Direct Lymph Node Vaccination of Lentivector/Prostate-Specific Antigen is Safe and Generates Tissue-Specific Responses in Rhesus Macaques
Abstract
:1. Introduction
2. Experimental Section
2.1. Lentivectors (LV)
2.2. Flow Cytometry
2.3. Murine rhPSA Tumor Cell Lines
2.4. Western Blot
2.5. Mice and Footpad Injections
2.6. Rhesus Macaque Surgeries and LV Injections
2.7. Quantitative PCR
2.8. Magnetic Resonance Imaging (MRI) for Prostate Volumes
2.9. Hematological Analyses
2.10. Cytokine and Chemokine Measurements
2.11. Pathology Analysis
2.12. Statistical Analyses
3. Results
3.1. LV/rhPSA/rhCD25 Vaccination Protects Mice from Relevant Tumor Challenge
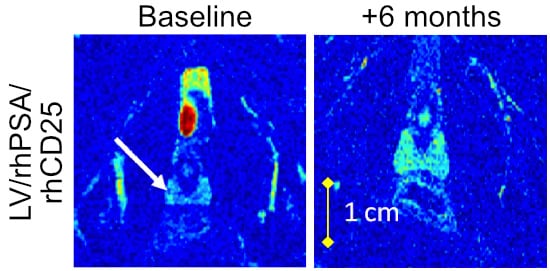
3.2. Direct Lymph Node Immunization with LV/rhPSA/rhCD25 in Rhesus Macaques
3.3. Prime-Boost Administration of Smaller LV Doses Ameliorates Acute Responses
3.4. Proviral Genomes Remained within the Site of Injection
3.5. Peripheral Blood Profiles Remained Normal after Direct LV Administration
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Mapara, M.Y.; Sykes, M. Tolerance and cancer: Mechanisms of tumor evasion and strategies for breaking tolerance. J. Clin. Oncol. 2004, 22, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef] [PubMed]
- Mercader, M.; Bodner, B.K.; Moser, M.T.; Kwon, P.S.; Park, E.S.; Manecke, R.G.; Ellis, T.M.; Wojcik, E.M.; Yang, D.; Flanagan, R.C.; et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc. Natl. Acad. Sci. USA 2011, 98, 14565–14570. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Bruno, T.C.; Meeker, A.K.; De Marzo, A.M.; Isaacs, W.B.; Drake, C.G. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate 2009, 69, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.; Sreekumar, A.; Varambally, S.; Shen, R.; Giacherio, D.; Mehra, R.; Monte, J.E.; Pienta, K.J.; Sanda, M.G.; et al. Autoantibody signatures in prostate cancer. N. Engl. J. Med. 2005, 353, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Vesalainen, S.; Lipponen, P.; Talja, M.; Syrjanen, K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur. J. Cancer 1994, 30a, 1797–1803. [Google Scholar] [CrossRef]
- McArdle, P.A.; Canna, K.; McMillan, D.C.; McNical, A.M.; Campbell, R.; Underwood, M.A. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br. J. Cancer 2004, 91, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, N.A.; Petrylak, D.; Kantoff, P.W.; Dela Rosa, C.; Stewart, F.P.; Kuan, L.Y.; Whoitmore, J.B.; Trager, J.B.; Poehlein, C.H.; Frohlich, M.W.; et al. Sipuleucel-T immune parameters correlate with survival: An analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol. Immunother. 2013, 62, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009, 115, 3670–3679. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Loisel-Meyer, S.; Foley, R.; Medin, J.A. Immuno-gene therapy approaches for cancer: From in vitro studies to clinical trials. Front. Biosci. 2008, 13, 3202–3214. [Google Scholar] [CrossRef] [PubMed]
- Mossoba, M.E.; Walia, J.S.; Rasaiah, V.I.; Buxhoeveden, N.; Head, R.; Ying, C.; Foley, J.E.; Bramson, J.L.; Fowler, D.H.; Medin, J.A. Tumor protection following vaccination with low doses of lentivirally transduced DCs expressing the self-antigen erbB2. Mol. Ther. 2008, 16, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Medin, J.A.; Liang, S.B.; Hou, J.W.; Kelley, L.S.; Peace, D.J.; Fowler, D.H. Efficient transfer of PSA and PSMA cDNAs into DCs generates antibody and T cell antitumor responses in vivo. Cancer Gene Ther. 2005, 12, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Loisel-Meyer, S.; Felizardo, T.; Mariotti, J.; Mossoba, M.E.; Foley, J.E.; Kammerer, R.; Mizue, N.; Keefe, R.; McCart, J.A.; Zimmermann, W.; et al. Potent induction of B- and T-cell immunity against human carcinoembryonic antigen-expressing tumors in human carcinoembryonic antigen transgenic mice mediated by direct lentivector injection. Mol. Cancer Ther. 2009, 8, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Yoshimitsu, M.; Sato, T.; Tao, K.; Walia, J.S.; Rasaiah, V.I.; Sleep, G.T.; Murray, G.J.; Poeppl, A.G.; Underwood, J.; West, L.; et al. Bioluminescent imaging of a marking transgene and correction of Fabry mice by neonatal injection of recombinant lentiviral vectors. Proc. Natl. Acad. Sci. USA 2004, 101, 16909–16914. [Google Scholar] [CrossRef] [PubMed]
