Gender-Related Differences in Trimethylamine and Oxidative Blood Biomarkers in Cardiovascular Disease Patients
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Samples Collection
2.3. Plasma TMA and TMAO
2.4. Erythrocyte Plasma Membrane Preparation
2.5. Erythrocyte Membrane Fluidity
2.6. DPPP Assay
2.7. Statistical Analysis
3. Results
3.1. Descriptive Statistics
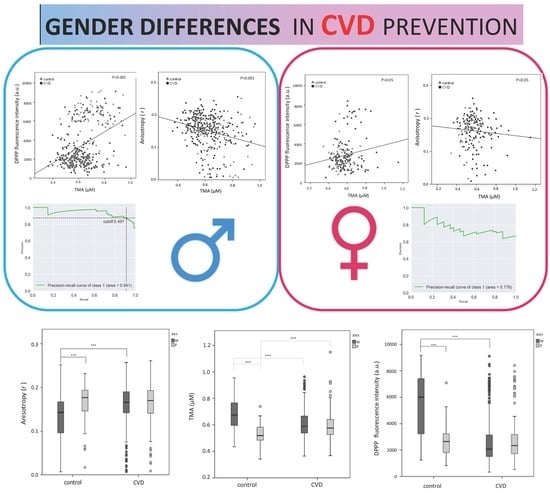
3.2. Gender Differences in the Plasma TMA Level
3.3. Gender Differences in the Erythrocyte Plasma Membrane Fluidity
3.4. Gender Differences in the Erythrocyte Lipid Hydroperoxide Level (DPPP)
3.5. Precision–Recall Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017. European Heart Network. Available online: http://www.ehnheart.org/images/CVD-statistics-report-August-2017.pdf (accessed on 15 February 2017).
- Mishra, R. Monica Determinants of cardiovascular disease and sequential decision-making for treatment among women: A Heckman’s approach. SSM Popul. Health. 2019, 7, 100365. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.L.; Pacheco, C.; Tweet, M.S.; Miller, V.M. Sex-Specific Physiology and Cardiovascular Disease. Adv. Exp. Med. Biol. 2018, 1065, 433–454. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J.P. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, D. The Developmental Origins of Adult Disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef] [PubMed]
- Calkins, K.; Devaskar, S.U. Fetal origins of adult disease. Curr. Probl. Pediatr. Adolesc. Health Care 2011, 41, 158–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.S.; Joles, J.A. Early determinants of cardiovascular disease. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 581–597. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141. [Google Scholar] [CrossRef]
- Bots, S.H.; Peters, S.A.E.; Woodward, M. Sex differences in coronary heart disease and stroke mortality: A global assessment of the effect of ageing between 1980 and 2010. BMJ Glob. Health 2017, 2, e000298. [Google Scholar] [CrossRef] [Green Version]
- Kawamoto, K.R.; Davis, M.B.; Duvernoy, C.S. Acute Coronary Syndromes: Differences in Men and Women. Curr. Atheroscler. Rep. 2016, 18, 73. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2016, 21, 1024–1032. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.B.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef]
- Bordoni, L.; Sawicka, A.K.; Szarmach, A.; Winklewski, P.J.; Olek, R.; Gabbianelli, R. A Pilot Study on the Effects of l-Carnitine and Trimethylamine-N-Oxide on Platelet Mitochondrial DNA Methylation and CVD Biomarkers in Aged Women. Int. J. Mol. Sci. 2020, 21, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosengren, A.; Hawken, S.; Ounpuu, S.; Sliwa, K.; Zubaid, M.; Almahmeed, W.A.; Blackett, K.N.; Sitthiamorn, C.; Sato, H.; Yusuf, S.; et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the IN- TERHEART study): Case-control study. Lancet 2004, 364, 953–962. [Google Scholar] [CrossRef]
- Winter, S.E.; A Lopez, C.; Baumler, A.J. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013, 14, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Chang, M.; Guo, Y.; Zhang, L.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018, 17, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeiro, M.H.; Ramirez, M.J.; Milagro, F.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Hassan, S.T.S.; Tenore, G.C.; Novellino, E. Effect of Grape Pomace Polyphenols With or Without Pectin on TMAO Serum Levels Assessed by LC/MS-Based Assay: A Preliminary Clinical Study on Overweight/Obese Subjects. Front. Pharmacol. 2019, 10, 575. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; De Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [Green Version]
