Stem Cell Therapies for Treatment of Liver Disease
Abstract
:1. Introduction
2. Stem Cell Sources for Liver Disease Therapy
2.1. Liver-Derived Stem Cells
2.2. Bone Marrow-Derived Stem Cells
2.3. Annex Stem Cells
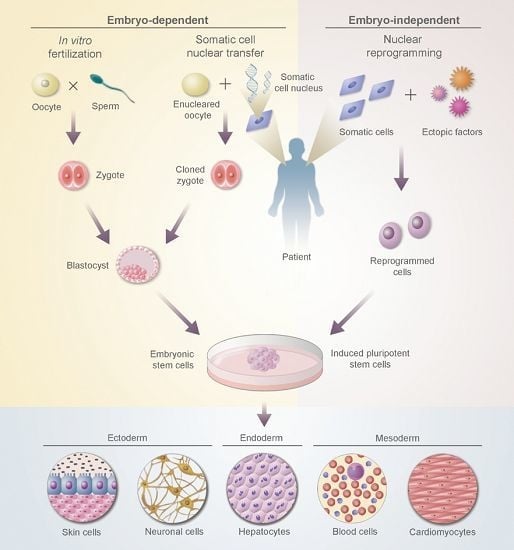
2.4. Embryonic Stem Cells (ESCs)
2.5. Induced Pluripotent Stem Cells (iPSCs)

3. Techniques in Stem Cell Use for Liver Disease Therapy

4. Potential Applications of Stem Cells in Liver Diseases
4.1. Hereditary Liver Diseases

4.2. Acute Liver Failure (ALF)
4.3. Cirrhosis
4.4. Liver Cancer
4.5. Liver Transplantation
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Taub, R. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Cantz, T.; Manns, M.P.; Ott, M. Stem cells in liver regeneration and therapy. Cell Tissue Res. 2008, 331, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Riehle, K.J.; Dan, Y.Y.; Campbell, J.S.; Fausto, N. New concepts in liver regeneration. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 1), S203–S212. [Google Scholar] [CrossRef] [PubMed]
- Rush, G.F.; Gorski, J.R.; Ripple, M.G.; Sowinski, J.; Bugelski, P.; Hewitt, W.R. Organic hydroperoxide-induced lipid peroxidation and cell death in isolated hepatocytes. Toxicol. Appl. Pharmacol. 1985, 78, 473–483. [Google Scholar] [CrossRef]
- Mitaka, T. The current status of primary hepatocyte culture. Int. J. Exp. Pathol. 1998, 79, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Boess, F.; Kamber, M.; Romer, S.; Gasser, R.; Muller, D.; Albertini, S.; Suter, L. Gene expression in two hepatic cell lines, cultured primary hepatocytes and liver slices compared to the in vivo liver gene expression in rats: Possible implications for toxicogenomics use of in vitro systems. Toxicol. Sci. 2003, 73, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.D.; Orr, S.; Skett, P.; Berry, D.P.; Dennison, A.R. Cryopreservation of hepatocytes: A review of current methods for banking. Cell Tissue Bank. 2003, 4, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Evarts, R.P.; Nagy, P.; Marsden, E.; Thorgeirsson, S.S. A precursor—Product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis 1987, 8, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, C.A.; Rhim, J.A.; Yamada, Y.; Fausto, N. Generation of hepatocytes from oval cell precursors in culture. Cancer Res. 1998, 58, 5514–5522. [Google Scholar] [PubMed]
- Kubota, H.; Reid, L.M. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc. Natl. Acad. Sci. USA 2000, 97, 12132–12137. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N.; Campbell, J.S. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 2003, 120, 117–130. [Google Scholar] [CrossRef]
- Oertel, M.; Rosencrantz, R.; Chen, Y.Q.; Thota, P.N.; Sandhu, J.S.; Dabeva, M.D.; Pacchia, A.L.; Adelson, M.E.; Dougherty, J.P.; Shafritz, D.A. Repopulation of rat liver by fetal hepatoblasts and adult hepatocytes transduced ex vivo with lentiviral vectors. Hepatology 2003, 37, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Haridass, D.; Yuan, Q.; Becker, P.D.; Cantz, T.; Iken, M.; Rothe, M.; Narain, N.; Bock, M.; Nörder, M.; Legrand, N.; et al. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. Am. J. Pathol. 2009, 175, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Mahieu-Caputo, D.; Allain, J.E.; Branger, J.; Coulomb, A.; Delgado, J.P.; Andreoletti, M.; Mainot, S.; Frydman, R.; Leboulch, P.; Di Santo, J.P.; et al. Repopulation of athymic mouse liver by cryopreserved early human fetal hepatoblasts. Hum. Gene Ther. 2004, 15, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Hayner, N.T.; Braun, L.; Yaswen, P.; Brooks, M.; Fausto, N. Isozyme profiles of oval cells, parenchymal cells, and biliary cells isolated by centrifugal elutriation from normal and preneoplastic livers. Cancer Res. 1984, 44, 332–338. [Google Scholar] [PubMed]
