Tumor Molecular Profiling for an Individualized Approach to the Treatment of Hepatocellular Carcinoma: A Patient Case Study
Abstract
:1. Introduction
2. Case Reports
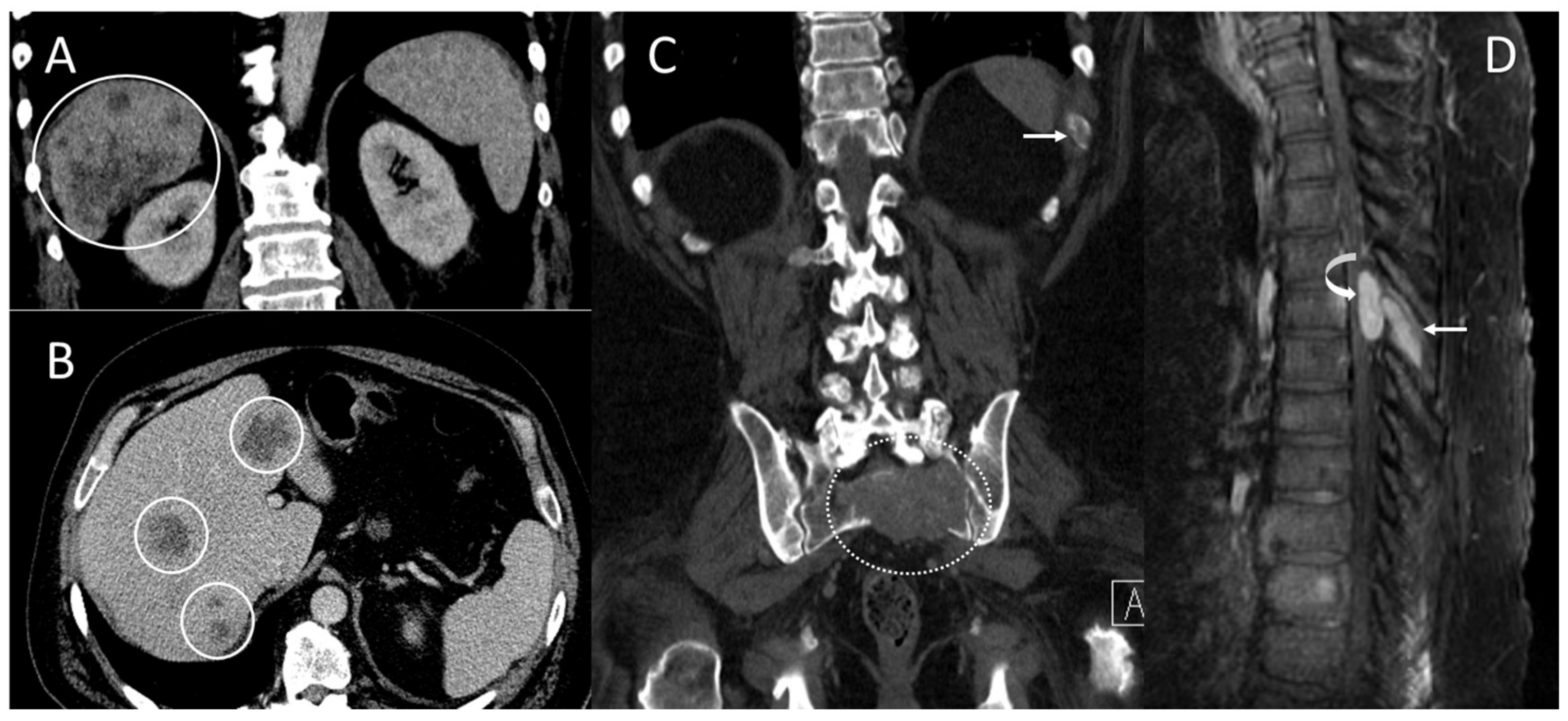
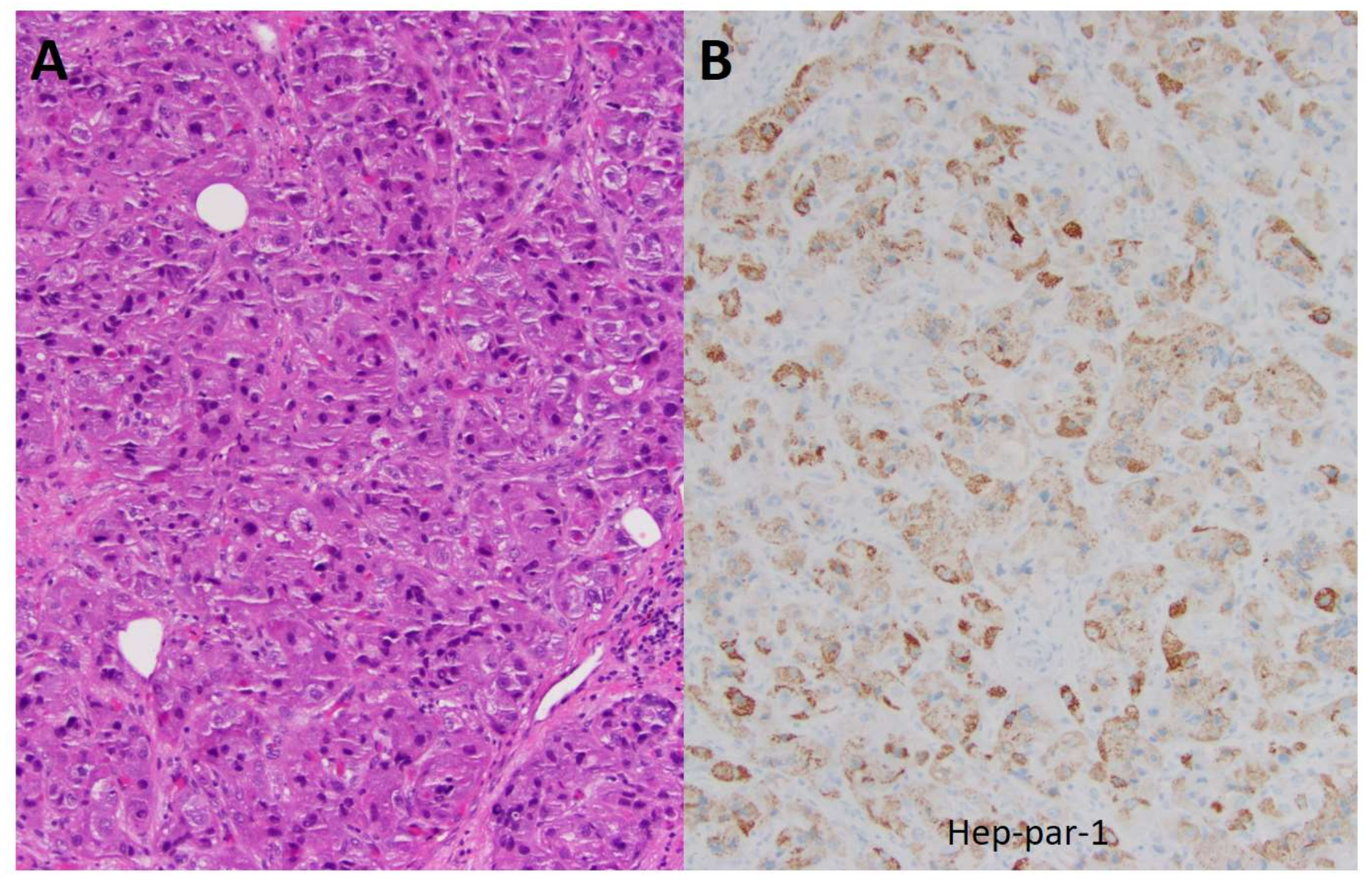
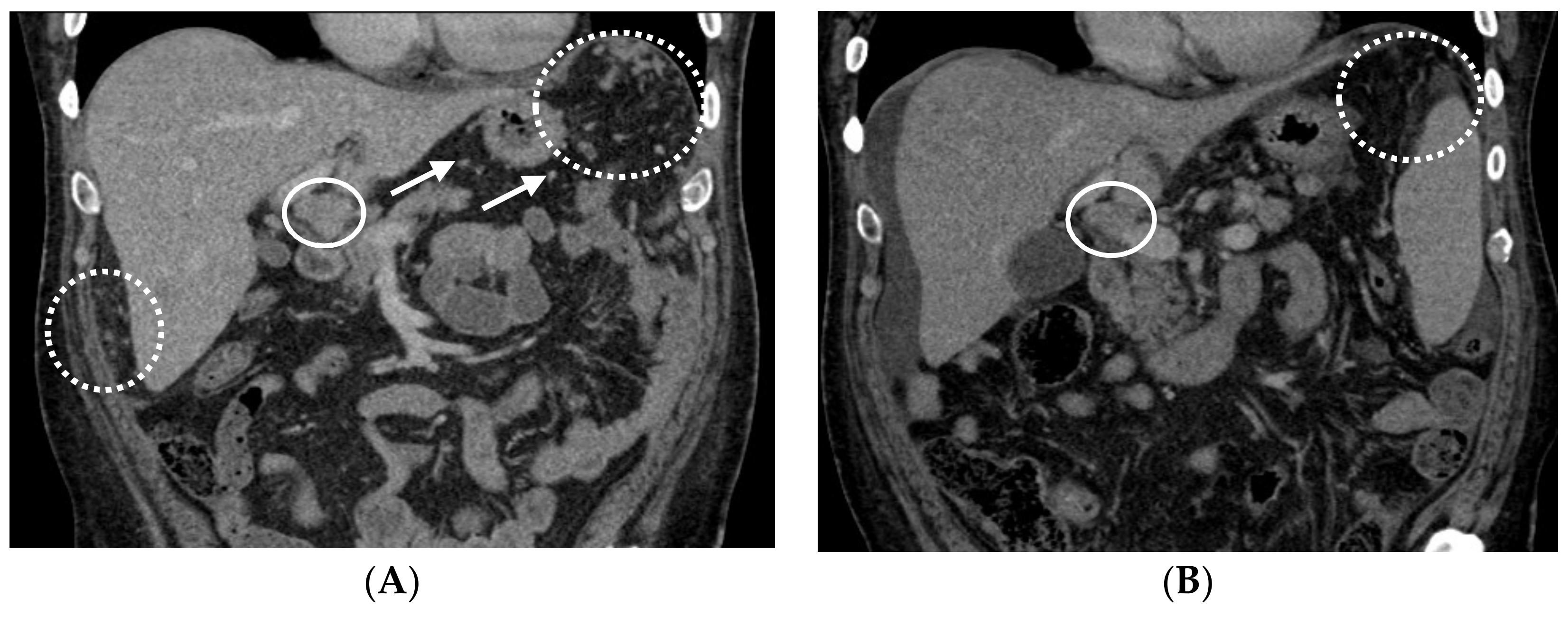
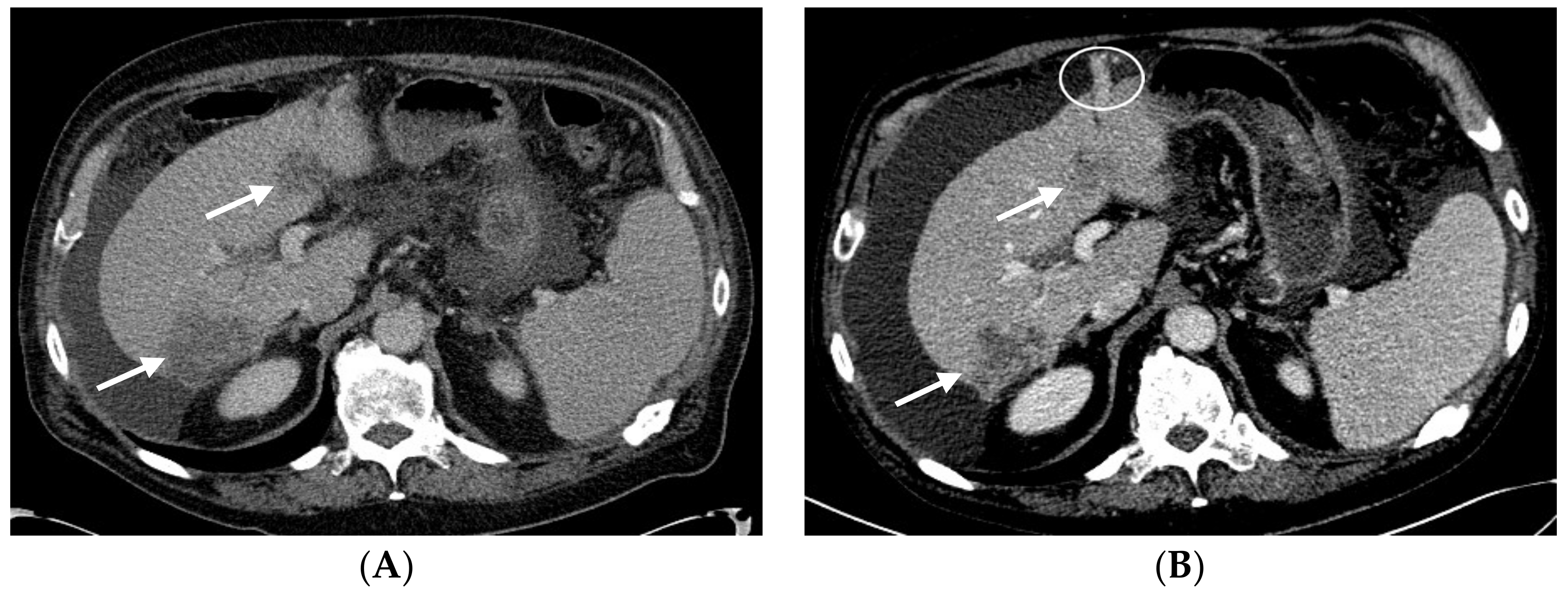
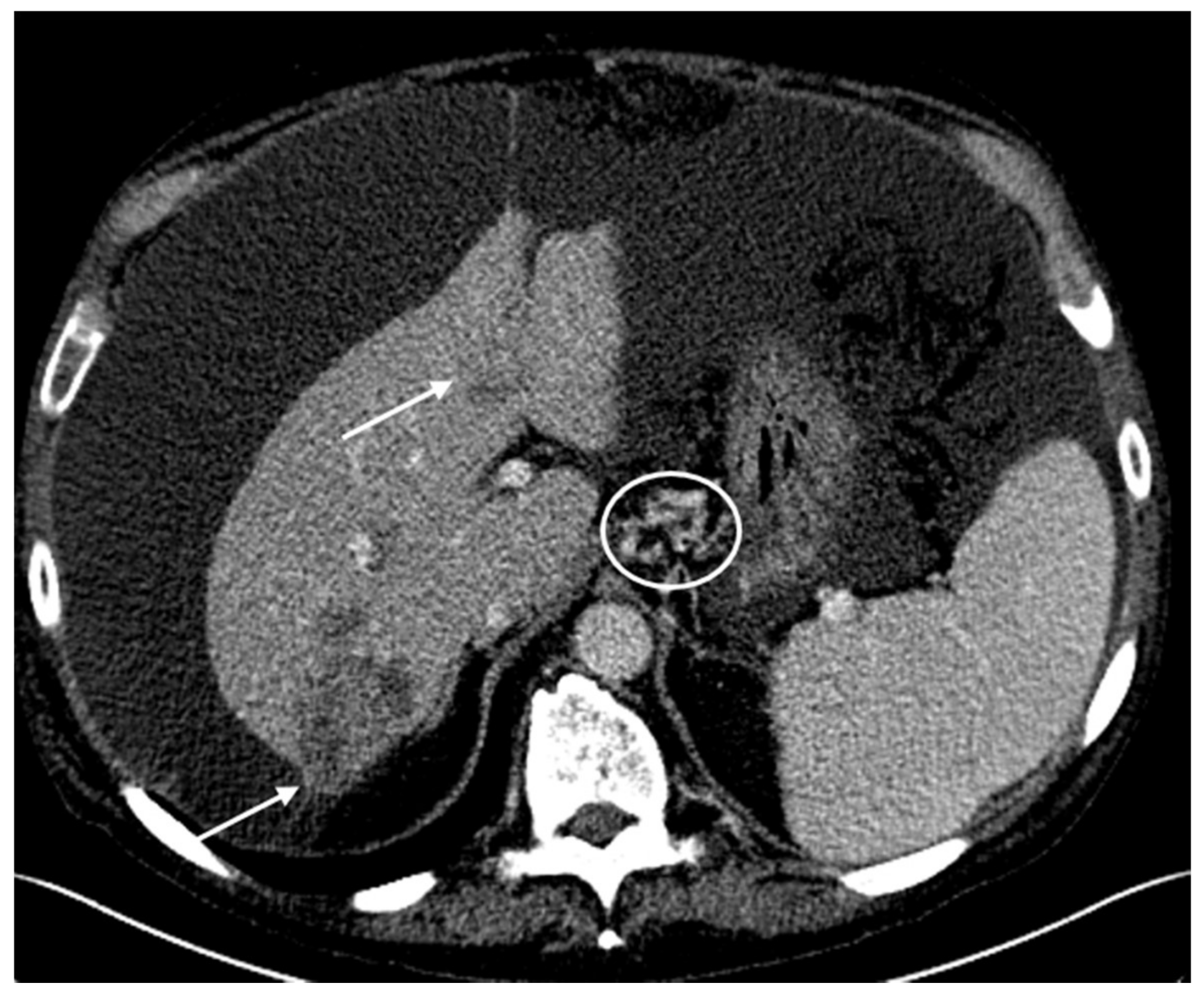
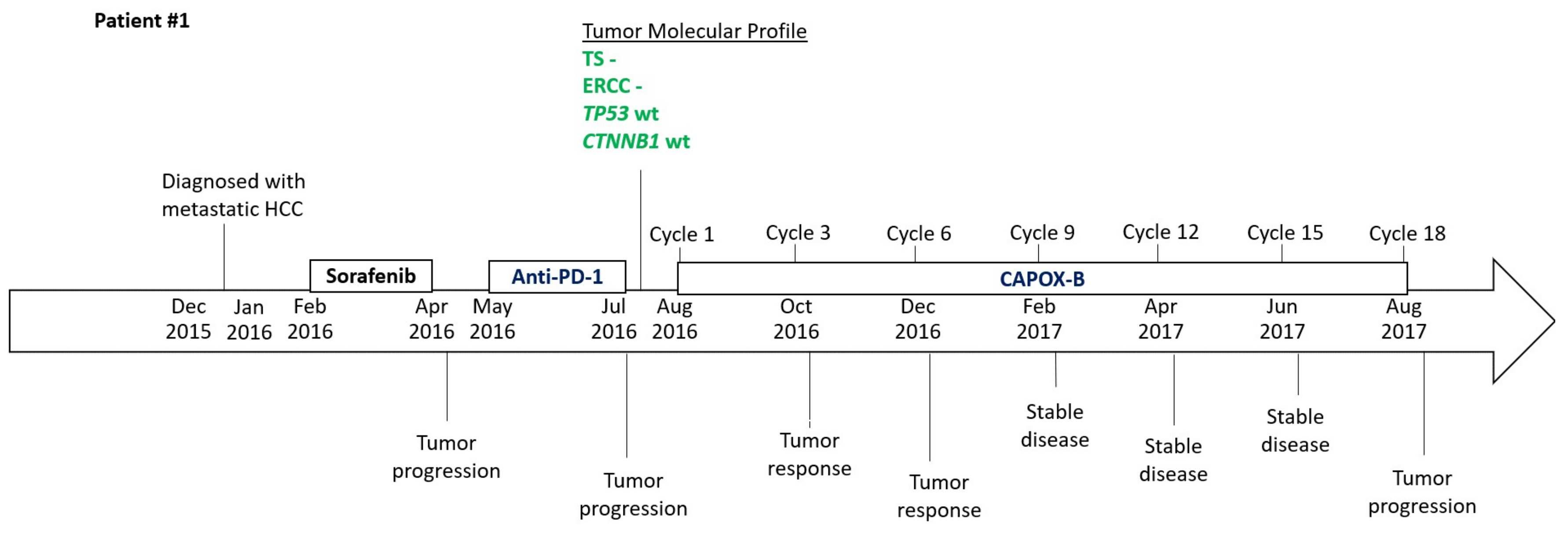
2.1. Patient #1
2.2. Patient #2
2.3. Timeline
3. Discussion
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Abrams, T.A.; Alberts, S.R.; Anaya, D.A.; Are, C.; Brown, D.B.; Chang, D.T.; Covey, A.M.; et al. NCCN Guidelines Insights Hepatobiliary cancers, Version 1.2017. Featured updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2017, 15, 563–573. [Google Scholar] [CrossRef]
- Marks, E.I.; Yee, N.S. Molecular genetics and targeted therapy in hepatocellular carcinoma. Curr. Cancer Drug Target 2015, 16, 53–70. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Benson, A.B.; Abrams, T.A.; Ben-Josef, E.; Bloomston, P.M.; Botha, J.F.; Clary, B.M.; Covey, A.; Curley, S.A.; D’Angelica, M.I.; Davila, R.; et al. The NCCN Hepatobiliary Cancers Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2009, 7, 350–391. [Google Scholar] [CrossRef]
- Nhieu, J.T.; Renard, C.A.; Wei, Y.; Cherqui, D.; Zafrani, E.S.; Buendia, M.A. Nuclear accumulation of mutated β-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am. J. Pathol. 1999, 155, 703–710. [Google Scholar] [CrossRef]
- Lai, P.B.; Chi, T.Y.; Chen, G.G. Different levels of p53 induced either apoptosis or cell cycle arrest in a doxycycline-regulated hepatocellular carcinoma cell line in vitro. Apoptosis 2007, 12, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Ji, Y.-N.; Yu, L.-K. TP53 mutation is associated with a poor outcome for patients with hepatocellular carcinoma: Evidence from a meta-analysis. Hepatobiliary Surg. Nutr. 2013, 2, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.; Miura, J.T.; Gamblin, T.C.; He, R.; Xiu, J.; Millis, S.Z.; Gatalica, Z.; Reddy, S.K.; Yee, N.S.; Abou-Alfa, G.K. Comprehensive multiplatform biomarker analysis of 350 hepatocellular carcinomas identifies potential novel therapeutic options. J. Surg. Oncol. 2016, 113, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.X.; Tang, Q.Y.; Bai, J.L.; Qian, X.P.; Li, R.T.; Liu, B.R.; Zheng, M.H. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine-based chemotherapy: Evidence from 24 studies. Int. J. Cancer 2008, 123, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fang, Y.-J.; Li, F.; Ou, Q.J.; Chen, G.; Ma, G. ERCC1, defective mismatch repair status as predictive biomarkers of survival for stage III colon cancer patients receiving oxaliplatin-based adjuvant chemotherapy. Br. J. Cancer 2013, 108, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Boige, V.; Raoul, J.-L.; Pignon, J.-P.; Bouche, O.; Blanc, J.F.; Dahan, L.; Jouve, J.L.; Dupouy, N.; Ducreux, M. Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br. J. Cancer 2007, 97, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sohal, D.; Haller, D.G.; Mykulowycz, K.; Rosen, M.; Soulen, M.C.; Caparro, M.; Teitelbaum, U.R.; Giantonio, B.; O’dwyer, P.J.; et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011, 117, 3187–3192. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Johnson, P.; Knox, J.J.; Capanu, M.; Davidenko, I.; Lacava, J.; Leung, T.; Gansukh, B.; Saltz, L.B. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: A randomized trial. JAMA 2010, 304, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.; Tang, V.; Chan, P.; Leung, R.; Wong, H.; Poon, R.T.P.; Fan, S.T.; Yau, T. The use of SECOX (sorafenib, oxaliplatin, capecitabine) as the treatment of advanced hepatocellular carcinoma (HCC)—A single center retrospective study. In Proceedings of the European Society for Medical Oncology, Vienna, Austria, 28 September–2 October 2012. [Google Scholar]
- Yau, T.C.; Cheung, F.Y.; Lee, F.; Choo, S.P.; Wong, H.; Toh, H.C.; Leung, A.K.; Chan, P.; Yau, T.K.; Wong, J.; et al. A multicenter phase II study of sorafenib, capecitabine, and oxaliplatin (SECOX) in patients with advanced hepatocellular carcinoma: Final results of Hong Kong-Singapore Hepatocellular Carcinoma Research Collaborative Group Study. J. Clin. Oncol. 2017. [Google Scholar] [CrossRef]

| Test | Method | Result | Value | Conditions for a Positive Results | Potential Benefit | Therapies |
|---|---|---|---|---|---|---|
| TS | IHC | Negative | 0 + 100% | ≥1 + ≥10% | Yes | capecitabine, fluorouracil, pemetrexed |
| ERCC1 | IHC | Negative | 2 + 5% | ≥3 + ≥10% or ≥2 + ≥50% | Yes | carboplatin, cisplatin, oxaliplatin |
| TUBB3 | IHC | Negative | 0 + 100% | ≥2 + ≥30% | Yes | docetaxel, nab-paclitaxel, paclitaxel |
| TOP2A TOPO1 | IHC IHC | Negative Positive | 2 + 3% 2 + 50% | ≥1 + ≥10% ≥2 + ≥30% | No Yes | doxorubicin, epirubicin, liposomal doxorubicin irinotecan, topotecan |
| HER2/Neu | CISH, IHC, NGS | Not amplified, Negative | No | adotrastuzumab emtansine (T-DM1), pertuzumab, trastuzumab, lapatinib | ||
