E.; Yoon, D.H.; Kim, K.N.; Ha, Y. Functional recovery after human umbilical cord blood cells transplantation with brain derived-neurotropic factor into the spinal cord injured rats. Acta Neurochir. (Wein.) 2005, 14, 985–992. [Google Scholar]
- Kang, K.-S.; Kim, S.W.; Oh, Y.H.; Yu, J.W.; Kim, K.-Y.; Park, H.K.; Song, C.-H.; Han, H. A thirty-seven-year old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood with improved sensory perception and mobility, both functionally and morphologically: A case study. Cytotherapy 2005, 7, 368–373. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, C.; Chopp, M.; Gosiewska, A.; Hong, K. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke 2011, 42, 1437–1444. [Google Scholar] [CrossRef]
- Hess, D.C.; Borlongan, C.V. Cell-based therapy in ischemic stroke. Expert Rev. Neurother. 2008, 8, 1193–1201. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Chiang, M.F.; Lin, S.Z.; Chang, Y.C.; Wang, H.J.; Su, C.Y.; Li, H. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol. Dis. 2007, 27, 339–353. [Google Scholar] [CrossRef]
- Koh, S.H.; Kim, K.S.; Choi, M.R.; Jung, K.H.; Park, K.S.; Chai, Y.G.; Roh, W.; Hwang, S.J.; Ko, H.J.; Huh, Y.M.; et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008, 1229, 233–248. [Google Scholar]
- Liao, W.; Zhong, J.; Yu, J.; Xie, J.; Liu, Y.; Du, L.; Yang, S.; Liu, P.; Xu, J.; Wang, J.; et al. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: Implications of anti-inflammation and angiogenesis. Cell. Physiol. Biochem. 2009, 24, 307–316. [Google Scholar] [CrossRef]
- Lim, J.H.; Byeon, Y.E.; Ryu, H.H.; Jeong, Y.H.; Lee, Y.W.; Kim, W.H.; Kang, K.S.; Kweon, O.K. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J. Vet. Sci. 2007, 8, 275–282. [Google Scholar] [CrossRef]
- Yang, C.C.; Shih, Y.H.; Ko, M.H.; Hsu, S.Y.; Cheng, H.; Fu, Y.S. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS One 2008, 3, e3336. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.T.; Hong, S.Q.; Ma, X.; Jiang, X.D.; Xu, R.X. Cografted Wharton’s jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem. Res. 2009, 34, 2030–2039. [Google Scholar] [CrossRef]
- Hu, S.-L.; Luo, H.-S.; Li, J.-T.; Xia, Y.-Z.; Li, L.; Zhang, L.-J.; Meng, H.; Cui, G.-Y.; Chen, Z.; Wu, N.; et al. Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit. Care Med. 2010, 38, 2181–2189. [Google Scholar] [CrossRef]
- Fu, Y.S.; Cheng, Y.C.; Lin, M.Y.; Cheng, H.; Chu, P.M.; Chou, S.C.; Shih, Y.H.; Ko, M.H.; Sung, M.S. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: Potential therapeutic application for Parkinsonism. Stem Cells 2006, 24, 115–124. [Google Scholar] [CrossRef]
- Liao, W.; Xie, J.; Zhong, J.; Liu, Y.; Du, L.; Zhou, B.; Xu, J.; Liu, P.; Yang, S.; Wang, J.; et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation 2009, 87, 350–359. [Google Scholar] [CrossRef]
- Copeland, N.; Harris, D.; Gaballa, M.A. Human umbilical cord blood stem cells, myocardial infarction and stroke. Clin. Med. 2009, 9, 342–345. [Google Scholar] [CrossRef]
- Botta, R.; Gao, E.; Stassi, G.; Bonci, D.; Pelosi, E.; Zwas, D.; Patti, M.; Colonna, L.; Baiocchi, M.; Coppola, S.; et al. Heart infarct in NOD-SCID mice: Therapeutic vasculogenesis by transplantation of human CD34+ cells ad low dose CD34+KDR+ cells. FASEB J. 2004, 18, 1392–1394. [Google Scholar]
- Henning, R.J.; Abu-Ali, H.; Balis, J.U.; Morgan, M.B.; Willing, A.E.; Sanberg, P.R. Human umbilical cord blood mononuclear cells for treatment of acute myocardial infarction. Cell Transplant. 2004, 13, 729–739. [Google Scholar] [CrossRef]
- Chen, H.K.; Hung, H.F.; Shyu, K.G.; Wang, B.W.; Sheu, J.R.; Liang, Y.J.; Chang, C.C.; Kuan, P. Combined cord blood cells and gene therapy enhances angiogenesis and improves cardiac performance in mouse after acute myocardial infarction. Eur. J. Clin. Invest. 2005, 35, 677–686. [Google Scholar] [CrossRef]
- Hirata, Y.; Sata, M.; Motomura, N.; Takanashi, M.; Suematsu, Y.; Ono, M.; Takamoto, S. Human umbilical cord blood cells improve cardiac function after myocardial infarction. Biochem. Biophys. Res. Commun. 2005, 327, 609–614. [Google Scholar]
- Kim, B.O.; Tian, H.; Prasongsukarn, K.; Wu, J.; Angoulvant, D.; Wnendt, S.; Muhs, A.; Spitkovsky, D.; Li, R.K. Cell transplantation improves ventricular function after a myocardial infarction: A preclinical study of human unrestricted somatic stem cells in a porcine model. Circulation 2006, 112, 196–204. [Google Scholar]
