In Vitro Anti-Oxidant and Anti-Microbial Potentiality Investigation of Different Fractions of Caryota urens Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection, Identification and Preparation of Materials
2.2. Extraction and Fractionation
2.3. Test Microorganisms
2.4. Preparation of Inoculum
2.5. Antimicrobial Susceptibility Test
2.6. Minimum Inhibitory Concentration (MIC) Determination Test
2.7. Antioxidant Activity Determination by DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Radical Scavenging Assay
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Jamison, D.T.; Breman, J.G.; Meashametal, A.R. Complementary and Alternative Medicine. In Disease Control Priorities in Developing Countries, 2nd ed.; World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Bhattacharjee, S.K. Handbook of Medicinal Plants, 3rd ed.; Pointer Pub: Jaipur, India, 2001; pp. 1–6. [Google Scholar]
- Sandhu, D.S.; Heinrich, M. The use of health foods, spices and other botanicals in the Sikh community in London. Phyto Res. 2005, 19, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.P.; Solis, P.N.; Calderon, A.I.; Guionneau-Sinclair, F.; Correa, M.; Galdames, C.; Guerra, C.; Espinosa, A.; Alvenda, G.I.; Robles, G.; et al. Medical ethnobotany of the Teribes of Bocas del Toro, Panama. J. Ethnopharmacol. 2005, 96, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.J.; Ochoa, V.J.; Ocampo, S.A.; Muñoz, J.F. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement. Altern. Med. 2006, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Mauricio, L.B.; Maria, G.T.; Eduardo, H.C. Infectious diseases epidemiology. J. Epidemiol. Community Health 2006, 60, 192–195. [Google Scholar]
- Boucher, H.W.; Talbot, G.H.; Bradleyetal, J.S. Badbugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hancock, E.W. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect. Dis. 2005, 5, 209–218. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microb. 2008, 124, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Marasini, B.P.; Baral, P.; Aryal, P.; Ghimire, K.R.; Neupane, S.; Dahal, N.; Singh, A.; Ghimire, L.; Shrestha, K. Evaluation of Antibacterial Activity of Some Traditionally Used Medicinal Plants against Human Pathogenic Bacteria. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar]
- Akarpat, A.; Tuthan, S.; Ustun, N.S. Effects of hot water extracts from myrtle, rosemary, nettle and lemon balm leaves on lipid oxidation and color of beef patties during frozen storage. J. Food Process. Preserv. 2008, 32, 117–132. [Google Scholar] [CrossRef]
- Pazos, M.; Alonso, A.; Sanchez, I.; Medina, I. Hydroxytyrosol prevents oxidative deterioration in foodstuffs rich in fish lipids. J. Agric. Food Chem. 2008, 56, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, N.M.A.; Tariq, P. In vitro antibacterial activities of kalonji, cumin and poppy seed. Pakistan J. Bot. 2008, 40, 461–467. [Google Scholar]
- Hussain, A.; Zaman, M.K.; Ramteke, M. Antibacterial activity of trunk bark of Alstoniascholaris. Asian J. Pharm. Clin. Res. 2010, 3, 46–47. [Google Scholar]
- Shan, B.; Cai, Y.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Chanda, S. Activity of some medicinal plants against certain pathogenic bacterial strains. Indian J. Pharmacol. 2006, 38, 142–144. [Google Scholar]
- Cohen, M.L. Changing patterns of infectious disease. Nature 2002, 406, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Premakumara, G.A.S.; Wijayarathna, C.D.; Ratnasooriya, W.D. Antioxidant activity of Caryota urens L. (Kithul) sap. Trop. Agric. Res. 2012, 23, 117–125. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Senguttuvan, J.; Krishnaswamy, T. Evaluation of phytocehmicals and in vitro antioxidant activities of some selected Indian medicinal fruits from Kannur city, Kerala. World J. Pharm. Pharm. Sci. 2013, 2, 4121–4138. [Google Scholar]
- Charles, A.; Ramani, V.A. Qualitative phytochemical screening, anti-oxidant and anti-microbial activity studies on ethanolic flowers extract of Caryota urens Linn. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 498–505. [Google Scholar]
- Charles, A.; Joseph, M.; Alex, V.R. Quanttitative estimation of primary and secondary metabolites on flowers of Caryota urens L. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 43–45. [Google Scholar]
- Lacmata, S.T.; Kuete, V.; Dzoyem, J.P.; Tankeo, S.B.; Teke, G.N.; Kuiate, J.R.; Pages, J.-M. Antibacterial activities of selected cameroonian plants and their synergistic effects with antibiotics against bacteria expressing MDR phenotypes. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial susceptibility testing. EUCAST disk diffusion method (Version 5.0, January 2015). Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/Manual_v_5.0_EUCAST_Disk_Test.pdf (accessed on 25 July 2016).