- Schmelzer, E.; Wauthier, E.; Reid, L.M. The phenotypes of pluripotent human hepatic progenitors. Stem Cells 2006, 24, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Liu, Z.C. Therapeutic potential of adult bone marrow stem cells in liver disease and delivery approaches. Stem Cell Rev. 2008, 4, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.A.; Ju, S.Y.; Cho, S.J.; Jung, Y.J.; Woo, S.Y.; Seoh, J.Y.; Han, H.S.; Ryu, K.H. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol. Int. 2009, 33, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, Z.W.; Wang, F.S. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin. Exp. Immunol. 2011, 164, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Kestendjieva, S.; Kyurkchiev, D.; Tsvetkova, G.; Mehandjiev, T.; Dimitrov, A.; Nikolov, A.; Kyurchiev, S. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol. Int. 2008, 32, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Yen, B.L.; Huang, H.I.; Chien, C.C.; Jui, H.Y.; Ko, B.S.; Yao, M.; Shun, C.T.; Yen, M.L.; Lee, M.C.; Chen, Y.C. Isolation of multipotent cells from human term placenta. Stem Cells 2005, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Di Campli, C.; Piscaglia, A.C.; Pierelli, L.; Rutella, S.; Bonanno, G.; Alison, M.R.; Mariotti, A.; Vecchio, F.M.; Nestola, M.; Monego, G.; et al. A human umbilical cord stem cell rescue therapy in a murine model of toxic liver injury. Dig Liver Dis. 2004, 36, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Zaret, K.S.; Grompe, M. Generation and regeneration of cells of the liver and pancreas. Science 2008, 322, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A. Ethical and social considerations of stem cell research. Nature 2001, 414, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Swijnenburg, R.J.; Schrepfer, S.; Govaert, J.A.; Cao, F.; Ransohoff, K.; Sheikh, A.Y.; Haddad, M.; Connolly, A.J.; Davis, M.M.; Robbins, R.C.; et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc. Natl. Acad. Sci. USA 2008, 105, 12991–12996. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Caron, J.; Hannoun, Z.; Antoni, M.; Lopez, S.; Burks, D.; Castell, J.V.; Weber, A.; Gomez-Lechon, M.J.; Dubart-Kupperschmitt, A. Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Res. Ther. 2015, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, D.G.; Nelson, T.J.; Mueller, P.S.; Hook, C.C. The science and ethics of induced pluripotency: What will become of embryonic stem cells? Mayo Clin. Proc. 2011, 86, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallier, L. Putting induced pluripotent stem cells to the test. Nat. Biotechnol. 2015, 33, 1145–1146. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, M.C.; Yeo, G.W.; Kainohana, O.; Marsala, M.; Gage, F.H.; Muotri, A.R. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS ONE 2009, 4, e7076. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ehrlich, L.I.; Yabuuchi, A.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Ye, Z.; Kim, Y.; Sharkis, S.; Jang, Y.Y. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology 2010, 51, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Okada, Y.; Aoi, T.; Okada, A.; Takahashi, K.; Okita, K.; Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Ohnuki, M.; et al. Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 2009, 27, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Kim, Y.; Liu, H.; Chaudhari, P.; Ye, Z.; Jang, Y.Y. Liver engraftment potential of hepatic cells derived from patient-specific induced pluripotent stem cells. Cell Cycle 2011, 10, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Toyoda, M.; Yamazaki-Inoue, M.; Fukawatase, Y.; Chikazawa, E.; Sakaguchi, H.; Akutsu, H.; Umezawa, A. DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genet. 2011, 7, e1002085. [Google Scholar] [CrossRef] [PubMed]
- Hartjes, K.A.; Li, X.; Martinez-Fernandez, A.; Roemmich, A.J.; Larsen, B.T.; Terzic, A.; Nelson, T.J. Selection via pluripotency-related transcriptional screen minimizes the influence of somatic origin on iPSC differentiation propensity. Stem Cells 2014, 32, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Bar-Nur, O.; Russ, H.A.; Efrat, S.; Benvenisty, N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 2011, 9, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Stadtfeld, M.; Nagaya, M.; Utikal, J.; Weir, G.; Hochedlinger, K. Induced pluripotent stem cells generated without viral integration. Science 2008, 322, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Fusaki, N.; Ban, H.; Nishiyama, A.; Saeki, K.; Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B 2009, 85, 348–362. [Google Scholar] [CrossRef]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, C.H.; Moon, J.I.; Chung, Y.G.; Chang, M.Y.; Han, B.S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R.; et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009, 4, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K.; et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hongling, L.; Ikeda, Y.; Amiot, B.; Rinaldo, P.; Duncan, S.; Nyberg, S.L. Hepatocyte-like cells differentiated from human induced pluripotent stem cells: Relevance to cellular therapies. Stem Cell Res. 2012, 9, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Pournasr, B.; Salekdeh, G.H.; Ghodsizadeh, A.; Ott, M.; Baharvand, H. Induced pluripotent stem cells: A new era for hepatology. J. Hepatol. 