Zebrafish Larvae as a Behavioral Model in Neuropharmacology
Abstract
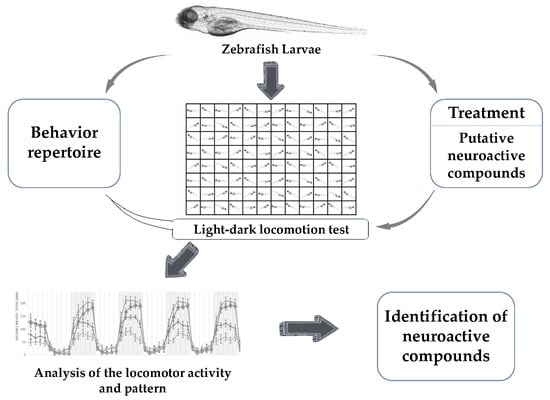
:1. Introduction
2. Connection Between Brain and Behavior
3. Behavior Repertoire in Zebrafish Larvae
3.1. Thigmotaxis
3.2. Startle Response
3.3. Optokinetic Response
3.4. Optomotor Response
3.5. Habituation
3.6. Prey Capture
3.7. Sleep/Awake Behavior
3.8. Locomotor Behavior
4. Identification of Neuroactive Compounds
4.1. Light-Dark Locomotion Test
4.2. Neuroactive Drugs
4.3. Metals, Metallic Ions, and Nanoparticles
4.4. Environmental Toxicants
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalueff, A.V.; Echevarria, D.J.; Stewart, A.M. Gaining translational momentum: More zebrafish models for neuroscience research. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 1–6. [Google Scholar] [CrossRef]
- Basnet, R.M.; Zizioli, D.; Guarienti, M.; Finazzi, D.; Memo, M. Methylxanthines induce structural and functional alterations of the cardiac system in zebrafish embryos. BMC Pharmacol. Toxicol. 2017, 18, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, C. The husbandry of zebrafish (danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, T.; Lambert, A.M.; Masino, M.A.; Downes, G.B. Mutation of zebrafish dihydrolipoamide branched-chain transacylase e2 results in motor dysfunction and models maple syrup urine disease. Dis. Models Mech. 2012, 5, 248–258. [Google Scholar] [CrossRef]
- Lange, M.; Norton, W.; Coolen, M.; Chaminade, M.; Merker, S.; Proft, F.; Schmitt, A.; Vernier, P.; Lesch, K.P.; Bally-Cuif, L. The adhd-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol. Psychiatry 2012, 17, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Levitas-Djerbi, T.; Appelbaum, L. Modeling sleep and neuropsychiatric disorders in zebrafish. Curr. Opin. Neurobiol. 2017, 44, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A. Zebrafish: A model system to examine the neurodevelopmental basis of schizophrenia. Prog. Brain Res. 2009, 179, 97–106. [Google Scholar]
- Norton, W.H. Toward developmental models of psychiatric disorders in zebrafish. Front. Neural Circuits 2013, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef]
- Lau, B.Y.; Mathur, P.; Gould, G.G.; Guo, S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 2581–2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucini, C.; D’Angelo, L.; Cacialli, P.; Palladino, A.; de Girolamo, P. BDNF, brain, and regeneration: Insights from zebrafish. Int. J. Mol. Sci. 2018, 19, 3155. [Google Scholar] [CrossRef]
- Perathoner, S.; Cordero-Maldonado, M.L.; Crawford, A.D. Potential of zebrafish as a model for exploring the role of the amygdala in emotional memory and motivational behavior. J. Neurosci. Res. 2016, 94, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Randlett, O.; Wee, C.L.; Naumann, E.A.; Nnaemeka, O.; Schoppik, D.; Fitzgerald, J.E.; Portugues, R.; Lacoste, A.M.; Riegler, C.; Engert, F.; et al. Whole-brain activity mapping onto a zebrafish brain atlas. Nat. Methods 2015, 12, 1039–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Tegelenbosch, R.A.J.; Noldus, L.P.J.J.; Richardson, M.K.; Ahmad, F. Zebrafish embryos and larvae in behavioural assays. Behaviour 2012, 149, 1241–1281. [Google Scholar] [CrossRef]
- Friedrich, R.W.; Jacobson, G.A.; Zhu, P. Circuit neuroscience in zebrafish. Curr. Biol. 2010, 20, R371–R381. [Google Scholar] [CrossRef] [PubMed]
