NF-κB, the Importance of Being Dynamic: Role and Insights in Cancer
Abstract
:1. Introduction: NF-κB, the Importance of Being Dynamic
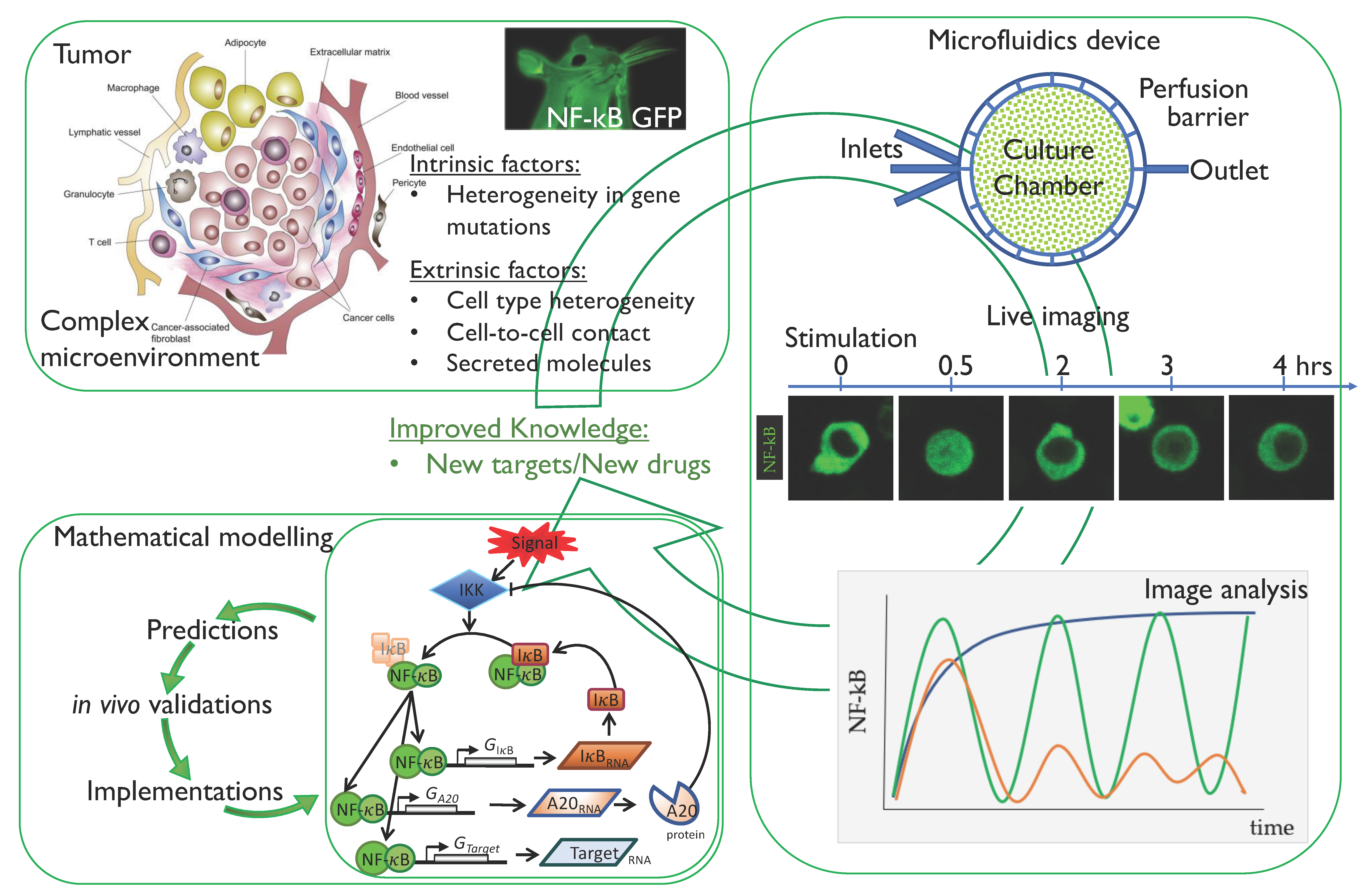
2. NF-κB and Cancer
3. Single-Cell Dynamics: Experimental Methodologies
3.1. Cell Systems and Fluorescent Tags
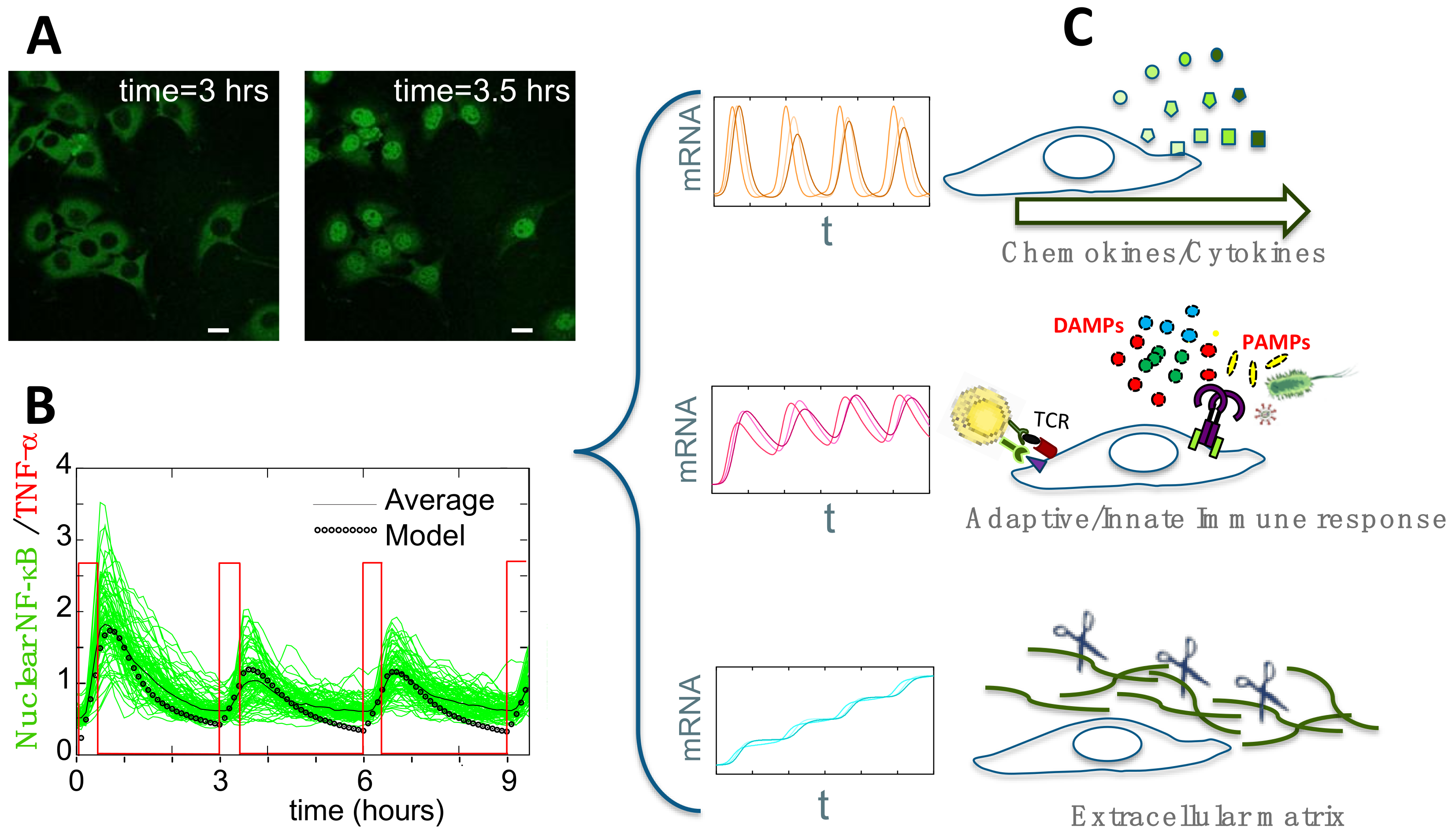
3.2. Experimental Observation and Quantification of Single-Cell NF-κB Dynamics
3.3. The Emerging Importance of Microfluidics Technologies
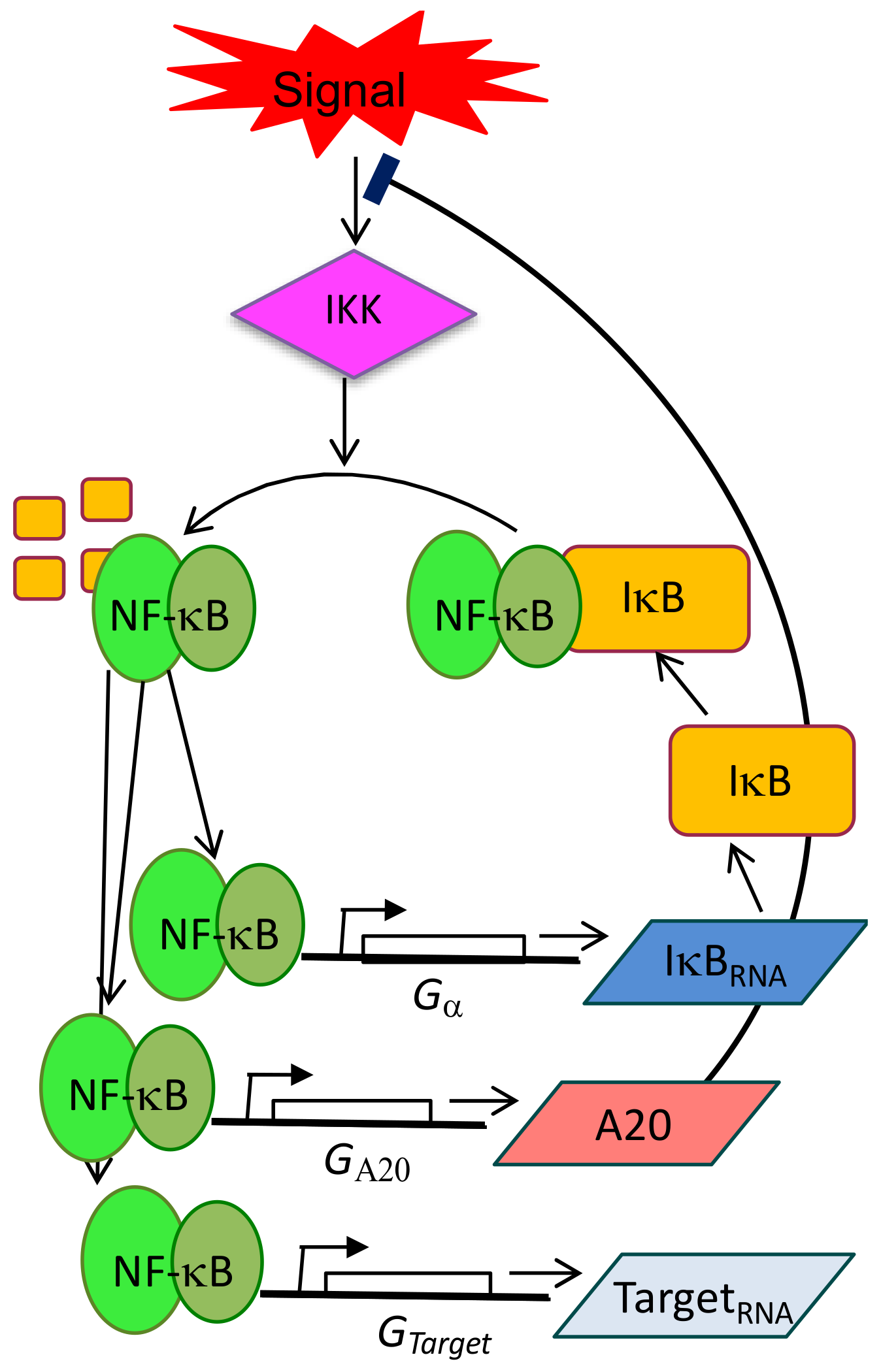
4. Single-Cell Dynamics: Main Insights on NF-κB Regulation of Gene Expression
4.1. Single-Cell NF-κB Dynamics: Main Insights
4.2. Connecting Dynamics to Transcription
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karin, M. Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, A.C.; Perkins, N.D. NF-κB and the cell cycle. Biochem. Soc. Trans. 2014, 42, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kearns, J.D.; Basak, S.; Werner, S.L.; Huang, C.S.; Hoffmann, A. IκBε provides negative feedback to control NF-κB oscillations, signaling dynamics, and inflammatory gene expression. J. Cell Biol. 2006, 173, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C. Non-canonical NF-κB signaling pathway. Cell Res. 2010, 21, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.E.; Ihekwaba, A.E.C.; Elliott, M.; Johnson, J.R.; Gibney, C.A.; Foreman, B.E.; Nelson, G.; See, V.; Horton, C.A.; Spiller, D.G.; et al. Oscillations in NF-kappaB Signaling Control the Dynamics of Gene Expression. Science 2004, 306, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Covert, M.W. High-throughput, single-cell NF-κB dynamics. Curr. Opin. Genet. Dev. 2010, 20, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Ashall, L.; Horton, C.A.; Nelson, D.E.; Paszek, P.; Harper, C.V.; Sillitoe, K.; Ryan, S.; Spiller, D.G.; Unitt, J.F.; Broomhead, D.S.; et al. Pulsatile stimulation determines timing and specificity of NF-κB-dependent transcription. Science 2009, 324, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.H.; Salvatore, L.; De Lorenzi, R.; Indrawan, A.; Pasparakis, M.; Hager, G.L.; Bianchi, M.E.; Agresti, A. Sustained oscillations of NF-κB produce distinct genome scanning and gene expression profiles. PLoS ONE 2009, 4, e7163. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E.C.; Walker, S.R.; Savery, K.; Frank, D.A.; Gaudet, S. Fold change of nuclear NF-κB determines TNF-induced transcription in single cells. Mol. Cell 2014, 53, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.; Boddington, C.; Downton, P.; Rowe, W.; Bagnall, J.; Lam, C.; Maya-Mendoza, A.; Schmidt, L.; Harper, C.V.; Spiller, D.G.; et al. Signal transduction controls heterogeneous NF-κB dynamics and target gene expression through cytokine-specific refractory states. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, S.; de Toma, I.; Piffer, A.; Bianchi, M.E.; Agresti, A. NF-κB oscillations translate into functionally related patterns of gene expression. Elife 2016, 5, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.H.; Lin, Y.; Elowitz, M.B. Functional roles of pulsing in genetic circuits. Science 2013, 342, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Purvis, J.E.; Karhohs, K.W.; Mock, C.; Batchelor, E.; Loewer, A.; Lahav, G. p53 Dynamics Control Cell Fate. Science 2012, 336, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.; Hughey, J.J.; Lee, T.K.; Lipniacki, T.; Quake, S.R.; Covert, M.W. Single-cell NF-κB dynamics reveal digital activation and analogue information processing. Nature 2010, 466, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, S.; Bianchi, M.E.; Agresti, A. High-throughput analysis of NF-kB dynamics in single cells reveals basal nuclear localization of NF-kB and spontaneous activation of oscillations. PLoS ONE 2014, 9, e90104. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A. The IkappaB–NF-kappaB Signaling Module : Temporal Control and Selective Gene Activation. Science 2002, 1241, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Mehling, M.; Tay, S. Microfluidic cell culture. Curr. Opin. Biotechnol. 2014, 25, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Paszek, P.; Ryan, S.; Ashall, L.; Sillitoe, K.; Harper, C.V.; Spiller, D.G.; Rand, D.A.; White, M.R.H. Population robustness arising from cellular heterogeneity. Proc. Natl. Acad. Sci. USA 2010, 107, 11644–11649. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lewis, D.J.; Duvic, M. Novel Mutations Involving NF-κB and B-Cell Signaling Pathways in Primary Cutaneous Large B-Cell Lymphoma, Leg-Type and Comparison with Sézary Syndrome. J. Investig. Dermatol. 