2010, 53, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Si-Tayeb, K.; Noto, F.K.; Nagaoka, M.; Li, J.; Battle, M.A.; Duris, C.; et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 2010, 51, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Duan, Y.; Tschudy-Seney, B.; Roll, G.; Behbahan, I.S.; Ahuja, T.P.; et al. Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem Cells Transl. Med. 2013, 2, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Tseng, C.Y.; Wang, H.W.; Kuo, H.C.; Yang, V.W.; Lee, O.K. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology 2012, 55, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.T.; Corbineau, S.; Hannan, N.; Marciniak, S.J.; Miranda, E.; Alexander, G.; Huang-Doran, I.; Griffin, J.; Ahrlung-Richter, L.; Skepper, J.; et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J. Clin. Investig. 2010, 120, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Moslem, M.; Bagheri-Lankarani, K.; Pournasr, B.; Miryounesi, M.; Baharvand, H. Differentiation and Transplantation of Human Induced Pluripotent Stem Cell-derived Hepatocyte-like Cells. Stem Cell Rev. 2013, 9, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, L.; Gao, Y.; He, Z.; Yao, D.; Wu, Z.; Cen, J.; Chen, X.; Liu, C.; Hu, Y.; et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 2014, 14, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, J.; Jia, J.; Song, N.; Xiang, C.; Xu, J.; Hou, Z.; Su, X.; Liu, B.; Jiang, T.; et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell 2014, 14, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, Y.; Lu, S.; Zhou, J.; Du, Z.; Duan, C.; Li, Z.; Wang, C. The tumourigenicity of iPS cells and their differentiated derivates. J. Cell. Mol. Med. 2013, 17, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, K.M.; Feldman, D.; Matthews, P.; Radfar, S.; Pickering, R.; Turkula, S.; Zebroski, H.; Dhodapkar, M.V. Natural immunity to pluripotency antigen OCT4 in humans. Proc. Natl. Acad. Sci. USA 2010, 107, 8718–8723. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, P.E.; Ransohoff, J.D.; Nahid, A.; Wu, J.C. Immunogenicity of pluripotent stem cells and their derivatives. Circ. Res. 2013, 112, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ooi, S.; Wang, L. Immunogenicity and tumorigenicity of pluripotent stem cells and their derivatives: Genetic and epigenetic perspectives. Curr. Stem Cell Res. Ther. 2014, 9, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kawabata, K.; Nagamoto, Y.; Kishimoto, K.; Tashiro, K.; Sakurai, F.; Tachibana, M.; Kanda, K.; Hayakawa, T.; Furue, M.K.; et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 2013, 34, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Jeong, D.; Xiao, J.; Schaffer, D.V. Developing defined and scalable 3D culture systems for culturing human pluripotent stem cells at high densities. Cell. Mol. Bioeng. 2014, 7, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L., 3rd; Hannan, N.R.; Bort, R.; Hanley, N.A.; Drake, R.A.; Cameron, G.W.; Wynn, T.A.; Valier, L. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS ONE 2014, 9, e86372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Rezvani, M.; Harbell, J.; Mattis, A.N.; Wolfe, A.R.; Benet, L.Z.; Willenbring, H.; Ding, S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature 2014, 508, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Wernig, M.; Meissner, A.; Cassady, J.P.; Jaenisch, R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2008, 2, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.G.; Winkler, T.; Wu, C.; Guo, V.; Pittaluga, S.; Nicolae, A.; Donahue, R.E.; Metzger, M.E.; Price, S.D.; Uchida, N.; et al. Path to the clinic: Assessment of iPSC-based cell therapies in vivo in a nonhuman primate model. Cell Rep. 2014, 7, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.A.; Ying, L.; Liesa, M.; Segeritz, C.P.; Mills, J.A.; Shen, S.S.; Jean, J.; Lonza, G.C.; Liberti, D.C.; Lang, A.H.; et