- Bennett, B.J.; Vallim, T.Q.D.A.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Koay, Y.C.; Chen, Y.-C.; Wali, A.J.; Luk, A.W.S.; Li, M.; Doma, H.; Reimark, R.; Zaldivia, M.T.K.; Habtom, H.T.; E Franks, A.; et al. Plasma levels of trimethylamine-N-oxide can be increased with ‘healthy’ and ‘unhealthy’ diets and do not correlate with the extent of atherosclerosis but with plaque instability. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Yang, C.; Wang, B.; Zhang, X.; Hu, T.; Gu, Y.; Li, J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed. Pharmacother. 2018, 97, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third Universal Definition of Myocardial Infarction. Glob. Hear. 2012, 7, 275–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Hear. J. 2015, 37, 267–315. [Google Scholar] [CrossRef]
- Grinberga, S.; Dambrova, M.; Latkovskis, G.; Strele, I.; Konrade, I.; Hartmane, D.; Sevostjanovs, E.; Liepinsh, E.; Pugovics, O. Determination of trimethylamine-N-oxide in combination with L-carnitine and γ-butyrobetaine in human plasma by UPLC/MS/MS. Biomed. Chromatogr. 2015, 29, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Awwad, H.M.; Geisel, J.; Obeid, R. Determination of trimethylamine, trimethylamine N-oxide, and taurine in human plasma and urine by UHPLC-MS/MS technique. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1038, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Shinitzky, M.; Barenholz, Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim. Biophys. Acta 1978, 515, 367–394. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; D’Ubaldo, A.; Gratton, E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 1990, 57, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Vadhana, D.; Carloni, M.; Fedeli, N.; Nasuti, C.; Gabbianelli, R. Perturbation of Rat Heart Plasma Membrane Fluidity Due to Metabolites of Permethrin Insecticide. Cardiovasc. Toxicol. 2011, 11, 226–234. [Google Scholar] [CrossRef]
- Bordoni, L.; Fedeli, N.; Nasuti, C.; Capitani, M.; Fiorini, D.; Gabbianelli, R. Permethrin pesticide induces NURR1 up-regulation in dopaminergic cell line: Is the pro-oxidant effect involved in toxicant-neuronal damage? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 201, 51–57. [Google Scholar] [CrossRef]
- Girotti, A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [PubMed]
- Sohn, J.-H.; Taki, Y.; Ushio, H.; Ohshima, T. Quantitative determination of total lipid hydroperoxides by a flow injection analysis system. Lipids 2005, 40, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.M.; Zhu, W.; Schugar, R.C.; Meng, Y.; Jia, X.; Miikeda, A.; Wang, Z.; Zieger, M.; Lee, R.; Graham, M.; et al. Genetic Deficiency of Flavin-Containing Monooxygenase 3 (Fmo3) Protects Against Thrombosis but Has Only a Minor Effect on Plasma Lipid Levels—Brief Report. Arter. Thromb. Vasc. Boil. 2019, 39, 1045–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A.; Espin, J.C. Back cover: Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1770016. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Roncal, C.; Martínez-Aguilar, E.; Orbe, J.; Ravassa, S.; Fernández-Montero, A.; Saenz-Pipaon, G.; Ugarte, A.; De Mendoza, A.E.-H.; Rodríguez, J.A.; Fernández-Alonso, S.; et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci. Rep. 2019, 9, 15580–15588. [Google Scholar] [CrossRef]