- Valgas, C.; de Souza, S.M.; Elza, F.A.S.; Artur, S., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Jennifer, M. Andrews: Determination of minimum inhibitory concentrtions. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, K.O.; Oluwa, O.K.; Omomigbehin, E.O. Antimicrobial activity of crude extracts of three medicinal plants used in south-west Nigerian folk medicine on some food borne bacterial pathogens. Afr. J. Tradit. CAM 2006, 3, 13–22. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Efflux Pumps of Gram-Negative Bacteria. J. Bacteriol. 1996, 178, 5853–5859. [Google Scholar] [PubMed]
- Li, X.-Z.; Nikaido, H. Efflux-Mediated Drug Resistance in Bacteria: An Update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef] [PubMed]
- Ananth, D.A.; Sivasudha, T.; Rameshkumar, A.; Jeyadevi, R.; Aseervatham, S.B. Chemical constituents, in vitro antioxidant and antimicrobial potential of Caryota urens L. Free Radic. Antioxid. 2013, 3, 107–112. [Google Scholar] [CrossRef]
- Dellavalle, P.D.; Cabrera, A.; Alem, D.; Larrañaga, P.; Ferreira, F.; Rizza, M.D. Antifungal Activity Of Medicinal Plant Extracts Against Phytopathogenic Fungus. Chil. J. Agric. Res. 2011, 71, 231–239. [Google Scholar] [CrossRef]
- Person, A.K.; Chudgar, S.M.; Norton, B.L.; Tong, B.C.; Stout, J.E. Aspergillus niger: An unusual cause of invasive pulmonary aspergillosis. J. Med. Microbiol. 2010, 59, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Bouza, E.; Cuenca-Estrella, M.; Eiros, J.M.; Pérez, M.J.; Sánchez-Somolinos, M.; Rincón, C.; Hortal, J.; Peláez, T. Saccharomyces cerevisiae Fungemia: An Emerging Infectious Disease. Clin. Infect. Dis. 2005, 40, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huyc, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Zengin, G.; Aktumsek, A. Investigation of Antioxidant Potentials of Solvent Extracts from Different Anatomical Parts of Asphodeline Anatolica E. Tuzlaci: An Endemic Plant to Turkey. Afr. J. Tradit Complement Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Oktaya, M.; Gulcin, İ.; Kufrevioglu, Ö. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Uddin, S.; Hasan, F.; Mamun, A.A.; Hossain, S.; Islam, T.; Asaduzzaman, M. In vitro estimation of antioxidant activity of caryota urens fruits. Indo Am. J. Pharm. Sci. 2015, 2, 1486–1490. [Google Scholar]
- Andrews, J.M. BSAC standardized disc susceptibility testing method (version 6). J. Antimicrob. Chemother. 2007, 60, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Mamun, A.A.; Khanum, S.; Begum, Y.; Alam, S. Analysis of in vitro antioxidant activity of Caryota. urens L. leaves: A traditional natural remedy. J. Coast. Life Med. 2016, 4, 483–489. [Google Scholar] [CrossRef]
- Kiyama, H.; Oono, T.; Huh, W.K. Actions of farnesol and xylitol against Staphylococcus aureus. Chemotherapy 2012, 48, 122–128. [Google Scholar]
- Jabra-Rizk, M.A.; Johnson, J.K.; Forrest, G.; Mankes, K.; Meiller, T.F.; Venezia, R.A. Prevalence of Candida dubliniensis Fungemia at a large teaching hospital. Clin. Infect. Dis. 2005, 41, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
| Diameter of Zone of Inhibition (mm) | ||||
|---|---|---|---|---|
| Test Organisms | Carbon Tetra Chloride (250 µg/disc) | Chloroform (500 µg/disc) | n-Hexane Extract (500 µg/disc) | Kanamycin (30 µg/disc) |
| Gram Positive Bacteria | ||||
| Bacillus subtilis | 18.67 ± 1.247 | 21.00 ± 1.414 | 24.67 ± 1.247 | 27.67 ± 1.700 |