al. Emergence of a stage-dependent human liver disease signature with directed differentiation of alpha-1 antitrypsin-deficient iPS cells. Stem Cell Rep. 2015, 4, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Sampaziotis, F.; Segeritz, C.P.; Vallier, L. Potential of human induced pluripotent stem cells in studies of liver disease. Hepatology 2015, 62, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagasse, E.; Connors, H.; Al-Dhalimy, M.; Rettsma, M.; Dohse, M.; Osborne, L.; Wang, X.; Finegold, M.; Weissman, I.L.; Grompe, M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000, 6, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Grompe, M.; Strom, S. Mice with human livers. Gastroenterology 2013, 145, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.; Mao, S.; Glorioso, J.; Lillegard, J.; Fisher, J.; Amiot, B.; Rinaldo, P.; Harding, C.O.; Marler, R.; Finegold, M.J.; et al. Fumarylacetoacetate hydrolase deficient pigs are a novel large animal model of metabolic liver disease. Stem Cell Res. 2014, 13, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Oh, H.J.; Chang, U.J.; Koo, S.K.; Jiang, J.X.; Hwang, S.Y.; Lee, J.D.; Yeoh, G.C.; Shin, H.S.; Lee, J.S.; et al. In vivo differentiation of mouse embryonic stem cells into hepatocytes. Cell Transplant. 2002, 11, 359–368. [Google Scholar] [PubMed]
- Hu, C.; Li, L. In vitro and in vivo hepatic differentiation of adult somatic stem cells and extraembryonic stem cells for treating end stage liver diseases. Stem Cells Int. 2015, 2015, 871972. [Google Scholar] [CrossRef] [PubMed]
- Cantz, T.; Sharma, A.D.; Ott, M. Concise review: Cell therapies for hereditary metabolic liver diseases-concepts, clinical results, and future developments. Stem Cells 2015, 33, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.J.; Chowdhury, J.R.; Kaufman, S.S.; Goertzen, T.C.; Chowdhury, N.R.; Warkentin, P.I.; Dorko, K.; Sauter, B.V.; Strom, S.C. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N. Engl. J. Med. 1998, 338, 1422–146. [Google Scholar] [PubMed]
- Dhawan, A.; Mitry, R.R.; Hughes, R.D. Hepatocyte transplantation for liver-based metabolic disorders. J. Inherit. Metab. Dis. 2006, 29, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Najimi, M.; Khuu, D.N.; Lysy, P.A.; Jazouli, N.; Abarca, J.; Sempoux, C.; Sokal, E.M. Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes? Cell Transplant. 2007, 16, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Garate, Z.; Davis, B.R.; Quintana-Bustamante, O.; Segovia, J.C. New frontier in regenerative medicine: Site-specific gene correction in patient-specific induced pluripotent stem cells. Hum. Gene Ther. 2013, 24, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Kim, Y.; Shim, J.S.; Park, J.T.; Wang, R.H.; Leach, S.D.; Liu, J.O.; Deng, C.; Ye, Z.; Jang, Y.Y. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology 2013, 57, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Eggenschwiler, R.; Loya, K.; Wu, G.; Sharma, A.D.; Sgodda, M.; Zychlinski, D.; Herr, C.; Steinemann, D.; Teckman, J.; Bals, R.; et al. Sustained knockdown of a disease-causing gene in patient-specific induced pluripotent stem cells using lentiviral vector-based gene therapy. Stem Cells Transl. Med. 2013, 2, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Chaudhari, P.; Jang, Y.Y. Applications of patient-specific induced pluripotent stem cells; focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int. J. Biol. Sci. 2010, 6, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Rashid, S.T.; Strick-Marchand, H.; Varela, I.; Liu, P.Q.; Paschon, D.E.; Miranda, E.; Ordóñez, A.; Hannan, N.R.; Rouhani, F.J.; et al. Targeted gene correction of a1-antitrypsin deficiency in induced pluripotent stem cells. Nature 2011, 478, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattahi, F.; Asgari, S.; Pournasr, B.; Seifinejad, A.; Totonchi, M.; Taei, A.; Aghdami, N.; Salekdeh, G.H.; Baharvand, H. Disease-corrected hepatocyte-like cells from familial hypercholesterolemia-induced pluripotent stem cells. Mol. Biotechnol. 2013, 54, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Raya, A.; Rodriguez-Piza, I.; Navarro, S.; Richard-Patin, Y.; Guenechea, G.; Sanchez-Sanes, A.; Consiglio, A.; Bueren, J.; Izpisua Belmonte, J.C. A protocol describing the genetic correction of somatic human cells and subsequent generation of iPS cells. Nat. Protoc. 