Clinical Significance of Electronegative Low-Density Lipoprotein Cholesterol in Atherothrombosis
Abstract
:1. Introduction
2. Characteristics of Electronegative Low-Density Lipoprotein (L5 LDL)
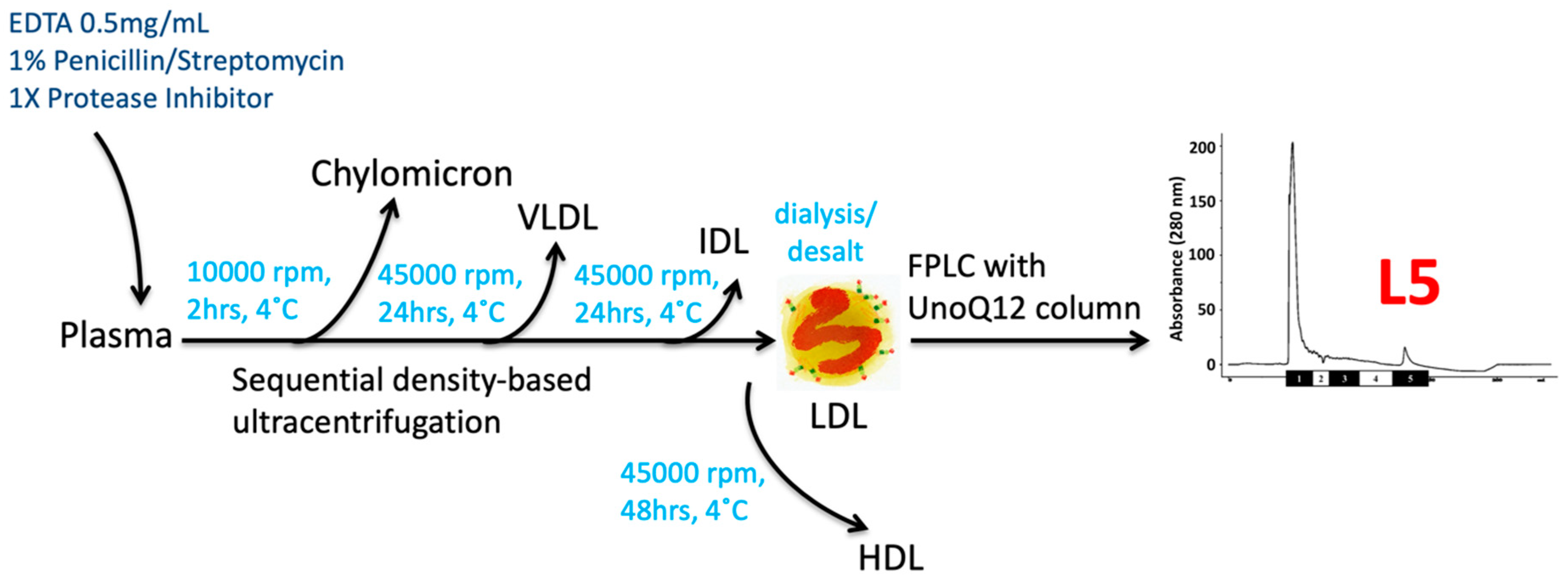
2.1. Definition and Methodolgy
2.2. Glycosylation of Apolipoproteins in L5 LDL
2.3. Atherogenic Lipid Moieties of L5 LDL
3. Cellular Signaling of L5 LDL
3.1. Signaling in Endothelial Cells
3.2. Signaling in Platelets
3.3. Signaling in Immune Cells
4. Clinical Significance of L5 LDL
4.1. Cardiometabolic Disorders
4.2. Acute Ischemic Events
4.3. Autoimmune Diseases
5. Implication of L5 LDL
5.1. Diagnostic Value of L5 LDL
5.2. Clinical Implication of L5 LDL
5.3. Drawback and Limitation of L5 LDL Quantification
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [PubMed] [Green Version]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Friera, L.; Fuster, V.; Lopez-Melgar, B.; Oliva, B.; Garcia-Ruiz, J.M.; Mendiguren, J.; Bueno, H.; Pocock, S.; Ibanez, B.; Fernandez-Ortiz, A.; et al. Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J. Am. Coll. Cardiol. 2017, 70, 2979–2991. [Google Scholar] [CrossRef]
- Toth, P.P.; Patti, A.M.; Giglio, R.V.; Nikolic, D.; Castellino, G.; Rizzo, M.; Banach, M. Management of Statin Intolerance in 2018: Still More Questions Than Answers. Am. J. Cardiovasc. Drugs 2018, 18, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Ke, L.Y.; Stancel, N.; Bair, H.; Chen, C.H. The underlying chemistry of electronegative LDL’s atherogenicity. Curr. Atheroscler. Rep. 2014, 16, 428. [Google Scholar] [CrossRef]
- Chu, C.S.; Chan, H.C.; Tsai, M.H.; Stancel, N.; Lee, H.C.; Cheng, K.H.; Tung, Y.C.; Chan, H.C.; Wang, C.Y.; Shin, S.J.; et al. Range of L5 LDL levels in healthy adults and L5’s predictive power in patients with hyperlipidemia or coronary artery disease. Sci. Rep. 2018, 8, 11866. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, W.; Yang, J.H.; Chang, P.Y.; Walterscheid, J.P.; Chen, H.H.; Marcelli, M.; Tang, D.; Lee, Y.T.; Liao, W.S.; et al. Electronegative LDL impairs vascular endothelial cell integrity in diabetes by disrupting fibroblast growth factor 2 (FGF2) autoregulation. Diabetes 2008, 57, 158–166. [Google Scholar] [CrossRef]
- Ke, L.Y.; Chan, H.C.; Chan, H.C.; Kalu, F.C.U.; Lee, H.C.; Lin, I.L.; Jhuo, S.J.; Lai, W.T.; Tsao, C.R.; Sawamura, T.; et al. Electronegative Low-Density Lipoprotein L5 Induces Adipose Tissue Inflammation Associated With Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 4615–4625. [Google Scholar] [CrossRef]
- Chan, H.C.; Ke, L.Y.; Chu, C.S.; Lee, A.S.; Shen, M.Y.; Cruz, M.A.; Hsu, J.F.; Cheng, K.H.; Chan, H.C.; Lu, J.; et al. Highly electronegative LDL from patients with ST-elevation myocardial infarction triggers platelet activation and aggregation. Blood 2013, 122, 3632–3641. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.Y.; Chen, F.Y.; Hsu, J.F.; Fu, R.H.; Chang, C.M.; Chang, C.T.; Liu, C.H.; Wu, J.R.; Lee, A.S.; Chan, H.C.; et al. Plasma L5 levels are elevated in ischemic stroke patients and enhance platelet aggregation. Blood 2016, 127, 1336–1345. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.C.; Bonnie Chan, H.C.; Liang, C.J.; Lee, H.C.; Su, H.; Lee, A.S.; Shiea, J.; Tsai, W.C.; Ou, T.T.; Wu, C.C.; et al. Role of Low-density Lipoprotein in Early Vascular Aging Associated With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2020, 72, 972–984. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, C.H.; Chen, Y.M.; Hsieh, T.Y.; Li, J.P.; Shen, M.Y.; Lan, J.L.; Chen, D.Y. Association between Negatively Charged Low-Density Lipoprotein L5 and Subclinical Atherosclerosis in Rheumatoid Arthritis Patients. J. Clin. Med. 2019, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.S.; Ke, L.Y.; Chan, H.C.; Chan, H.C.; Chen, C.C.; Cheng, K.H.; Lee, H.C.; Kuo, H.F.; Chang, C.T.; Chang, K.C.; et al. Four Statin Benefit Groups Defined by the 2013 ACC/AHA New Cholesterol Guideline are Characterized by Increased Plasma Level of Electronegative Low-Density Lipoprotein. Acta Cardiol. Sin. 2016, 32, 667–675. [Google Scholar]
- Chu, C.S.; Wang, Y.C.; Lu, L.S.; Walton, B.; Yilmaz, H.R.; Huang, R.Y.; Sawamura, T.; Dixon, R.A.; Lai, W.T.; Chen, C.H.; et al. Electronegative low-density lipoprotein increases C-reactive protein expression in vascular endothelial cells through the LOX-1 receptor. PLoS ONE 2013, 8, e70533. [Google Scholar] [CrossRef] [Green Version]
- Hoff, H.F.; Bradley, W.A.; Heideman, C.L.; Gaubatz, J.W.; Karagas, M.D.; Gotto, A.M., Jr. Characterization of low density lipoprotein-like particle in the human aorta from grossly normal and atherosclerotic regions. Biochim. Biophys. Acta 1979, 573, 361–374. [Google Scholar] [CrossRef]
- Avogaro, P.; Bittolo Bon, G.; Cazzolato, G. Meaning of a modified LDL in humans. Adv. Exp. Med. Biol. 1987, 210, 209–212. [Google Scholar] [PubMed]
- Avogaro, P.; Bon, G.B.; Cazzolato, G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis 1988, 8, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.Y.; Raya, J.L.; Chen, H.H.; Chen, C.H.; Abe, Y.; Pownall, H.J.; Taylor, A.A.; Smith, C.V. Isolation, characterization, and functional assessment of oxidatively modified subfractions of circulating low-density lipoproteins. Arter. Thromb Vasc. Biol. 2003, 23, 1083–1090. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Jiang, T.; Yang, J.H.; Jiang, W.; Lu, J.; Marathe, G.K.; Pownall, H.J.; Ballantyne, C.M.; McIntyre, T.M.; Henry, P.D.; et al. Low-density lipoprotein in hypercholesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcription. Circulation 2003, 107, 2102–2108. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Quesada, J.L.; Vinagre, I.; de Juan-Franco, E.; Sanchez-Hernandez, J.; Blanco-Vaca, F.; Ordonez-Llanos, J.; Perez, A. Effect of improving glycemic control in patients with type 2 diabetes mellitus on low-density lipoprotein size, electronegative low-density lipoprotein and lipoprotein-associated phospholipase A2 distribution. Am. J. Cardiol. 2012, 110, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.Y.; Engler, D.A.; Lu, J.; Matsunami, R.K.; Chan, H.C.; Wang, G.J.; Yang, C.Y.; Chang, J.G.; Chen, C.H. Chemical composition-oriented receptor selectivity of L5, a naturally occurring atherogenic low-density lipoprotein. Pure Appl. Chem. 2011, 83, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Bancells, C.; Canals, F.; Benitez, S.; Colome, N.; Julve, J.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Proteomic analysis of electronegative low-density lipoprotein. J. Lipid Res. 2010, 51, 3508–3515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.Y.; Chen, Y.F.; Chan, H.C.; Chung, C.H.; Peng, H.Y.; Ho, Y.C.; Chen, C.H.; Chang, K.C.; Tang, C.H.; Lee, A.S. Role of apolipoprotein E in electronegative low-density lipoprotein-induced mitochondrial dysfunction in cardiomyocytes. Metabolism 2020, 107, 154227. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.Y.; Chan, H.C.; Chen, C.C.; Chang, C.F.; Lu, P.L.; Chu, C.S.; Lai, W.T.; Shin, S.J.; Liu, F.T.; Chen, C.H. Increased APOE glycosylation plays a key role in the atherogenicity of L5 low-density lipoprotein. FASEB J. 2020. [Google Scholar] [CrossRef]