- Kokel, D.; Bryan, J.; Laggner, C.; White, R.; Cheung, C.Y.; Mateus, R.; Healey, D.; Kim, S.; Werdich, A.A.; Haggarty, S.J.; et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 2010, 6, 231–237. [Google Scholar] [CrossRef]
- Kokel, D.; Peterson, R.T. Chemobehavioural phenomics and behaviour-based psychiatric drug discovery in the zebrafish. Brief. Funct. Genom. Proteom. 2008, 7, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Fleming, A.; National Centre for the Replacement, Refinement, and Reduction of Animals in Research (Great Britain). Zebrafish as an Alternative Model Organism for Disease Modelling and Drug Discovery: Implications for the 3Rs; National Centre for the Replacement, Refinement and Reduction of Animals in Research: London, UK, 2007. [Google Scholar]
- Lorenzetti, S.; Altieri, I.; Arabi, S.; Balduzzi, D.; Bechi, N.; Cordelli, E.; Galli, C.; Ietta, F.; Modina, S.C.; Narciso, L.; et al. Innovative non-animal testing strategies for reproductive toxicology: The contribution of italian partners within the eu project reprotect. Annali dell’Istituto Superiore di Sanita 2011, 47, 429–444. [Google Scholar]
- Palmer, T.; Ek, F.; Enqvist, O.; Olsson, R.; Astrom, K.; Petersson, P. Action sequencing in the spontaneous swimming behavior of zebrafish larvae - implications for drug development. Sci. Rep. 2017, 7, 3191. [Google Scholar] [CrossRef] [PubMed]
- Orger, M.B.; de Polavieja, G.G. Zebrafish behavior: Opportunities and challenges. Annu. Rev. Neurosci. 2017, 40, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Saint-Amant, L.; Drapeau, P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Buss, R.R.; Pierre, D. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J. Neurophysiol. 2001, 86, 197–210. [Google Scholar] [CrossRef]
- Drapeau, P.; Saint-Amant1, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Brustein, E. Development of the locomotor network in zebrafish. Prog. Neurobiol. 2002, 68, 85–111. [Google Scholar] [CrossRef]
- Brustein, E.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Drapeau, P. Steps during the development of the zebrafish locomotor network. J. Physiol. (Paris) 2003, 97, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.A.; Granato, M. Modulation of locomotor activity in larval zebrafish during light adaptation. J. Exp. Biol. 2007, 210, 2526–2539. [Google Scholar] [CrossRef] [Green Version]
- Lowery, L.A.; Sive, H. Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech. Dev. 2004, 121, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Tropepe, V.; Sive, H.L. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003, 2, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panula, P.; Chen, Y.C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish cns systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.; Mahler, J.; Schweitzer, J.; Driever, W. Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J. Comp. Neurol. 2010, 518, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Kozol, R.A.; Abrams, A.J.; James, D.M.; Buglo, E.; Yan, Q.; Dallman, J.E. Function over form: Modeling groups of inherited neurological conditions in zebrafish. Front. Mol. Neurosci. 2016, 9, 55. [Google Scholar] [CrossRef]
- Jarvis, E.D.; Gunturkun, O.; Bruce, L.; Csillag, A.; Karten, H.; Kuenzel, W.; Medina, L.; Paxinos, G.; Perkel, D.J.; Shimizu, T.; et al. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 2005, 6, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Fosque, B.F.; Sun, Y.; Dana, H.; Yang, C.T.; Ohyama, T.; Tadross, M.R.; Patel, R.; Zlatic, M.; Kim, D.S.; Ahrens, M.B.; et al. Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science (New York) 2015, 347, 755–760. [Google Scholar] [CrossRef]