| Bacillus megaterium | 23.33 ± 2.055 | 17.67 ± 2.494 | 21.00 ± 3.266 | 26.33 ± 1.247 |
| Bacillus cereus | 19.33 ± 0.943 | 20.33 ± 0.471 | 24.00 ± 3.266 | 27.33 ± 2.494 |
| Sarina lutea | 21.00 ± 0.816 | 22.00 ± 1.633 | 21.00 ± 1.633 | 24.67 ± 1.247 |
| Gram Negative Bacteria | ||||
| Vibrio mimicus | 17.00 ± 1.633 | 23.00 ± 2.449 | 22.33 ± 1.700 | 28.67 ± 1.247 |
| Shigella boydii | 17.33 ± 2.494 | 25.83 ± 0.850 | 21.17 ± 1.434 | 28.17 ± 1.027 |
| Escherichia coli | Not significant | 25.67 ± 1.247 | 20.67 ± 0.943 | 34.33 ± 3.300 |
| Pseudomonas aeruginosa | 20.33 ± 2.055 | 22.67 ± 2.055 | 21.33 ± 1.247 | 26.00 ± 2.944 |
| Fungi | ||||
| Aspergillus niger | 18.67 ± 2.055 | 26.67 ± 1.247 | 21.67 ± 1.247 | 23.00 ± 0.816 |
| Saccharomyces cerevisiae | 22.33 ± 0.943 | 23.33 ± 0.471 | 24.00 ± 0.816 | 25.33 ± 0.471 |
| Sample | Concentrations of n-Hexane Extracts (µg/mL) | Observation of the Growth Results of the Microorganisms at Different Concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bs | Bm | Bc | Sl | Sb | Pa | Ec | Vm | An | Sc | ||
| 1 | 512 | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| 2 | 256 | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| 3 | 128 | NG | NG | NG | NG | SG | SG | NG | NG | NG | NG |
| 4 | 64 | NG | SG | SG | NG | SG | SG | SG | NG | SG | SG |
| 5 | 32 | SG | G | G | SG | G | G | G | SG | G | G |
| 6 | 16 | G | G | G | G | G | G | G | G | G | G |
| 7 | 8 | G | G | G | G | G | G | G | G | G | G |
| 8 | 4 | G | G | G | G | G | G | G | G | G | G |
| 9 | 2 | G | G | G | G | G | G | G | G | G | G |
| Cs | 512 | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| Ci | 0 | G | G | G | G | G | G | G | G | G | G |
| Cm | 0 | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| MIC (µg/mL) determined from n-hexane extract | 64 | 128 | 128 | 64 | 256 | 256 | 128 | 64 | 128 | 128 | |