2010, 5, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.W.; Dorrell, C.; Grompe, M. Stem cells and liver regeneration. Gastroenterology 2009, 137, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, A.; Chung, R.T. Acute liver failure: Mechanisms of hepatocyte injury and regeneration. Semin. Liver Dis. 2008, 28, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Millis, J.M.; Losanoff, J.E. Technology insight: Liver support systems. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Pareja, E.; Cortes, M.; Bonora, A.; Fuset, P.; Orbis, F.; Lopez, R.; Mir, J. New alternatives to the treatment of acute liver failure. Transplant. Proc. 2010, 42, 2959–2961. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Liu, X.; Zhao, W.; Wang, Y.; Wang, X. Transplantation of immortalized human fetal hepatocytes prevents acute liver failure in 90% hepatectomized mice. Transplant. Proc. 2010, 42, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; P, B.P.; He, Z.; Holgersson, J.; Olausson, M.; Sumitran-Holgersson, S. Fetal liver-derived mesenchymal stromal cells augment engraftment of transplanted hepatocytes. Cytotherapy 2012, 14, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.L.; Zhang, Y.; Gu, J.Y.; Ding, Y.T. Coencapsulation of hepatocytes with bone marrow mesenchymal stem cells improves hepatocyte-specific functions. Transplantation 2009, 88, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; He, B.; Zhou, X.; Ren, J. Effects of transplanted bone-marrow-derived mesenchymal stem cells in animal models of acute hepatitis. Cell Tissue Res. 2013, 351, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Cui, J.; Zhu, J.; Ma, Y.; Yuan, X.; Shi, J.; Guo, D.; Li, C. Bone marrow-derived mesenchymal stem cells suppress NK cell recruitment and activation in PolyI:C-induced liver injury. Biochem. Biophys. Res. Commun. 2015, 466, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Parekkadan, B.; van Poll, D.; Suganuma, K.; Carter, E.A.; Berthiaume, F.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE 2007, 2, e941. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S. Bridging the gap: Advances in artificial liver support. Liver Transpl. 2012, 18, S10–S14. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, T.J.; Lillegard, J.B.; Nyberg, S.L. Artificial and bioartificial liver support. Semin. Liver Dis. 2008, 28, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, A.A.; Brown, R.S., Jr.; Busuttil, R.W.; Fair, J.; McGuire, B.M.; Rosenthal, P.; Am Esch, J.S., 2nd; Lerut, J.; Nyberg, S.L.; Salizzoni, M.; et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann. Surg. 2004, 239, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, B.; Gautier, A.; Legallais, C. Artificial and bioartificial liver devices: Present and future. Gut 2009, 58, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.J.; Hughes, R.D.; Wendon, J.A.; Dunne, J.; Langley, P.G.; Kelly, J.H.; Gislason, G.T.; Sussman, N.L.; Williams, R. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology 1996, 24, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Kjaergard, L.; Liu, J.; Als-Nielsen, B.; Gluud, C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: A systematic review. JAMA 2003, 289, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, X.; Li, Z.; Ma, X. Artificial and bioartificial liver support systems for acute and acute-on-chronic hepatic failure: A meta-analysis and meta-regression. Exp Ther Med. 2013, 6, 929–936. [Google Scholar] [PubMed]
- Parent, R.; Marion, M.J.; Furio, L.; Trepo, C.; Petit, M.A. Origin and characterization of a human bipotent liver progenitor cell line. Gastroenterology 2004, 126, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, R.; Nibourg, G.A.; van der Hoeven, T.V.; Ackermans, M.T.; Hakvoort, T.B.; van Gulik, T.M.; Lamers, W.H.; Elferink, L.P.; Chamuleau, R.A. The HepaRG cell line is suitable for bioartificial liver application. Int. J. Biochem. Cell Biol. 2011, 43, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Nibourg, G.A.; Hoekstra, R.; van der Hoeven, T.V.; Ackermans, M.T.; Hakvoort, T.B.; van Gulik, T.M.; Chamuleau, R.A. Increased hepatic functionality of the human hepatoma cell line HepaRG cultured in the AMC bioreactor. Int. J. Biochem. Cell