- Panier, T.; Romano, S.A.; Olive, R.; Pietri, T.; Sumbre, G.; Candelier, R.; Debregeas, G. Fast functional imaging of multiple brain regions in intact zebrafish larvae using selective plane illumination microscopy. Front. Neural Circuits 2013, 7, 65. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.M.; Kafaligonul, H. Zebrafish-a model organism for studying the neurobiological mechanisms underlying cognitive brain aging and use of potential interventions. Front. Cell Dev. Biol. 2018, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Heffern, K.; Tierney, K.; Gallagher, E.P. Comparative effects of cadmium, zinc, arsenic and chromium on olfactory-mediated neurobehavior and gene expression in larval zebrafish (danio rerio). Aquat. Toxicol. (Amsterdam) 2018, 201, 83–90. [Google Scholar] [CrossRef]
- Liu, C.X.; Li, C.Y.; Hu, C.C.; Wang, Y.; Lin, J.; Jiang, Y.H.; Li, Q.; Xu, X. Crispr/cas9-induced shank3b mutant zebrafish display autism-like behaviors. Mol. Autism 2018, 9, 23. [Google Scholar] [CrossRef]
- Norton, W.; Bally-Cuif, L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010, 11, 90. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, danio rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. [Google Scholar] [CrossRef]
- Khatri, D.; Zizioli, D.; Trivedi, A.; Borsani, G.; Monti, E.; Finazzi, D. Overexpression of human mutant pank2 proteins affects development and motor behavior of zebrafish embryos. Neuromol. Med. 2018. [Google Scholar] [CrossRef]
- Muniandy, Y. The use of larval zebrafish (danio rerio) model for identifying new anxiolytic drugs from herbal medicine. Zebrafish 2018, 15, 321–339. [Google Scholar] [CrossRef]
- Bruni, G.; Lakhani, P.; Kokel, D. Discovering novel neuroactive drugs through high-throughput behavior-based chemical screening in the zebrafish. Front. Pharmacol. 2014, 5, 153. [Google Scholar] [CrossRef]
- Fontana, B.D.; Mezzomo, N.J.; Kalueff, A.V.; Rosemberg, D.B. The developing utility of zebrafish models of neurological and neuropsychiatric disorders: A critical review. Exp. Neurol. 2018, 299, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish models in neuropsychopharmacology and cns drug discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef] [PubMed]
- Lamprea, M.R.; Cardenas, F.P.; Setem, J.; Morato, S. Thigmotactic responses in an open-field. Braz. J. Med. Biol. Res. = Revista Brasileira de Pesquisas Medicas e Biologicas 2008, 41, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef]
- Colwill, R.M.; Creton, R. Imaging escape and avoidance behavior in zebrafish larvae. Rev. Neurosci. 2011, 22, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Lin, J.; Zhang, Y.; Peng, X.; Guo, N.; Li, Q. Effects of diphenylhydantoin on locomotion and thigmotaxis of larval zebrafish. Neurotoxicol. Teratol. 2016, 53, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, J.; Peng, X.; Zhang, Q.; Zhang, Y.; Guo, N.; Zhou, S.; Li, Q. Effects of picrotoxin on zebrafish larvae behaviors: A comparison study with ptz. Epilepsy Behav. 2017, 70, 224–231. [Google Scholar] [CrossRef]
- Lundegaard, P.R.; Anastasaki, C.; Grant, N.J.; Sillito, R.R.; Zich, J.; Zeng, Z.; Paranthaman, K.; Larsen, A.P.; Armstrong, J.D.; Porteous, D.J.; et al. Mek inhibitors reverse camp-mediated anxiety in zebrafish. Chem. Biol. 2015, 22, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Fetcho, J.R.; McLean, D.L. Startle response. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; pp. 375–379. [Google Scholar]