2017, 137, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, A.; Navarro, M.; Suárez-Cabrera, C.; Bravo, A.; Ramirez, A. Context-dependent role of IKKβ in cancer. Genes 2017, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Basseres, D.S.; Baldwin, A.S. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 2006, 25, 6817–6830. [Google Scholar] [CrossRef] [PubMed]
- Keats, J.J.; Fonseca, R.; Chesi, M.; Schop, R.; Baker, A.; Chng, W.J.; Van Wier, S.; Tiedemann, R.; Shi, C.X.; Sebag, M.; et al. Promiscuous Mutations Activate the Noncanonical NF-κB Pathway in Multiple Myeloma. Cancer Cell 2007, 12, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, P.; Zhao, L.; Huang, L.; Zhang, Z.; Zhao, S.; Huang, J. NF-κB Expression and Outcomes in Solid Tumors: A Systematic Review and Meta-Analysis; Wolters Kluwer Health: Philadelphia, PA, USA, 2015; Volume 94, p. e1687. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Staudt, L.M. Oncogenic activation of NF-kappaB. Cold Spring Harb. Perspect. Biol. 2010, 2, a000109. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, C.M.; Davis, R.E.; Demchenko, Y.; Bellamy, W.; Gabrea, A.; Zhan, F.; Lenz, G.; Hanamura, I.; Wright, G.; Xiao, W.; et al. Frequent Engagement of the Classical and Alternative NF-κB Pathways by Diverse Genetic Abnormalities in Multiple Myeloma. Cancer Cell 2007, 12, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Neri, P.; Tassone, P.; Yasui, H.; Ishitsuka, K.; Raje, N.; Chauhan, D.; Podar, K.; Mitsiades, C.; Dang, L.; et al. MLN120B, a novel IkappaB kinase beta inhibitor, blocks multiple myeloma cell growth in vitro and in vivo. Clin. Cancer Res. 2006, 12, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, M.; Moreaux, J.; Vos, J.D.; Hose, D.; Mahtouk, K.; Abouladze, M.; Robert, N.; Baudard, M.; Rème, T.; Romanelli, A.; et al. Targeting NF-kappaB pathway with an IKK2 inhibitor induces inhibition of multiple myeloma cell growth. Br. J. Haematol. 2007, 138, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, T.; Perkins, N.D.; Wilson, C.L. NFKB1: A suppressor of inflammation, ageing and cancer. FEBS J. 2016, 283, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.; Mallon, E.A.; Horgan, P.G.; Paul, A.; McMillan, D.C.; Edwards, J. The relationship between members of the canonical NF-κB pathway, components of tumour microenvironment and survival in patients with invasive ductal breast cancer. Oncotarget 2017, 8, 33002–33013. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, Y.N.; Brents, L.A.; Li, Z.; Bergsagel, L.P.; McGee, L.R.; Kuehl, M.W. Novel inhibitors are cytotoxic for myeloma cells with NFkB inducing kinase-dependent activation of NFkB. Oncotarget 2014, 5, 4554–4566. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-C.; Huang, C.-H.; Yang, S.-F.; Li, C.-C.; Chang, L.-L.; Lin, H.-H.; Ke, H.-L.; Wei, Y.-C.; Wu, W.-J. Nuclear factor-κB activation predicts an unfavourable outcome in human upper urinary tract urothelial carcinoma. BJU Int. 2010, 106, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Al-Saad, S.; Al-Shibli, K.; Donnem, T.; Persson, M.; Bremnes, R.M.; Busund, L.T. The prognostic impact of NF-κB p105, vimentin, E-cadherin and Par6 expression in epithelial and stromal compartment in non-small-cell lung cancer. Br. J. Cancer 2008, 99, 1476–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, Y.; Richmond, A. NF-κB activation in melanoma. Pigment Cell Res. 2006, 19, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Peri, S.; Devarajan, K.; Yang, D.H.; Knudson, A.G.; Balachandran, S. Meta-Analysis Identifies NF-κB as a Therapeutic Target in Renal Cancer. PLoS ONE 2013, 8, e76746. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P. Colorectal cancer and NF-κB signaling pathway. Gastroenterol. Hepatol. Bed Bench 2011, 4, 127–132. [Google Scholar] [PubMed]
- Jin, R.; Yi, Y.; Yull, F.E.; Blackwell, T.S.; Clark, P.E.; Koyama, T.; Smith, J.A.; Matusik, R.J. NF-κB gene signature predicts prostate cancer progression. Cancer Res. 2014, 74, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Didonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Nagel, D.; Vincendeau, M.; Eitelhuber, A.C.; Krappmann, D. Mechanisms and consequences of constitutive NF-kappaB activation in B-cell lymphoid malignancies. Oncogene 2014, 33, 5655–5665. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, E.S.; Askautrud, H.A.; Kees, T.; Park, J.-H.; Plaks, V.; Ewald, A.J.; Fein, M.; Rasch, M.G.; Tan, Y.-X.; Qiu, J.; et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 2012, 21, 488–503. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F. Cancer: Fibroblasts for all seasons. Nature 2016, 530, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Bouyssou, J.M.C.; Ghobrial, I.M.; Roccaro, A.M. Targeting SDF-1 in multiple myeloma tumor microenvironment. Cancer Lett. 2016, 380, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Boyle, W.J.; Simonet, W.S.; Kostenuik, P.J.; Dougall, W.C.; Sullivan, J.K.; Martin, J.S.; Dansey, R. Bench to bedside: Elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 2012, 11, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Dougall, W.C.; Holen, I.; González Suárez, E. Targeting RANKL in metastasis. Bonekey Rep. 2014, 3, 519. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E.C.; Qasaimeh, M.A.; Xia, X.; Juncker, D.; Gaudet, S. NF-κB signalling and cell fate decisions in response to a short pulse of tumour necrosis factor. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Di, Z.; Herpers, B.; Fredriksson, L.; Yan, K.; van de Water, B.; Verbeek, F.J.; Meerman, J.H.N. Automated Analysis ofNF-κB Nuclear Translocation Kinetics in High-Throughput Screening. PLoS ONE 2012, 7, e52337. [Google Scholar] [CrossRef] [PubMed]
- Sero, J.E.; Sailem, H.Z.; Ardy, R.C.; Almuttaqi, H.; Zhang, T.; Bakal, C. Cell shape and the microenvironment regulate nuclear translocation of NF-κB in breast epithelial and tumor cells. Mol. Syst. Biol. 2015, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Gapuzan, M.E.R.; Schmah, O.; Pollock, A.D.; Hoffmann, A.; Gilmore, T.D. Immortalized fibroblasts from NF-κB RelA knockout mice show phenotypic heterogeneity and maintain increased sensitivity to tumor necrosis factor α after transformation by v-Ras. Oncogene 2005, 24, 6574–6583. [Google Scholar] [CrossRef] [PubMed]
- James, C.D.; Moorman, M.W.; Carson, B.D.; Branda, C.S.; Lantz, J.W.; Manginell, R.P.; Martino, A.; Singh, A.K. Nuclear translocation kinetics of NF-κB in macrophages challenged with pathogens in a microfluidic platform. Biomed. Microdevices 2009, 11, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Li, N.; Lao, Q.; Gottschalk, R.A.; Hager, G.L.; Fraser, I.D.C. Switching of the Relative Dominance Between Feedback Mechanisms in Lipopolysaccharide-Induced NF-κB Signaling. Sci. Signal. 2014, 7, ra6. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.; Van Valen, D.; DeFelice, M.M.; Macklin, D.N.; Kudo, T.; Jaimovich, A.; Carr, A.; Meyer, T.; Pe’er, D.; Boutet, S.C.; et al. Measuring Signaling and RNA-Seq in the Same Cell Links Gene Expression to Dynamic Patterns of NF-κB Activation. Cell Syst. 2017, 4, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, R.A.; Tay, S. Noise facilitates transcriptional control under dynamic inputs. Cell 2015, 160, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Heltberg, M.; Kellogg, R.A.; Krishna, S.; Tay, S.; Jensen, M.H. Noise Induces Hopping between NF-κB Entrainment Modes. Cell Syst. 2016, 3, 532–539. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, R.; Gareus, R.; Fengler, S.; Pasparakis, M. GFP-p65 knock-in mice as a tool to study NF-κB dynamics in vivo. Genesis 2009, 47, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Lin, G.; Li, J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J. 2016, 283, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, L.; Siggers, T.; Tiana, G.; Caprara, G.; Notarbartolo, S.; Corona, T.; Pasparakis, M.; Milani, P.; Bulyk, M.L.; Natoli, G. Noncooperative Interactions between Transcription Factors and Clustered DNA Binding Sites Enable Graded Transcriptional Responses to Environmental Inputs. Mol. Cell 2010, 37, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, S.; Bianchi, M.E.; Agresti, A. A simple model of NF-κB dynamics reproduces experimental observations. J. Theor. Biol. 2014, 347, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sjöberg, R.; Leyrat, A.A.; Pirone, D.M.; Chen, C.S.; Quake, S.R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 2007, 79, 8557–8563. [Google Scholar] [CrossRef] [PubMed]
- Cheong, R.; Wang, C.J.; Levchenko, A. High Content Cell Screening in a Microfluidic Device. Mol. Cell. Proteom. 2009, 8, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Menolascina, F.; Fiore, G.; Orabona, E.; De Stefano, L.; Ferry, M.; Hasty, J.; di Bernardo, M.; di Bernardo, D. In-Vivo Real-Time Control of Protein Expression from Endogenous and Synthetic Gene Networks. PLoS Comput. Biol. 2014, 10, e1003625. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Marquès, E.; Zambrano, S.; Tiérrez, A.; Bianchi, M.E.; Agresti, A.; García-del Portillo, F. Single-cell analyses reveal an attenuated NF-κB response in the Salmonella-infected fibroblast. Virulence 2017, 8, 719–740. [Google Scholar] [CrossRef] [PubMed]