- Santos-Ferreira, C.; Baptista, R.; Oliveira-Santos, M.; Costa, R.; Pereira Moura, J.; Goncalves, L. Apolipoprotein E2 Genotype Is Associated with a 2-Fold Increase in the Incidence of Type 2 Diabetes Mellitus: Results from a Long-Term Observational Study. J. Lipids 2019, 2019, 1698610. [Google Scholar] [CrossRef] [Green Version]
- Safieh, M.; Korczyn, A.D.; Michaelson, D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Ke, L.Y.; Chan, H.C.; Chen, C.C.; Lu, J.; Marathe, G.K.; Chu, C.S.; Chan, H.C.; Wang, C.Y.; Tung, Y.C.; McIntyre, T.M.; et al. Enhanced Sphingomyelinase Activity Contributes to the Apoptotic Capacity of Electronegative Low-Density Lipoprotein. J. Med. Chem. 2016, 59, 1032–1040. [Google Scholar]
- Sanchez-Quesada, J.L.; Camacho, M.; Anton, R.; Benitez, S.; Vila, L.; Ordonez-Llanos, J. Electronegative LDL of FH subjects: Chemical characterization and induction of chemokine release from human endothelial cells. Atherosclerosis 2003, 166, 261–270. [Google Scholar] [CrossRef]
- Benitez, S.; Perez, A.; Sanchez-Quesada, J.L.; Wagner, A.M.; Rigla, M.; Arcelus, R.; Jorba, O.; Ordonez-Llanos, J. Electronegative low-density lipoprotein subfraction from type 2 diabetic subjects is proatherogenic and unrelated to glycemic control. Diabetes Metab. Res. Rev. 2007, 23, 26–34. [Google Scholar] [CrossRef]
- Ho, C.I.; Chen, J.Y.; Chen, S.Y.; Tsai, Y.W.; Weng, Y.M.; Tsao, Y.C.; Li, W.C. Relationship between TG/HDL-C ratio and metabolic syndrome risk factors with chronic kidney disease in healthy adult population. Clin. Nutr. 2015, 34, 874–880. [Google Scholar] [CrossRef]
- Gaubatz, J.W.; Gillard, B.K.; Massey, J.B.; Hoogeveen, R.C.; Huang, M.; Lloyd, E.E.; Raya, J.L.; Yang, C.Y.; Pownall, H.J. Dynamics of dense electronegative low density lipoproteins and their preferential association with lipoprotein phospholipase A(2). J. Lipid Res. 2007, 48, 348–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Quesada, J.L.; Vinagre, I.; De Juan-Franco, E.; Sanchez-Hernandez, J.; Bonet-Marques, R.; Blanco-Vaca, F.; Ordonez-Llanos, J.; Perez, A. Impact of the LDL subfraction phenotype on Lp-PLA2 distribution, LDL modification and HDL composition in type 2 diabetes. Cardiovasc. Diabetol. 2013, 12, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.Y.; Chen, H.H.; Huang, M.T.; Raya, J.L.; Yang, J.H.; Chen, C.H.; Gaubatz, J.W.; Pownall, H.J.; Taylor, A.A.; Ballantyne, C.M.; et al. Pro-apoptotic low-density lipoprotein subfractions in type II diabetes. Atherosclerosis 2007, 193, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, A.; Macphee, C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: Biology, epidemiology, and possible therapeutic target. Arter. Thromb. Vasc. Biol. 2005, 25, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Perry, D.K.; Obeid, L.M.; Hannun, Y.A. Ceramide and the regulation of apoptosis and the stress response. Trends Cardiovasc. Med. 1996, 6, 158–162. [Google Scholar] [CrossRef]