- Ganzen, L.; Venkatraman, P.; Pang, C.P.; Leung, Y.F.; Zhang, M. Utilizing zebrafish visual behaviors in drug screening for retinal degeneration. Int. J. Mol. Sci. 2017, 18, 1185. [Google Scholar] [CrossRef] [PubMed]
- Best, J.D.; Berghmans, S.; Hunt, J.J.; Clarke, S.C.; Fleming, A.; Goldsmith, P.; Roach, A.G. Non-associative learning in larval zebrafish. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 1206–1215. [Google Scholar] [CrossRef]
- Zeddies, D.G.; Fay, R.R. Development of the acoustically evoked behavioral response in zebrafish to pure tones. J. Exp. Biol. 2005, 208, 1363–1372. [Google Scholar] [CrossRef] [Green Version]
- Mena, A.; Ruiz-Salas, J.C.; Puentes, A.; Dorado, I.; Ruiz-Veguilla, M.; De la Casa, L.G. Reduced prepulse inhibition as a biomarker of schizophrenia. Front. Behav. Neurosci. 2016, 10, 202. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Neuhauss, S.C. The optokinetic response in zebrafish and its applications. Front. Biosci. J. Virtual Library 2008, 13, 1899–1916. [Google Scholar] [CrossRef]
- Easter, S.S., Jr.; Nicola, G.N. The development of eye movements in the zebrafish (danio rerio). Dev. Psychobiol. 1997, 31, 267–276. [Google Scholar] [CrossRef]
- Brockerhoff, S.E. Measuring the optokinetic response of zebrafish larvae. Nat. Protoc. 2006, 1, 2448–2451. [Google Scholar] [CrossRef] [PubMed]
- Cahill, H.; Nathans, J. The optokinetic reflex as a tool for quantitative analyses of nervous system function in mice: Application to genetic and drug-induced variation. PLoS ONE 2008, 3, e2055. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, V.C.; Neuhauss, S.C. Visual behavior in zebrafish. Zebrafish 2006, 3, 191–201. [Google Scholar] [CrossRef]
- Orger, M.B.; Baier, H. Channeling of red and green cone inputs to the zebrafish optomotor response. Vis. Neurosci. 2005, 22, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeser, T.; Baier, H. Visuomotor behaviors in larval zebrafish after gfp-guided laser ablation of the optic tectum. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 3726–3734. [Google Scholar] [CrossRef]
- Thompson, R.F.; Spencer, W.A. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 1966, 73, 16–43. [Google Scholar] [CrossRef] [PubMed]
- Wolman, M.A.; Jain, R.A.; Marsden, K.C.; Bell, H.; Skinner, J.; Hayer, K.E.; Hogenesch, J.B.; Granato, M. A genome-wide screen identifies papp-aa-mediated igfr signaling as a novel regulator of habituation learning. Neuron 2015, 85, 1200–1211. [Google Scholar] [CrossRef]
- Muto, A.; Kawakami, K. Prey capture in zebrafish larvae serves as a model to study cognitive functions. Front. Neural Circuits 2013, 7, 110. [Google Scholar] [CrossRef]
- Borla, M.A.; Palecek, B.; Budick, S.; O’Malley, D.M. Prey capture by larval zebrafish: Evidence for fine axial motor control. Brain Behav. Evol. 2002, 60, 207–229. [Google Scholar] [CrossRef]
- Gahtan, E.; Tanger, P.; Baier, H. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 9294–9303. [Google Scholar] [CrossRef] [PubMed]
- Sorribes, A.; Thornorsteinsson, H.; Arnardottir, H.; Johannesdottir, I.; Sigurgeirsson, B.; de Polavieja, G.G.; Karlsson, K.A.E. The ontogeny of sleep-wake cycles in zebrafish: A comparison to humans. Front. Neural Circuits 2013, 7, 178. [Google Scholar] [CrossRef]