| ALK | RNA-Seq | Fusion not detected | No | ceritinib, crizotinib | ||
| ROS1 | RNA-Seq | Fusion not detected | No | crizotinib |
| ABL1 | BRCA2 | EGFR | HRAS | NF1 | RET |
|---|---|---|---|---|---|
| AKT1 | c-KIT | HER2/Neu (ERBB2) | IDH1 | NOTCH1 | ROS1 |
| ALK | CDK4 | ERBB3 | IDH2 | NRAS | SMO |
| Androgen Receptor | CDKN2A | FGFR1 | JAK2 | NTRK1 | SRC |
| APC | CHEK1 | FGFR2 | KDR (VEGFR2) | PDGFRA | TP53 |
| ARAF | CHEK2 | FGFR3 | KRAS | PDGFRB | VHL |
| ATM | cMET | FLT3 | MEK1 | PIK3CA | WT1 |
| BAP1 | CSF1R | GNA11 | MEK2 | PTCH1 | |
| BRAF | CTNNB1 | GNAQ | MLH1 | PTEN | |
| BRCA1 | DDR2 | GNAS | MPL | RAF1 |
| Test | Method | Result | Value | Potential Benefit | Therapies |
|---|---|---|---|---|---|
| TS ERCC1 | IHC IHC | Positive Negative | 1 + 10% 2 + 35% | No Yes | capecitabine, fluorouracil, pemetrexed carboplatin, cisplatin, oxaliplatin |
| TUBB3 | IHC | Negative | 1 + 90% | Yes | docetaxel, nab-paclitaxel, paclitaxel |
| TOP2A | IHC | Positive | 2 + 20% | Yes | doxorubicin, epirubicin, liposomal doxorubicin |
| TOPO1 | IHC | Positive | 2 + 80% | Yes | irinotecan, topotecan |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posadas, K.; Ankola, A.; Yang, Z.; Yee, N.S. Tumor Molecular Profiling for an Individualized Approach to the Treatment of Hepatocellular Carcinoma: A Patient Case Study. Biomedicines 2018, 6, 46. https://doi.org/10.3390/biomedicines6020046
Posadas K, Ankola A, Yang Z, Yee NS. Tumor Molecular Profiling for an Individualized Approach to the Treatment of Hepatocellular Carcinoma: A Patient Case Study. Biomedicines. 2018; 6(2):46. https://doi.org/10.3390/biomedicines6020046
Chicago/Turabian StylePosadas, Kristine, Anita Ankola, Zhaohai Yang, and Nelson S. Yee. 2018. "Tumor Molecular Profiling for an Individualized Approach to the Treatment of Hepatocellular Carcinoma: A Patient Case Study" Biomedicines 6, no. 2: 46. https://doi.org/10.3390/biomedicines6020046