Biol. 2013, 45, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Nibourg, G.A.; Hoekstra, R.; van der Hoeven, T.V.; Ackermans, M.T.; Hakvoort, T.B.; van Gulik, T.M.; Chamuleau, R.A. Effects of acute-liver-failure-plasma exposure on hepatic functionality of HepaRG-AMC-bioartificial liver. Liver Int. 2013, 33, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Nibourg, G.A.; Chamuleau, R.A.; van der Hoeven, T.V.; Maas, M.A.; Ruiter, A.F.; Lamers, W.H.; Oude Elferink, R.P.; van Gulik, T.M.; Hoekstra, R. Liver progenitor cell line HepaRG differentiated in a bioartificial liver effectively supplies liver support to rats with acute liver failure. PLoS ONE 2012, 7, e38778. [Google Scholar] [CrossRef] [PubMed]
- Glorioso, J.; Mao, S.; Rodysill, B.; Mounajjed, T.; Kremers, W.; Elgilani, F.; Hickey, R.D.; Haugaa, H.; Rose, C.F.; Amiot, B.; et al. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J. Hepatol. 2015, 63, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, X.; Nyberg, S.L. Potential and Challenges of Induced Pluripotent Stem Cells in Liver Diseases Treatment. J Clin Med. 2014, 3, 997–1017. [Google Scholar] [CrossRef] [PubMed]
- Soto-Gutierrez, A.; Kobayashi, N.; Rivas-Carrillo, J.D.; Navarro-Alvarez, N.; Zhao, D.; Okitsu, T.; Noguchi, H.; Basma, H.; Tabata, Y.; Chen, Y.; et al. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat. Biotechnol. 2006, 24, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Iwamuro, M.; Shiraha, H.; Nakaji, S.; Furutani, M.; Kobayashi, N.; Takaki, A.; Yamamoto, K. A preliminary study for constructing a bioartificial liver device with induced pluripotent stem cell-derived hepatocytes. Biomed. Eng. Online 2012, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Terai, S.; Ishikawa, T.; Omori, K.; Aoyama, K.; Marumoto, Y.; Urata, Y.; Yokoyama, Y.; Uchida, K.; Yamasaki, T.; Fujii, Y.; et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 2006, 24, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Yannaki, E.; Anagnostopoulos, A.; Kapetanos, D.; Xagorari, A.; Iordanidis, F.; Batsis, I.; Kaloyannidis, P.; Athanasiou, E.; Dourvas, G.; Kitis, G.; et al. Lasting amelioration in the clinical course of decompensated alcoholic cirrhosis with boost infusions of mobilized peripheral blood stem cells. Exp Hematol. 2006, 34, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yan, L.; Han, G.; Zhou, X.; Hong, L.; Yin, Z.; Zhang, X.; Wang, S.; Wang, J.; Sun, A.; et al. Controlled trials in hepatitis B virus-related decompensate liver cirrhosis: Peripheral blood monocyte transplant versus granulocyte-colony-stimulating factor mobilization therapy. Cytotherapy 2008, 10, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Lukashyk, S.P.; Tsyrkunov, V.M.; Isaykina, Y.I.; Romanova, O.N.; Shymanskiy, A.T.; Aleynikova, O.V.; Kravchuk, R.I. Mesenchymal Bone Marrow-derived Stem Cells Transplantation in Patients with HCV Related Liver Cirrhosis. J. Clin. Transl. Hepatol. 2014, 2, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kantarcioglu, M.; Demirci, H.; Avcu, F.; Karslioglu, Y.; Babayigit, M.A.; Karaman, B.; Ozturk, K.; Gurel, H.; Akdogan Kayhan, M.; Kaçar, S.; et al. Efficacy of autologous mesenchymal stem cell transplantation in patients with liver cirrhosis. Turk. J. Gastroenterol. 2015, 26, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.P.; Akahoshi, T.; Piao, J.S.; Narahara, S.; Murata, M.; Kawano, T.; Hamano, N.; Ikeda, T.; Hashizume, M. Basic fibroblast growth factor-treated adipose tissue-derived mesenchymal stem cell infusion to ameliorate liver cirrhosis via paracrine hepatocyte growth factor. J. Gastroenterol. Hepatol. 2015, 30, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Chiou, T.W.; Lin, Z.S.; Huang, K.C.; Lin, Y.C.; Huang, P.C.; Syu, W.S.; Harn, H.J.; Lin, S.Z. A proposed novel stem cell therapy protocol for liver cirrhosis. Cell Transplant. 2015, 24, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Uhm, Y.K.; Lim, Y.J.; Yim, S.V. Human umbilical cord blood-derived mesenchymal stem cells improve glucose homeostasis in rats with liver cirrhosis. Int. J. Oncol. 2011, 39, 137–143. [Google Scholar] [PubMed]