- Zhdanova, I.V. Sleep in zebrafish. Zebrafish 2006, 3, 215–226. [Google Scholar] [CrossRef]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science (New York) 2010, 327, 348–351. [Google Scholar] [CrossRef]
- Rihel, J.; Schier, A.F. Behavioral screening for neuroactive drugs in zebrafish. Dev. Neurobiol. 2012, 72, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Sigurgeirsson, B.; Thorsteinsson, H.; Arnardottir, H.; Johannesdottir, I.T.; Karlsson, K.A. Effects of modafinil on sleep-wake cycles in larval zebrafish. Zebrafish 2011, 8, 133–140. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Hou, Y.-Y.; Sun, M.-Z.; Zhang, C.-Y.; Bai, G.; Zhao, X.; Feng, X.-Z. Behavioural screening of zebrafish using neuroactive traditional chinese medicine prescriptions and biological targets. Sci. Rep. 2014, 4, 5311. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.E.; Galitan, L.; Cameron, J.; Goodwin, N.; Ramakrishnan, L. Delay of initial feeding of zebrafish larvae until 8 days postfertilization has no impact on survival or growth through the juvenile stage. Zebrafish 2018, 15, 515–518. [Google Scholar] [CrossRef]
- Grillner, S.; Markram, H.; De Schutter, E.; Silberberg, G.; LeBeau, F.E. Microcircuits in action—From cpgs to neocortex. Trends Neurosci. 2005, 28, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Horzmann, K.A.; Freeman, J.L. Zebrafish get connected: Investigating neurotransmission targets and alterations in chemical toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, J.; Zhang, Y.; Liu, X.; Chen, X.Q.; Xu, M.Q.; He, L.; Li, S.; Guo, N. Differential behavioral responses of zebrafish larvae to yohimbine treatment. Psychopharmacology 2015, 232, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Irons, T.D.; MacPhail, R.C.; Hunter, D.L.; Padilla, S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, R.C.; Brooks, J.; Hunter, D.L.; Padnos, B.; Irons, T.D.; Padilla, S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Vignet, C.; Begout, M.L.; Pean, S.; Lyphout, L.; Leguay, D.; Cousin, X. Systematic screening of behavioral responses in two zebrafish strains. Zebrafish 2013, 10, 365–375. [Google Scholar] [CrossRef]
- Ali, S.; Champagne, D.L.; Richardson, M.K. Behavioral profiling of zebrafish embryos exposed to a panel of 60 water-soluble compounds. Behav. Brain Res. 2012, 228, 272–283. [Google Scholar] [CrossRef]
- Bilotta, J. Effects of abnormal lighting on the development of zebrafish visual behavior. Behav. Brain Res. 2000, 116, 81–87. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Khezri, A.; Jusdado, J.G.H.; Lewandowska-Sabat, A.M.; Henry, T.; Ropstad, E. Toxicant induced behavioural aberrations in larval zebrafish are dependent on minor methodological alterations. Toxicol. Lett. 2017, 276, 62–68. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, T.; Huang, G.; Yin, D.; Zhang, Q.; Yang, X. Neurobehavioral effects of two metabolites of bde-47 (6-oh-bde-47 and 6-meo-bde-47) on zebrafish larvae. Chemosphere 2018, 200, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.D.; Seibert, J.; Soanes, K.H. Distinct models of induced hyperactivity in zebrafish larvae. Brain Res. 2012, 1449, 46–59. [Google Scholar] [CrossRef]