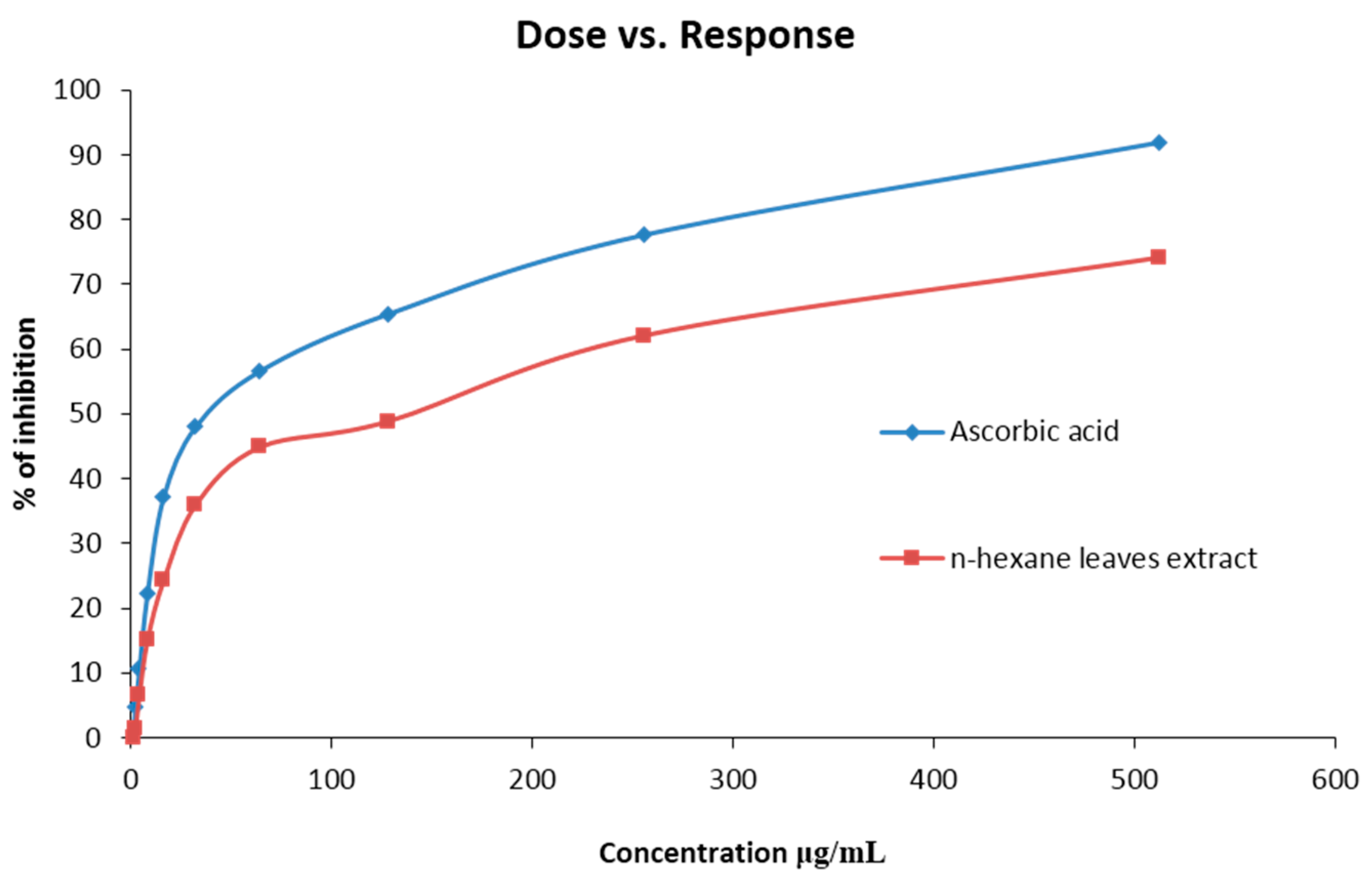
| Sample | Concentration (µg/mL) | % of Inhibition | IC50 Value (µg/mL) |
|---|---|---|---|
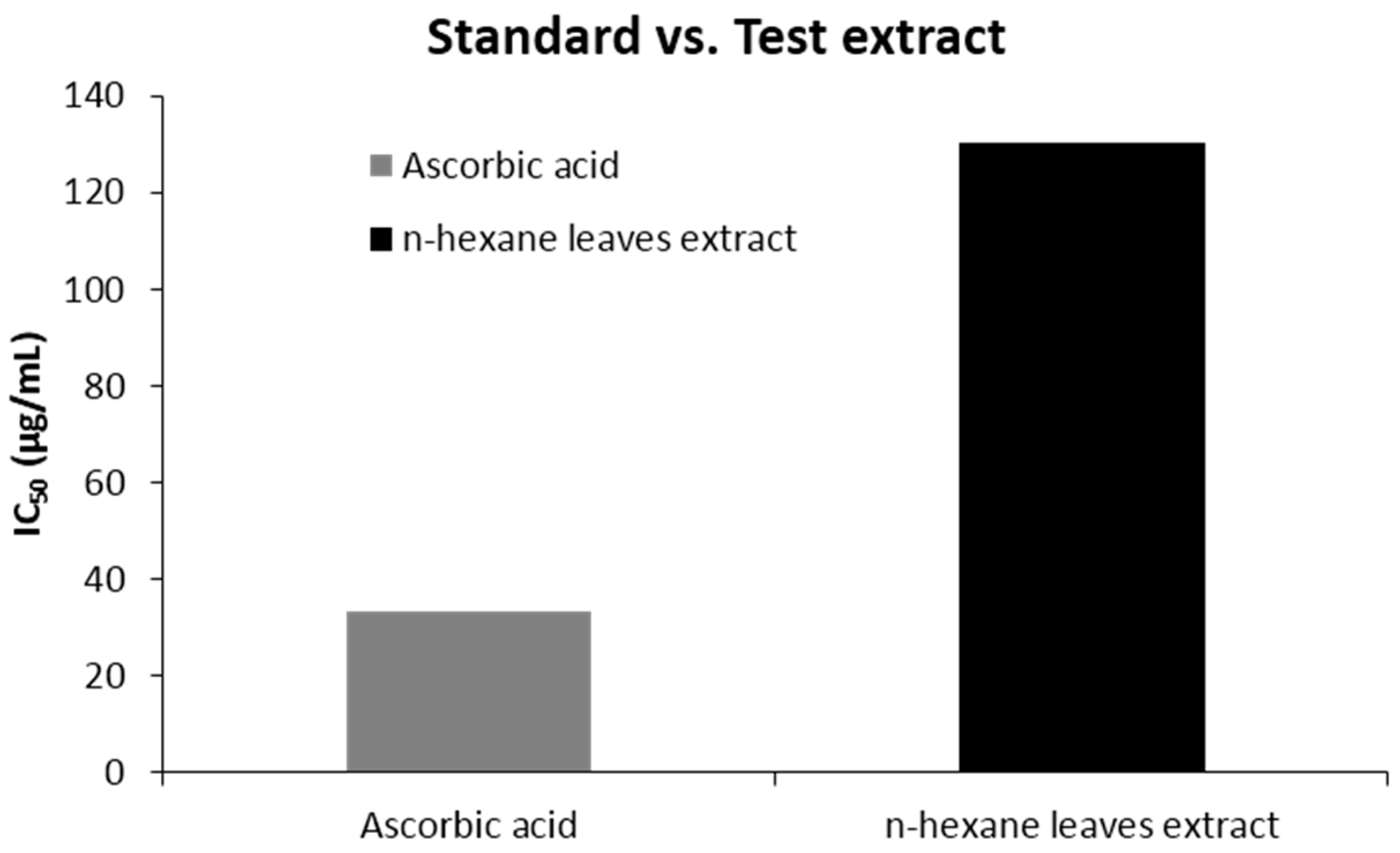
| Ascorbic acid | 1 | 0.95 | 33.18 |
| 2 | 4.55 | ||
| 4 | 10.36 | ||
| 8 | 22.45 | ||
| 16 | 37.15 | ||
| 32 | 48.22 | ||
| 64 | 57.85 | ||
| 128 | 65.56 | ||
| 265 | 78.22 | ||
| 512 | 92.45 | ||
| n-Hexane leave extract of C. urens | 1 | 0.35 | 130.32 |
| 2 | 1.23 | ||
| 4 | 6.45 | ||
| 8 | 15.24 | ||
| 16 | 25.36 | ||
| 32 | 36.47 | ||
| 64 | 45.25 | ||
| 128 | 49.11 | ||
| 265 | 62.45 | ||
| 512 | 74.12 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam, S.; Mahmud, M.K.; Naquib, M.H.; Hossain, S.M.; Alam, M.N.; Uddin, M.J.; Sajid, I.; Hossain, M.S.; Karim, M.S.; Hasan, M.A. In Vitro Anti-Oxidant and Anti-Microbial Potentiality Investigation of Different Fractions of Caryota urens Leaves. Biomedicines 2016, 4, 17. https://doi.org/10.3390/biomedicines4030017
Azam S, Mahmud MK, Naquib MH, Hossain SM, Alam MN, Uddin MJ, Sajid I, Hossain MS, Karim MS, Hasan MA. In Vitro Anti-Oxidant and Anti-Microbial Potentiality Investigation of Different Fractions of Caryota urens Leaves. Biomedicines. 2016; 4(3):17. https://doi.org/10.3390/biomedicines4030017
Chicago/Turabian StyleAzam, Shofiul, Md. Kayes Mahmud, Md. Hamza Naquib, Saad Mosharraf Hossain, Mohammad Nazmul Alam, Md. Josim Uddin, Irfan Sajid, Muhammad Sazzad Hossain, Md. Salimul Karim, and Md. Ali Hasan. 2016. "In Vitro Anti-Oxidant and Anti-Microbial Potentiality Investigation of Different Fractions of Caryota urens Leaves" Biomedicines 4, no. 3: 17. https://doi.org/10.3390/biomedicines4030017