- Leor, J.; Guetta, E.; Feinberg, M.S.; Galski, H.; Bar, I.; Holbova, R.; Miller, L.; Zarin, P.; Castel, D.; Barbash, I.M.; et al. Human umbilical cord blood-derived CD133+ cells enhance function and repair of the infracted myocardium. Stem Cells 2006, 24, 772–780. [Google Scholar] [CrossRef]
- Ma, N.; Stamm, C.; Kaminski, A.; Li, W.; Kleine, H.D.; Müller-Hilke, B.; Zhang, L.; Ladilov, Y.; Egger, D.; Steinhoff, G. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid mice. Cardiovasc. Res. 2005, 66, 45–54. [Google Scholar] [CrossRef]
- Bonnano, G.; Mariotti, A.; Procoli, A.; Rutella, M.; Pessina, S.; Scambia, G.; Mancuso, G.; Pierelli, S.; Luca, P. Human cord blood CD133+ cells imunoselected by a clinical-grade apparatus differentiate in vitro into endothelial- and cardiomyocyte-like cells. Transfusion 2007, 47, 280–289. [Google Scholar] [CrossRef]
- Schmidt, D.; Breymann, C.; Weber, A.; Guenter, C.I.; Neuenschwander, S.; Zund, G.; Turina, M.; Hoerstrup, S.P. Umbilical cord blood derived endothelial progenitor cells for tissue engineering of vascular grafts. Soc. Thorac. Surg. 2004, 78, 2094–2098. [Google Scholar] [CrossRef]
- Murga, M.; Yao, L.; Tosato, G. Derivation of endothelial cells from CD34− umbilical cord blood. Stem Cells 2004, 22, 385–395. [Google Scholar] [CrossRef]
- Hoerstrup, S.P.; Kadner, A.; Breymann, C.; Maurus, C.F.; Guenter, C.I.; Sodian, R.; Visjager, J.F.; Zund, G.; Turina, M.I. Living, autologous pulmonary artery conduits tissue engineered from human umbilical cord cells. Ann. Thorac. Surg. 2002, 74, 46–52. [Google Scholar] [CrossRef]
- Schmidt, D.; Mol, A.; Neuenschwander, S.; Breymann, C.; Gössi, M.; Zund, G.; Turina, M.; Hoerstrup, S.P. Living patches engineered from human umbilical cord derived fibroblasts and endothelial progenitor cells. Eur. J. Cardiothorac. Surg. 2005, 27, 795–800. [Google Scholar] [CrossRef]
- Murohara, T.; Ikeda, H.; Duan, J.; Shintani, S.; Sasaki, Ki.; Eguchi, H.; Onitsuka, I.; Matsui, K.; Imaizumi, T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J. Clin. Invest. 2000, 105, 1527–1536. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Laughlin, M.J. UC blood hematopoietic stem cells and therapeutic angiogenesis. Cytotherapy 2007, 9, 4–13. [Google Scholar] [CrossRef]
- Nieda, M.; Nicol, A.; Denning-Kendall, P.; Sweetenham, J.; Bradley, B.; Hows, J. Endothelial cell precursors are normal components of human umbilical cord blood. Br. J. Hematol. 1997, 98, 775–777. [Google Scholar]
- Murohara, T. Therapeutic vasculogenesis using human cord blood-derived endothelial progenitors. Trends Cardiovasc. Med. 2001, 11, 303–307. [Google Scholar] [CrossRef]
- Ikeda, Y.; Fukuda, N.; Wada, M.; Matsumoto, T.; Satomi, A.; Yokoyama, S.; Saito, S.; Matsumoto, K.; Kanmatsuse, K.; Mugishima, H. Development of angiogenic cell and gene therapy by transplantation of umbilical cord blood with vascular endothelial growth factor gene. Hypertens. Res. 2004, 27, 119–128. [Google Scholar] [CrossRef]
- Cho, S.W.; Gwak, S.J.; Kang, S.W.; Bhang, S.H.; Won Song, K.W.; Yang, Y.S.; Choi, C.Y.; Kim, B.S. Enhancement of angiogenic efficacy of human cord blood cell transplantation. Tissue Eng. 2006, 12, 1651–1661. [Google Scholar] [CrossRef]
- Finney, M.R.; Greco, N.J.; Haynesworth, S.E.; Martin, J.M.; Hedrick, D.P.; Swan, J.Z.; Winter, D.G.; Kadereit, S.; Joseph, M.E.; Fu, P.; et al. Direct comparison of umbilical cord blood versus bone marrow-derived endothelial precursor cells in mediating neovascularization in response to vascular ischemia. Biol. Blood Marrow Transplant. 2006, 12, 585–593. [Google Scholar] [CrossRef]
- Pesce, M.; Orlandi, A.; Iachininoto, M.G.; Straino, S.; Torella, A.R.; Rizzuti, V.; Pompilio, G.; Bonanno, G.; Scambia, G.; Capogrossi, M.C. Myoendothelial differentiation of human umbilical cord blood-derived stem cells in ischemic limb tissue. Circ. Res. 2003, 93, 51–62. [Google Scholar] [CrossRef]
- Wang, F.S.; Yang, K.D.; Wang, C.J. Shockwave stimulates oxygen radical-mediated osteogenesis of the mesenchymal cells from human umbilical cord blood. J. Bone Miner. Res. 2004, 19, 973–982. [Google Scholar] [CrossRef]
- Szivek, J.A.; Wiley, D.; Cox, L.; Harris, D.T.; Margolis, D.S.; Grana, W.A. Stem cells grown in dynamic culture on micropatterned surfaces can be used to engineer cartilage like tissue. In Proceedings of the Orthopaedic Research Society Meeting, Chicago, IL, USA, 19–22 March 2006.