- Asmonaite, G.; Boyer, S.; Souza, K.B.; Wassmur, B.; Sturve, J. Behavioural toxicity assessment of silver ions and nanoparticles on zebrafish using a locomotion profiling approach. Aquat. Toxicol. (Amsterdam) 2016, 173, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Irons, T.D.; Kelly, P.E.; Hunter, D.L.; Macphail, R.C.; Padilla, S. Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol. Biochem. Behav. 2013, 103, 792–813. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, L.J.; Bailey, J.M.; Levin, E.D.; Stapleton, H.M. Persisting effects of a pbde metabolite, 6-oh-bde-47, on larval and juvenile zebrafish swimming behavior. Neurotoxicol. Teratol. 2015, 52, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Colon-Cruz, L.; Kristofco, L.; Crooke-Rosado, J.; Acevedo, A.; Torrado, A.; Brooks, B.W.; Sosa, M.A.; Behra, M. Alterations of larval photo-dependent swimming responses (PDR): New endpoints for rapid and diagnostic screening of aquatic contamination. Ecotoxicol. Environ. Saf. 2018, 147, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Thit, A.; Skjolding, L.M.; Selck, H.; Sturve, J. Effects of copper oxide nanoparticles and copper ions to zebrafish (danio rerio) cells, embryos and fry. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2017, 45, 89–100. [Google Scholar] [CrossRef]
- De Esch, C.; van der Linde, H.; Slieker, R.; Willemsen, R.; Wolterbeek, A.; Woutersen, R.; De Groot, D. Locomotor activity assay in zebrafish larvae: Influence of age, strain and ethanol. Neurotoxicol. Teratol. 2012, 34, 425–433. [Google Scholar] [CrossRef]
- Ramcharitar, J.; Ibrahim, R.M. Ethanol modifies zebrafish responses to abrupt changes in light intensity. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2013, 20, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Dipp, V.R.; Valles, S.; Ortiz-Kerbertt, H.; Suarez, J.V.; Bardullas, U. Neurobehavioral alterations in zebrafish due to long-term exposure to low doses of inorganic arsenic. Zebrafish 2018, 15, 575–585. [Google Scholar] [CrossRef]
- Li, F.; Lin, J.; Liu, X.; Li, W.; Ding, Y.; Zhang, Y.; Zhou, S.; Guo, N.; Li, Q. Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs. Ann. Transl. Med. 2018, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Lin, J.; Zhu, Y.; Liu, X.; Zhang, Y.; Ji, Y.; Yang, X.; Zhang, Y.; Guo, N.; Li, Q. Anxiety-related behavioral responses of pentylenetetrazole-treated zebrafish larvae to light-dark transitions. Pharmacol. Biochem. Behav. 2016, 145, 55–65. [Google Scholar] [CrossRef]
- Spulber, S.; Kilian, P.; Wan Ibrahim, W.N.; Onishchenko, N.; Ulhaq, M.; Norrgren, L.; Negri, S.; Di Tuccio, M.; Ceccatelli, S. Pfos induces behavioral alterations, including spontaneous hyperactivity that is corrected by dexamfetamine in zebrafish larvae. PLoS ONE 2014, 9, e94227. [Google Scholar] [CrossRef]
- Chen, X.; Huang, C.; Wang, X.; Chen, J.; Bai, C.; Chen, Y.; Chen, X.; Dong, Q.; Yang, D. Bde-47 disrupts axonal growth and motor behavior in developing zebrafish. Aquat. Toxicol. (Amsterdam) 2012, 120-121, 35–44. [Google Scholar] [CrossRef]
- Liang, X.; Souders, C.L., 2nd; Zhang, J.; Martyniuk, C.J. Tributyltin induces premature hatching and reduces locomotor activity in zebrafish (danio rerio) embryos/larvae at environmentally relevant levels. Chemosphere 2017, 189, 498–506. [Google Scholar] [CrossRef]
- Thompson, W.A.; Arnold, V.I.; Vijayan, M.M. Venlafaxine in embryos stimulates neurogenesis and disrupts larval behavior in zebrafish. Environ. Sci. Technol. 2017, 51, 12889–12897. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Adrenaline and noradrenaline. eLS 2010. [Google Scholar] [CrossRef]
- Carter, K.M.; Woodley, C.M.; Brown, R.S. A review of tricaine methanesulfonate for anesthesia of fish. Rev. Fish Biol. Fish. 2010, 21, 51–59. [Google Scholar] [CrossRef]
