Deubiquitinylase USP47 Promotes RelA Phosphorylation and Survival in Gastric Cancer Cells
Abstract
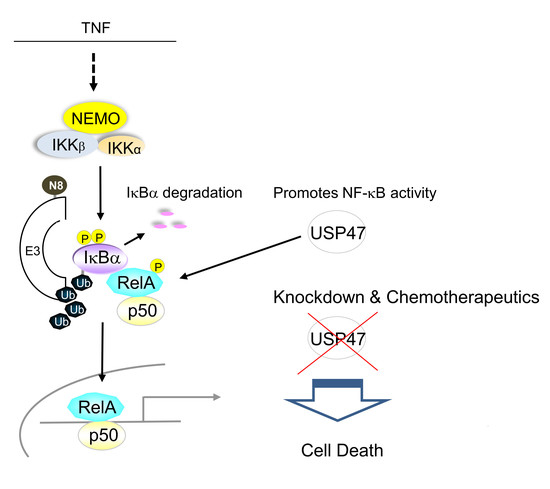
:1. Introduction
2. Experimental Section
2.1. Cell Culture and siRNA Transfection
2.2. Subcellular Fractionation
2.3. SDS-PAGE and Immunoblotting
2.4. Apoptosis Detection
2.5. Cell Viability Assay
2.6. Statistics
3. Results
3.1. Depletion of USP47 Decreased Phospho-RelA and βTrCP Protein Levels
3.2. USP47 Promotes Chemoresistance and Cell Viability in NCI-N87 Gastric Carcinoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Orditura, M.; Galizia, G.; Sforza, V.; Gambardella, V.; Fabozzi, A.; Laterza, M.M.; Andreozzi, F.; Ventriglia, J.; Savastano, B.; Mabilia, A.; et al. Treatment of gastric cancer. World J. Gastroenterol. 2014, 20, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.-C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.; Naumann, M. NF-κB Signaling in Gastric Cancer. Toxins 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Naumann, M. Beyond IκBs: Alternative regulation of NF-κB activity. FASEB J. 2007, 21, 2642–2654. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Omata, M. Inflammation and cancer: Role of nuclear factor-kappaB activation. Cancer Sci. 2008, 99, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-C.; Kim, S.-H.; Oh, S.Y.; Lee, S.; Lee, J.H.; Jang, J.S.; Kim, M.C.; Kim, K.H.; Kim, S.-J.; Kim, S.-G.; et al. Clinicopathologic significance of expression of nuclear factor-κB RelA and its target gene products in gastric cancer patients. World J. Gastroenterol. 2012, 18, 4744–4750. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fu, X.-Q.; Zhang, L.-L.; Zhang, J.; Huang, X.; Lu, X.-H.; Shen, L.; Liu, B.-N.; Liu, J.; Luo, H.-S.; et al. The AKT1/NF-kappaB/Notch1/PTEN axis has an important role in chemoresistance of gastric cancer cells. Cell Death Dis. 2013, 4, e847. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, J.; Fushida, S.; Harada, S.; Makino, I.; Nakamura, K.; Oyama, K.; Fujita, H.; Ninomiya, I.; Fujimura, T.; Kayahara, M.; et al. PSK enhances the efficacy of docetaxel in human gastric cancer cells through inhibition of nuclear factor-κB activation and survivin expression. Int. J. Oncol. 2010, 36, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Clague, M.J.; Barsukov, I.; Coulson, J.M.; Liu, H.; Rigden, D.J.; Urbé, S. Deubiquitylases from Genes to Organism. Physiol. Rev. 2013, 93, 1289–1315. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, J.A.; Jacq, X.; Martin, N.M.; Jackson, S.P. Deubiquitylating enzymes and drug discovery: Emerging opportunities. Nat. Rev. Drug Discov. 2017, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bengtson, M.H.; Ulbrich, A.; Matsuda, A.; Reddy, V.A.; Orth, A.; Chanda, S.K.; Batalov, S.; Joazeiro, C.A.P. Genome-Wide and Functional Annotation of Human E3 Ubiquitin Ligases Identifies MULAN, a Mitochondrial E3 that Regulates the Organelle’s Dynamics and Signaling. PLoS ONE 2008, 3, e1487. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E.; Dixit, V.M. Signaling to NF-κB: Regulation by Ubiquitination. Cold Spring Harb. Perspect. Biol. 2010, 2, a003350. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, K.; Bozko, P.M.; Dubiel, W.; Naumann, M. CSN controls NF-κB by deubiquitinylation of IκBα. EMBO J. 2007, 26, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, K.; Naumann, M. CSN-associated USP48 confers stability to nuclear NF-κB/RelA by trimming K48-linked Ub-chains. Biochim. Biophys. Acta 2015, 1853, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Erstad, D.J.; Cusack, J.C. Targeting the NF-κB Pathway in Cancer Therapy. Transl. Cancer Res. Surg. 2013, 22, 705–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yin, Y.; Hu, Y.; Zhang, J.; Bian, Z.; Song, M.; Hua, D.; Huang, Z. MicroRNA-204-5p inhibits gastric cancer cell proliferation by downregulating USP47 and RAB22A. Med. Oncol. 2014, 32, 331. [Google Scholar] [CrossRef] [PubMed]
- Peschiaroli, A.; Skaar, J.; Pagano, M.; Melino, G. The ubiquitin-specific protease USP47 is a novel β-TRCP interactor regulating cell survival. Oncogene 2010, 29. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, E.E.; Ashkenazi, A. Tumor Necrosis Factor: An Apoptosis JuNKie? Cell 2004, 116, 491–497. [Google Scholar] [CrossRef]
- Habraken, Y.; Piette, J. NF-κB activation by double-strand breaks. Biochem. Pharmacol. 2006, 72, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Jeong, S. PI3-Kinase and PDK-1 Regulate HDAC1-mediated Transcriptional Repression of Transcription Factor NF-κB. Mol. Cells 2005, 20, 241–246. [Google Scholar] [PubMed]
- He, M.; Zhou, Z.; Wu, G.; Chen, Q.; Wan, Y. Emerging role of DUBs in tumor metastasis and apoptosis: Therapeutic implication. Pharmacol. Ther. 2017, 177, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.L.; Dianova, I.I.; Khoronenkova, S.V.; Edelmann, M.J.; Kessler, B.M.; Dianov, G.L. USP47 Is a Deubiquitylating Enzyme that Regulates Base Excision Repair by Controlling Steady-State Levels of DNA Polymerase β. Mol. Cell 2011, 41, 609–615. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naghavi, L.; Schwalbe, M.; Ghanem, A.; Naumann, M. Deubiquitinylase USP47 Promotes RelA Phosphorylation and Survival in Gastric Cancer Cells. Biomedicines 2018, 6, 62. https://doi.org/10.3390/biomedicines6020062
Naghavi L, Schwalbe M, Ghanem A, Naumann M. Deubiquitinylase USP47 Promotes RelA Phosphorylation and Survival in Gastric Cancer Cells. Biomedicines. 2018; 6(2):62. https://doi.org/10.3390/biomedicines6020062
Chicago/Turabian StyleNaghavi, Lara, Martin Schwalbe, Ahmed Ghanem, and Michael Naumann. 2018. "Deubiquitinylase USP47 Promotes RelA Phosphorylation and Survival in Gastric Cancer Cells" Biomedicines 6, no. 2: 62. https://doi.org/10.3390/biomedicines6020062