- Yovchev, M.I.; Xue, Y.; Shafritz, D.A.; Locker, J.; Oertel, M. Repopulation of the fibrotic/cirrhotic rat liver by transplanted hepatic stem/progenitor cells and mature hepatocytes. Hepatology 2014, 59, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Carpino, G.; Gentile, R.; Napoletano, C.; Rahimi, H.; Franchitto, A.; Semeraro, R.; Nuti, M.; Onori, P.; Berloco, P.B.; et al. Transplantation of human fetal biliary tree stem/progenitor cells into two patients with advanced liver cirrhosis. BMC Gastroenterol. 2014, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, F.S. Stem cell therapies for liver failure and cirrhosis. J Hepatol. 2013, 59, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Y.; Tai, G.; Zhang, S. Could co-transplantation of iPS cells derived hepatocytes and MSCs cure end-stage liver disease? Cell Biol. Int. 2009, 33, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Espejel, S.; Roll, G.R.; McLaughlin, K.J.; Lee, A.Y.; Zhang, J.Y.; Laird, D.J.; Okita, K.; Yamanaka, S.; Willenbring, H. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J. Clin. Investig. 2010, 120, 3120–3126. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Viganò, L.; Polastri, R.; Muratore, A.; Eminefendic, H.; Regge, D.; Capussotti, L. Postoperative liver dysfunction and future remnant liver: Where is the limit? World J. Surg. 2007, 31, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Am Esch, J.S., 2nd; Knoefel, W.T.; Klein, M.; Ghodsizad, A.; Fuerst, G.; Poll, L.W.; Piechaczek, C.; Burchardt, E.R.; Feifel, N.; Stoldt, V.; et al. Portal application of autologous CD133+ bone marrow cells to the liver: A novel concept to support hepatic regeneration. Stem Cells 2005, 23, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Furst, G.; Schulte am Esch, J.; Poll, L.W.; Hosch, S.B.; Fritz, L.B.; Klein, M.; Godehardt, E.; Krieg, A.; Wecker, B.; Stoldt, V.; et al. Portal vein embolization and autologous CD133+ bone marrow stem cells for liver regeneration: Initial experience. Radiology 2007, 243, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Fouad, O.; Abdelnasser, A.; Chowdhury, A.; Selim, A. Stem cell therapy improves the outcome of liver resection in cirrhotics. J. Gastrointest. Cancer 2010, 41, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R.; Remberger, M.; Cederlund, K.; Ringden, O.; Barkholt, L. A comparison between low intensity and reduced intensity conditioning in allogeneic hematopoietic stem cell transplantation for solid tumors. Haematologica 2008, 93, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. 2012, 12, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Sekine, T.; Makuuchi, M.; Yamasaki, S.; Kosuge, T.; Yamamoto, J.; Shimada, K.; Sakamoto, M.; Hirohashi, S.; Ohashi, Y.; et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: A randomised trial. Lancet 2000, 356, 802–807. [Google Scholar] [CrossRef]
- Lei, F.; Zhao, B.; Haque, R.; Xiong, X.; Budgeon, L.; Christensen, N.D.; Wu, Y.; Song, J. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res. 2011, 71, 4742–4747. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Zhang, Z.; Feng, X.; Li, T.; Liu, N.; Lai, J.; Shuai, L.; Xiong, Q.; Fu, C.; Zou, H.; et al. TRAIL-secreting mesenchymal stem cells promote apoptosis in heat-shock-treated liver cancer cells and inhibit tumor growth in nude mice. Gene Ther. 2014, 21, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Sharkis, S.J.; Jones, R.J.; Civin, C.; Jang, Y.Y. Pluripotent stem cell-based cancer therapy: Promise and challenges. Sci. Transl. Med. 2012, 4, 127ps9. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Morisaki, Y.; Kuno, S.; Nagamoto, Y.; Harada, K.; Furukawa, N.; Ohtaka, M.; Nishimura, K.; Imagawa, K.; Sakurai, F.; et al. Prediction of interindividual differences in hepatic functions and drug sensitivity by using human iPS-derived hepatocytes. Proc. Natl. Acad. Sci. USA 2014, 111, 16772–16777. [Google Scholar] [CrossRef] [PubMed]