- Holdstock, L.; de Wit, H. Individual differences in the biphasic effects of ethanol. Alcohol. Clin. Exp. Res. 1998, 22, 1903–1911. [Google Scholar] [CrossRef]
- Bremner, J.D.; Mletzko, T.; Welter, S.; Quinn, S.; Williams, C.; Brummer, M.; Siddiq, S.; Reed, L.; Heim, C.M.; Nemeroff, C.B. Effects of phenytoin on memory, cognition and brain structure in post-traumatic stress disorder: A pilot study. J. Psychopharmacol. (Oxford) 2005, 19, 159–165. [Google Scholar] [CrossRef]
- Hidaka, N.; Suemaru, K.; Li, B.; Araki, H. Effects of repeated electroconvulsive seizures on spontaneous alternation behavior and locomotor activity in rats. Biol. Pharm. Bull. 2008, 31, 1928–1932. [Google Scholar] [CrossRef]
- Tonelli, D.A.; Pereira, M.; Siba, I.P.; Martynhak, B.J.; Correia, D.; Casarotto, P.C.; Biojone, C.; Guimaraes, F.S.; Joca, S.L.; Andreatini, R. The antimanic-like effect of phenytoin and carbamazepine on methylphenidate-induced hyperlocomotion: Role of voltage-gated sodium channels. Fundam. Clin. Pharmacol. 2013, 27, 650–655. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Belyaev, N.D.; Turner, A.J. Sodium valproate: An old drug with new roles. Trends Pharmacol. Sci. 2009, 30, 509–514. [Google Scholar] [CrossRef]
- Bogi, E.; Belovicova, K.; Ujhazy, E.; Mach, M.; Koprdova, R.; Zilava, L.; Garafova, A.; Jezova, D.; Dubovicky, M. Perinatal exposure to venlafaxine leads to lower anxiety and depression-like behavior in the adult rat offspring. Behav. Pharmacol. 2018, 29, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.S.; Jamwal, S.; Kumar, P.; Deshmukh, R. Sertraline and venlafaxine improves motor performance and neurobehavioral deficit in quinolinic acid induced huntington’s like symptoms in rats: Possible neurotransmitters modulation. Pharmacol. Rep. 2017, 69, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Mora-Zamorano, F.X.; Svoboda, K.R.; Carvan, M.J., 3rd. The nicotine-evoked locomotor response: A behavioral paradigm for toxicity screening in zebrafish (danio rerio) embryos and eleutheroembryos exposed to methylmercury. PLoS ONE 2016, 11, e0154570. [Google Scholar] [CrossRef] [PubMed]
- Goulet, S.; Dore, F.Y.; Mirault, M.E. Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol. Teratol. 2003, 25, 335–347. [Google Scholar] [CrossRef]
- Onishchenko, N.; Tamm, C.; Vahter, M.; Hokfelt, T.; Johnson, J.A.; Johnson, D.A.; Ceccatelli, S. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol. Sci. Off. J. Soc. Toxicol. 2007, 97, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Berto-Junior, C.; de Carvalho, D.P.; Soares, P.; Miranda-Alves, L. Tributyltin and zebrafish: Swimming in dangerous water. Front. Endocrinol. 2018, 9, 152. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Stage and Well Plate | Protocol | References |
|---|---|---|---|
| Aconitine Pentylenetrazol 4-aminopyridine | 4–6 dpf, 48 and 96 well plates | 4 successive cycles of 10 min alternating light and dark. | [90] |
| Ag+ and AgNPs (silver nanoparticles) | 5 dpf, 48 well plate | 18 alternating dark and light cycles of 5 min each. | [91] |
| Apomorphine SKF-38393 Quinpirole Butaclamol SCH-23390 Haloperidol | 6 dpf, 96 well plate | 10 min acclimatization in dark followed by 2 cycles of 10 min of light and 20 mins of dark. | [92] |
| Bisphenol A (BPA) Tetrabromobisphenol A (TBBPA) | 4–5 dpf, 96 well plate | 20 mins of light followed by 10 min of dark and 10 min of light. | [88] |