- Walia, J.S.; Neschadim, A.; Lopez-Perez, O.; Alayoubi, A.; Fan, X.; Carpentier, S.; Madden, M.; Lee, C.J.; Cheung, F.; Jaffray, D.A.; et al. Autologous transplantation of lentivector/acid ceramidase-transduced hematopoietic cells in nonhuman primates. Hum. Gene Ther. 2011, 22, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Estcourt, M.J.; Ramsay, A.J.; Brooks, A.; Thomson, S.A.; Medveckzy, C.J.; Ramshaw, I.A. Prime-boost immunization generates a high frequency, high-avidity CD8+ cytotoxic T lymphocyte population. Int. Immunol. 2002, 14, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Woodberry, T.; Gardner, J.; Elliot, S.L.; Leyrer, S.; Purdie, D.M.; Chaplin, P.; Suhrbier, A. Prime boost vaccination strategies: CD8 T cell numbers, protection, and Th1 bias. J. Immunol. 2003, 170, 2599–2604. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Valladeau-Guilemond, J.; Donnadieu, M.H.; Sastre-Garau, X.; Soumelis, V.; Amigorena, S. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 2012, 209, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Wuest, S.C.; Edwan, J.H.; Martin, J.F.; Han, S.; Perry, J.S.; Cartagena, C.M.; Matsuura, E.; Maric, D.; Waldmann, T.A.; Bielekova, B. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med. 2011, 17, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Driesen, J.; Popov, A.; Schultze, J.L. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology 2008, 213, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Candore, G.; Cigna, D.; Colucci, A.T.; Modica, M.A. Biological significance of soluble IL-2 receptor. Mediat. Inflamm. 1993, 2, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, C.C.; Rouhani, S.J.; Srinivasan, N.; Engelhard, V.H. Peripheral tissue homing receptors enable T cell entry into lymph nodes and affect the anatomical distribution of memory cells. J. Immunol. 2013, 191, 2412–2425. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Aarntzen, E.H.; De Vries, I.J.; Lesterhuis, W.J.; Schuurhuis, D.; Jacobs, J.F.; Bol, K.; Schreibelt, G.; Mus, R.; De Wilt, J.H.; Haanen, J.B.; et al. Targeting CD4+ T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res. 2013, 73, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Boswell, W.; Smith, J.; Hersh, E.; Snively, J.; Diaz, M.; Miles, S.; Liu, X.; Obracea, M.; Qiu, Z.; et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J. Immunother. 2008, 31, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Spaner, D.E.; Astsaturov, I.; Vogul, T.; Petrella, T.; Elias, I.; Burdett-Radoux, S.; Verma, S.; Iscoe, N.; Hamilton, P.; Bernstein, N.L. Enhanced viral and tumor immunity with intranodal injection of canary pox viruses expressing the melanoma antigen, gp100. Cancer 2006, 106, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Felizardo, T.C.; Wang, J.C.; McGray, R.A.; Evelegh, C.; Spaner, D.E.; Fowler, D.H.; Bramson, J.L.; Medin, J.A. Differential immune responses mediated by adenovirus- and lentivirus-transduced DCs in a HER-2/neu overexpressing tumor model. Gene Ther. 2011, 18, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Amos, S.M.; Duong, C.P.; Westwood, J.A.; Ritchie, D.S.; Junghans, R.P.; Darcy, P.K.; Kershaw, M.H. Autoimmunity associated with immunotherapy of cancer. Blood 2011, 118, 499–509. [Google Scholar] [CrossRef] [PubMed]





| Organ | LV/rhPSA/rhCD25 Single Injection | LV/rhPSA/rhCD25 Prime-Boost | LV/eGFP Prime-Boost | |||
|---|---|---|---|---|---|---|
| 7680JM | 7663VL | 7612BN | 7593DN | 7597KN | 7685DZ | |
| Heart | − | − | − | − | N.D. | N.D. |
| Liver | − | − | − | − | N.D. | N.D. |
| Thymus | − | − | − | − | N.D. | N.D. |
| Lung | − | − | − | − | N.D. | N.D. |
| Testicles | − | − | − | − | N.D. | N.D. |
| Spleen | − | − | − | − | N.D. | N.D. |
| Mesenteric LN | − | − | − | − | + | + |
| Right Inguinal LN | − | − | − | − | + | − |
| Left Axillary LN | − | − | − | − | − | − |
| Right Axillary LN | − | − | − | − | − | − |
| Left Popliteal LN | + | − | − | − | − | − |
| Left Inguinal LN | − | − | − | − | + | − |
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Au, B.C.; Lee, C.-J.; Lopez-Perez, O.; Foltz, W.; Felizardo, T.C.; Wang, J.C.M.; Huang, J.; Fan, X.; Madden, M.; Goldstein, A.; et al. Direct Lymph Node Vaccination of Lentivector/Prostate-Specific Antigen is Safe and Generates Tissue-Specific Responses in Rhesus Macaques. Biomedicines 2016, 4, 6. https://doi.org/10.3390/biomedicines4010006
Au BC, Lee C-J, Lopez-Perez O, Foltz W, Felizardo TC, Wang JCM, Huang J, Fan X, Madden M, Goldstein A, et al. Direct Lymph Node Vaccination of Lentivector/Prostate-Specific Antigen is Safe and Generates Tissue-Specific Responses in Rhesus Macaques. Biomedicines. 2016; 4(1):6. https://doi.org/10.3390/biomedicines4010006
Chicago/Turabian StyleAu, Bryan C., Chyan-Jang Lee, Orlay Lopez-Perez, Warren Foltz, Tania C. Felizardo, James C.M. Wang, Ju Huang, Xin Fan, Melissa Madden, Alyssa Goldstein, and et al. 2016. "Direct Lymph Node Vaccination of Lentivector/Prostate-Specific Antigen is Safe and Generates Tissue-Specific Responses in Rhesus Macaques" Biomedicines 4, no. 1: 6. https://doi.org/10.3390/biomedicines4010006