- Yersiz, H.; Cameron, A.; Carmody, I.; Zimmerman, M.; Kelly, B.; Ghobrial, R.; Farmer, D.G.; Busuttil, R.W. Split liver transplantation. Transplant. Proc. 2006, 38, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Dahm, F.; Georgiev, P.; Clavien, P.A. Small-for-Size Syndrome After Partial Liver Transplantation: Definition, Mechanisms of Disease and Clinical Implications. Am. J. Transplant. 2005, 5, 2605–2610. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.D.; Liu, Z.W.; Cashman, S.; Fusai, G.K. Small for size syndrome following living donor and split liver transplantation. World J. Gastrointest. Surg. 2010, 2, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Schwabe, R.F.; Kai, Y.; He, L.; Yang, L.; Bunzendahl, H.; Brenner, D.A.; Lemasters, J.J. Liver regeneration is suppressed in small-for-size liver grafts after transplantation: Involvement of c-Jun N-terminal kinase, cyclin D1, and defective energy supply. Transplantation 2006, 82, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Lv, X.; Liang, R.; Wang, L.; Liu, Q. Suppression of graft regeneration, not ischemia/reperfusion injury, is the primary cause of small-for-size syndrome after partial liver transplantation in mice. PLoS ONE 2014, 9, e93636. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yao, A.-.H.; Chen, N.; Pu, L.-.Y.; Fan, Y.; Lv, L.; Sun, B.C.; Li, G.Q.; Wang, X.H. Mesenchymal Stem Cells Over-expressing Hepatocyte Growth Factor Improve Small-for-size Liver Grafts Regeneration. Mol. Ther. 2007, 15, 1382–1389. [Google Scholar] [PubMed]
- Yu, Y.; Lu, L.; Qian, X.; Chen, N.; Yao, A.; Pu, L.; Zhang, F.; Li, X.; Kong, L.; Sun, B.; et al. Antifibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem Cells Dev. 2010, 19, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, Z.; Yan, J.; Ma, D.; Shi, M.; Zhang, M.; Peng, C.; Li, H. Mesenchymal stem cells promote liver regeneration and prolong survival in small-for-size liver grafts: Involvement of C-Jun N-terminal kinase, cyclin D1, and NF-kappaB. PLoS ONE 2014, 9, e112532. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wei, C.; Cheng, K.; Han, B.; Yan, J.; Zhang, M.; Peng, C.; Liu, Y. Mesenchymal stem cell-conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. J. Surg. Res. 2013, 183, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.; Newsome, P.N. Mesenchymal stromal cell therapy in liver disease: Opportunities and lessons to be learnt? Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G791–G800. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.E.; Yarmush, M.L.; Uygun, K. Application of whole-organ tissue engineering in hepatology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Fukumitsu, K.; Yasuchika, K.; Adachi, K.; Kawase, E.; Suemori, H.; Nakatsuji, N.; Ikai, I.; Uemoto, S. Effects of extracellular matrixes and growth factors on the hepatic differentiation of human embryonic stem cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G313–G321. [Google Scholar] [CrossRef] [PubMed]
- Palakkan, A.A.; Hay, D.C.; Anil Kumar, P.R.; Kumary, T.V.; Ross, J.A. Liver tissue engineering and cell sources: Issues and challenges. Liver Int. 2013, 33, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.J.; Duncan, S.A. Engineering liver tissue from induced pluripotent stem cells: A first step in generating new organs for transplantation? Hepatology 2013, 58, 2198–2201. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, J.A.; Baptista, P.M.; Soto-Gutierrez, A. Cellular therapy and bioartificial approaches to liver replacement. Curr. Opin. Organ. Transplant. 2012, 17, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pati, N.T.; Sarin, S.K. Use of stem cells for liver diseases-current scenario. J. Clin. Exp. Hepatol. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Hiura, H.; Toyoda, M.; Okae, H.; Sakurai, M.; Miyauchi, N.; Sato, A.; Kiyokawa, N.; Okita, H.; Miyagawa, Y.; Akutsu, H.; et al. Stability of genomic imprinting in human induced pluripotent stem cells. BMC Genet. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.; Roberts, R.M.; Mirochnitchenko, O. Large animal models for stem cell therapy. Stem Cell Res. Ther. 2013, 4, 23. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolas, C.; Wang, Y.; Luebke-Wheeler, J.; Nyberg, S.L. Stem Cell Therapies for Treatment of Liver Disease. Biomedicines 2016, 4, 2. https://doi.org/10.3390/biomedicines4010002
Nicolas C, Wang Y, Luebke-Wheeler J, Nyberg SL. Stem Cell Therapies for Treatment of Liver Disease. Biomedicines. 2016; 4(1):2. https://doi.org/10.3390/biomedicines4010002
Chicago/Turabian StyleNicolas, Clara, Yujia Wang, Jennifer Luebke-Wheeler, and Scott L. Nyberg. 2016. "Stem Cell Therapies for Treatment of Liver Disease" Biomedicines 4, no. 1: 2. https://doi.org/10.3390/biomedicines4010002
APA StyleNicolas, C., Wang, Y., Luebke-Wheeler, J., & Nyberg, S. L. (2016). Stem Cell Therapies for Treatment of Liver Disease. Biomedicines, 4(1), 2. https://doi.org/10.3390/biomedicines4010002