| Chlorpyrifos 6-hydroxy-2,2’,4,4’ tetrabromodiphenyl ether (6-OH-BDE-47) | 6 dpf, 96 well plate | 10 min acclimatization followed by 10 min alternating light and dark for 2 times. | [93] |
| Cocaine Ethanol D-Amphetamine | 6 dpf, 96 well plate | 20 mins acclimatization in dark followed by 10 min of alternating light and dark for 70 min. | [83] |
| Copper | 5 dpf, 96 well plate | 4 successive cycles of 10 min alternating light and dark. | [94] |
| Copper ions, copper oxide nanoparticles | 4 dpf, 24 well plate | 18 alternating cycles of 5 min of light and 5 min of dark. | [95] |
| Diphenylhydantoin | 5 dpf, 24 well plate | 10 min acclimatization followed by 30 min of light and 5 min of dark. | [52] |
| Ethanol | 6 dpf, 96 well plate | 20 mins acclimatization in dark followed by 10 min in dark and 10 min in light, then 20 mins in dark, and then another cycle of 10 min of light and 20 mins of dark. | [84] |
| Ethanol | 6 dpf, 96 well plate | 15 min in dark followed by 15 min in light and 15 min in dark. | [96] |
| Ethanol | 9-10 dpf, 24 well plate | 5 min acclimatization in light followed by 15 min of dark and 5 min of light. | [97] |
| Inorganic arsenic | 7 dpf, 24 well plate | Acclimatization for 10 min followed by 2 successive cycles of 10 min of light and 10 min of dark. | [98] |
| MK-801; Pentylenetetrazole; Valproic acid sodium salt; Yohimbine hydrochloride; 5,5-Diphenylhydantoin sodium salt Sulpiride | 7 dpf, 24 well plate | 60 min in light followed by 5 min in dark. The activities of zebrafish larvae during the last 10 min of the light period and the 5 min of the dark period were analyzed. | [99] |
| Pentylenetetrazole | 5 and 7 dpf, 24 well plate | 10 min acclimatization in light followed by 40 min of light and then 3 successive cycles of 10 min of light and 5 min of dark. | [100] |
| Pentylenetetrazole Picrotoxin | 5 dpf, 24 well plate | 25 min of acclimatization in environment followed by 5 min in light and 5 min in dark. | [54] |
| Perfluorooctane sulphate (PFOS) | 6 dpf, 48 well plate | 15 min acclimatization followed by 10 min in dark and 10 min in light. | [101] |
| Polybrominated diphenyl ethers and their hydroxyl metabolites (OH-BDEs MeO-BDEs) | 5, 6 and 7 dpf, 48 well plate | 10 min light adaptation followed by two repeated cycles of 10 min of dark and 10 min of light. | [89] |
| 2,2’,4,4’-Tetrabromodiphenyl ether (BDE-47) | 5 dpf, 24 well plate | 70 min of alternating 10 min of light and 10 min of dark starting with a light cycle. | [102] |
| Tributyltin | 4 dpf, 96 well plate | 50 min of alternating 10 min of light and 10 min of dark starting with a dark cycle. | [103] |
| Venlafaxine | 5 dpf, 96 well plate | Acclimatization for 1 h followed by 60 min of alternating cycles of 7.5 min of light and 7.5 min of dark. | [104] |
| Yohimbine | 5 and 7 dpf, 24 well plate | 10 min of acclimatization with light followed by 40 min of light and three 15 min cycles of 10 min of light and 5 min of dark. | [82] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. https://doi.org/10.3390/biomedicines7010023
Basnet RM, Zizioli D, Taweedet S, Finazzi D, Memo M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines. 2019; 7(1):23. https://doi.org/10.3390/biomedicines7010023
Chicago/Turabian StyleBasnet, Ram Manohar, Daniela Zizioli, Somrat Taweedet, Dario Finazzi, and Maurizio Memo. 2019. "Zebrafish Larvae as a Behavioral Model in Neuropharmacology" Biomedicines 7, no. 1: 23. https://doi.org/10.3390/biomedicines7010023