- Covert, M.W.; Leung, T.H.; Gaston, J.E.; Baltimore, D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science 2005, 309, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Denny, E.M.; Sanghvi, J.C.; Gaston, J.E.; Maynard, N.D.; Hughey, J.J.; Covert, M.W. A Noisy Paracrine Signal Determines the Cellular NF-κB Response to Lipopolysaccharide. Sci. Signal. 2009, 2, ra65. [Google Scholar] [CrossRef] [PubMed]
- Barken, D.; Wang, C.J.; Kearns, J.; Cheong, R.; Hoffmann, A.; Levchenko, A. Comment on “Oscillations in NF-kB Signaling Control the Dynamics of Gene Expression”. Science 2005, 308, 52a. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.E. Response to Comment on “Oscillations in NF-κB Signaling Control the Dynamics of Gene Expression. ” Science 2005, 308, 52. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Wang, Q.Y.; Ke, Y.X.; Liu, S.Y.; Ju, J.Q.; Lim, W.A.; Tang, C.; Wei, P. Design of Tunable Oscillatory Dynamics in a Synthetic NF-κB Signaling Circuit. Cell Syst. 2017, 5, 460–470.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gupta, S.; Schipper, D.L.; Kowalczyk, G.J.; Mancini, A.E.; Faeder, J.R.; Lee, R.E.C.; Zhang, Q.; Gupta, S.; Schipper, D.L.; et al. NF-kB Dynamics Discriminate between TNF Doses in Single Cells. Cell Syst. 2017, 5, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Elowitz, M.B.; Levine, A.J.; Siggia, E.D.; Swain, P.S. Stochastic gene expression in a single cell. Science 2002, 297, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.; Lambert, D.W. Cancer-associated fibroblasts—Not-so-innocent bystanders in metastasis to bone? J. Bone Oncol. 2016, 5, 128–131. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, F.; Zambrano, S.; Agresti, A. NF-κB, the Importance of Being Dynamic: Role and Insights in Cancer. Biomedicines 2018, 6, 45. https://doi.org/10.3390/biomedicines6020045
Colombo F, Zambrano S, Agresti A. NF-κB, the Importance of Being Dynamic: Role and Insights in Cancer. Biomedicines. 2018; 6(2):45. https://doi.org/10.3390/biomedicines6020045
Chicago/Turabian StyleColombo, Federica, Samuel Zambrano, and Alessandra Agresti. 2018. "NF-κB, the Importance of Being Dynamic: Role and Insights in Cancer" Biomedicines 6, no. 2: 45. https://doi.org/10.3390/biomedicines6020045