- Arthritis Foundation. Available online: http://www.arthritis.org (accessed on 1 December 2011).
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W.; et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef]
- Eliopoulos, N.; Stagg, J.; Lejeune, L.; Pommey, S.; Galipeau, J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood 2005, 106, 4057–4065. [Google Scholar] [CrossRef]
- Nauta, A.J.; Westerhuis, G.; Kruisselbrink, A.B.; Lurvink, E.G.A.; Willemze, R.; Fibbe, W.E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006, 108, 2114–2120. [Google Scholar] [CrossRef]
- Zangi, L.; Margalit, R.; Reich-Zeliger, S.; Bachar-Lustig, E.; Beilhack, A.; Negrin, R.; Reisner, Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009, 27, 2865–2874. [Google Scholar] [CrossRef]
- Buja, L.M.; Vela, D. Immunologic and inflammatory reactions to exogenous stem cells implications for experimental studies and clinical trials for myocardial repair. J. Am. Coll. Cardiol. 2010, 56, 1693–1700. [Google Scholar] [CrossRef]
- Pérez-Simon, J.A.; López-Villar, O.; Andreu, E.J.; Rifón, J.; Muntion, S.; Campelo, M.D.; Sánchez-Guijo, F.M.; Martinez, C.; Valcarcel, D.; Cañizo, C.D. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graftversus host disease: Results of a phase I/II clinical trial. Haematologica 2011, 96, 1072–1076. [Google Scholar] [CrossRef]
- Dimmeler, S.; Leri, A. Aging and disease as modifiers of efficacy of cell therapy. Circ. Res. 2008, 102, 1319–1330. [Google Scholar] [CrossRef]
- Scheubel, R.J.; Zorn, H.; Silber, R.E.; Kuss, O.; Morawietz, H.; Holtz, J.; Simm, A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J. Am. Coll. Cardiol. 2003, 42, 2073–2080. [Google Scholar] [CrossRef]
- Alt, E.U.; Senst, C.; Murthy, S.N.; Slakey, D.P.; Dupin, C.L.; Chaffin, A.E.; Kadowitz, P.J.; Izadpanah, R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012, 8, 215–225. [Google Scholar] [CrossRef]
- Zhu, M.; Kohan, E.; Bradley, J.; Hedrick, M.; Benhaim, P.; Zuk, P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J. Tissue Eng. Regen. Med. 2009, 3, 290–301. [Google Scholar] [CrossRef]
- Khan, W.S.; Adesida, A.B.; Tew, S.R.; Andrew, J.G.; Hardingham, T.E. The epitope characterization and the osteogenic differentiation potential of human fat pad-derived stem cells is maintained with ageing in later life. Injury 2009, 40, 150–157. [Google Scholar] [CrossRef]
- Harris, D.T.; Hilgaertner, J.; Simonson, C.; Ablin, R.J.; Badowski, M. Cell based therapy for epithelial wounds. Cytotherapy 2012, 14, 802–810. [Google Scholar] [CrossRef]
- Harris, D.T.; Badowski, M.; Ahmad, N.; Gaballa, M. The potential of cord blood stem cells for use in regenerative medicine. Expert Opin. Biol. Ther. 2007, 7, 1311–1322. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Harris, D.T. Stem Cell Banking for Regenerative and Personalized Medicine. Biomedicines 2014, 2, 50-79. https://doi.org/10.3390/biomedicines2010050
Harris DT. Stem Cell Banking for Regenerative and Personalized Medicine. Biomedicines. 2014; 2(1):50-79. https://doi.org/10.3390/biomedicines2010050
Chicago/Turabian StyleHarris, David T. 2014. "Stem Cell Banking for Regenerative and Personalized Medicine" Biomedicines 2, no. 1: 50-79. https://doi.org/10.3390/biomedicines2010050