- Falls, J.G.; Ryu, D.-Y.; Cao, Y.; Levi, P.E.; Hodgson, E. Regulation of Mouse Liver Flavin-Containing Monooxygenases 1 and 3 by Sex Steroids. Arch. Biochem. Biophys. 1997, 342, 212–223. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Mi, M. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Hear. Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016. [Google Scholar] [CrossRef] [Green Version]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater oxidative stress in healthy young men compared with premenopausal women. Arter. Thromb. Vasc. Boil. 2002, 22, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Félix, R.; Valentão, P.; Andrade, P.B.; Félix, C.; Novais, S.C.; Lemos, M.F. Evaluating the In Vitro Potential of Natural Extracts to Protect Lipids from Oxidative Damage. Antioxidants 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamon, I.; Valdès, V.; Franck, P.; Buchweiller, M.-C.; Fresson, J.; Hascoët, J.M. Différences liées au sexe dans le métabolisme du glutathion (GSH) du grand prématuré. Archives de Pédiatrie 2011, 18, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Brilakis, E.S.; Khera, A.; McGuire, D.K.; See, R.; Banerjee, S.; Murphy, S.A.; De Lemos, J.A. Influence of race and sex on lipoprotein-associated phospholipase A2 levels: Observations from the Dallas Heart Study. Atherosclerosis 2008, 199, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Kuslys, T.; Vishwanath, B.S.; Frey, F.J.; Frey, B.M. Differences in phospholipase A 2 activity between males and females and Asian Indians and Caucasians. Eur. J. Clin. Investig. 1996, 26, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Fedeli, D.; Fiorini, D.; Gabbianelli, R. Extra Virgin Olive Oil and Nigella sativa Oil Produced in Central Italy: A Comparison of the Nutrigenomic Effects of Two Mediterranean Oils in a Low-Grade Inflammation Model. Antioxidants 2019, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Jaworska, K.; Hering, D.; Mosieniak, G.; Bielak-Zmijewska, A.; Pilz, M.; Konwerski, M.; Gasecka, A.; Kapłon-Cieślicka, A.; Filipiak, K.; Sikora, E.; et al. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins 2019, 11, 490. [Google Scholar] [CrossRef] [Green Version]
- Jaworska, K.; Konop, M.; Hutsch, T.; Perlejewski, K.; Radkowski, M.; Grochowska, M.; Bielak-Zmijewska, A.; Mosieniak, G.; Sikora, E.; Ufnal, M. Trimethylamine But Not Trimethylamine Oxide Increases With Age in Rat Plasma and Affects Smooth Muscle Cells Viability. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2019, 75, 1276–1283. [Google Scholar] [CrossRef]





| Control F = 64/M = 89 | CVD F = 125/M = 269 | |
|---|---|---|
| Obesity (BMI ≥ 30) | 14/30 | 46/98 |
| Hypertension | 22/41 | 102/199 |
| Hyperlipidemia | 16/24 | 78/186 |
| Diabetes mellitus | 5/16 | 41/77 |
| Current or past smokers | 7/52 | 43/153 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordoni, L.; Fedeli, D.; Piangerelli, M.; Pelikant-Malecka, I.; Radulska, A.; Samulak, J.J.; Sawicka, A.K.; Lewicki, L.; Kalinowski, L.; Olek, R.A.; et al. Gender-Related Differences in Trimethylamine and Oxidative Blood Biomarkers in Cardiovascular Disease Patients. Biomedicines 2020, 8, 238. https://doi.org/10.3390/biomedicines8080238
Bordoni L, Fedeli D, Piangerelli M, Pelikant-Malecka I, Radulska A, Samulak JJ, Sawicka AK, Lewicki L, Kalinowski L, Olek RA, et al. Gender-Related Differences in Trimethylamine and Oxidative Blood Biomarkers in Cardiovascular Disease Patients. Biomedicines. 2020; 8(8):238. https://doi.org/10.3390/biomedicines8080238
Chicago/Turabian StyleBordoni, Laura, Donatella Fedeli, Marco Piangerelli, Iwona Pelikant-Malecka, Adrianna Radulska, Joanna J. Samulak, Angelika K. Sawicka, Lukasz Lewicki, Leszek Kalinowski, Robert A. Olek, and et al. 2020. "Gender-Related Differences in Trimethylamine and Oxidative Blood Biomarkers in Cardiovascular Disease Patients" Biomedicines 8, no. 8: 238. https://doi.org/10.3390/biomedicines8080238