- Spiegel, S.; Cuvillier, O.; Edsall, L.; Kohama, T.; Menzeleev, R.; Olivera, A.; Thomas, D.; Tu, Z.; Van Brocklyn, J.; Wang, F. Roles of sphingosine-1-phosphate in cell growth, differentiation, and death. Biochemistry 1998, 63, 69–73. [Google Scholar]
- Sassoli, C.; Pierucci, F.; Zecchi-Orlandini, S.; Meacci, E. Sphingosine 1-Phosphate (S1P)/ S1P Receptor Signaling and Mechanotransduction: Implications for Intrinsic Tissue Repair/Regeneration. Int. J. Mol. Sci. 2019, 20, 5545. [Google Scholar] [CrossRef] [Green Version]
- Filippov, V.; Song, M.A.; Zhang, K.; Vinters, H.V.; Tung, S.; Kirsch, W.M.; Yang, J.; Duerksen-Hughes, P.J. Increased ceramide in brains with Alzheimer’s and other neurodegenerative diseases. J. Alzheimer’s Dis. 2012, 29, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Is There a Role for Bioactive Lipids in the Pathobiology of Diabetes Mellitus? Front. Endocrinol. 2017, 8, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; Marz, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Huang, Y.H.; Schafer-Elinder, L.; Wu, R.; Claesson, H.E.; Frostegard, J. Lysophosphatidylcholine (LPC) induces proinflammatory cytokines by a platelet-activating factor (PAF) receptor-dependent mechanism. Clin. Exp. Immunol. 1999, 116, 326–331. [Google Scholar] [CrossRef]
- Takahara, N.; Kashiwagi, A.; Maegawa, H.; Shigeta, Y. Lysophosphatidylcholine stimulates the expression and production of MCP-1 by human vascular endothelial cells. Metabolism 1996, 45, 559–564. [Google Scholar] [CrossRef]
- Murugesan, G.; Sandhya Rani, M.R.; Gerber, C.E.; Mukhopadhyay, C.; Ransohoff, R.M.; Chisolm, G.M.; Kottke-Marchant, K. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J. Mol. Cell Cardiol. 2003, 35, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Lee, J.J.; Chen, Y.J.; Lin, S.I.; Lin, L.D.; Jein-Wen Liou, E.; Huang, W.L.; Chan, C.P.; Huang, C.C.; Jeng, J.H. Lysophosphatidylcholine induces cytotoxicity/apoptosis and IL-8 production of human endothelial cells: Related mechanisms. Oncotarget 2017, 8, 106177–106189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugiyama, K.; Kerns, S.A.; Morrisett, J.D.; Roberts, R.; Henry, P.D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature 1990, 344, 160–162. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, J.A.; Park, M.H.; Jung, S.C.; Suh, S.H.; Pang, M.G.; Kim, Y.J. Lysophosphatidylcholine induces endothelial cell injury by nitric oxide production through oxidative stress. J. Matern. Fetal Neonatal Med. 2009, 22, 325–331. [Google Scholar] [CrossRef]
- Li, B.; Tian, S.; Liu, X.; He, C.; Ding, Z.; Shan, Y. Sulforaphane protected the injury of human vascular endothelial cell induced by LPC through up-regulating endogenous antioxidants and phase II enzymes. Food Funct. 2015, 6, 1984–1991. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Colles, S.M.; Damron, D.S.; Graham, L.M. Lysophosphatidylcholine inhibits endothelial cell migration by increasing intracellular calcium and activating calpain. Arter. Thromb. Vasc. Biol. 2003, 23, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Rikitake, Y.; Kawashima, S.; Yamashita, T.; Ueyama, T.; Ishido, S.; Hotta, H.; Hirata, K.; Yokoyama, M. Lysophosphatidylcholine inhibits endothelial cell migration and proliferation via inhibition of the extracellular signal-regulated kinase pathway. Arter. Thromb. Vasc. Biol. 2000, 20, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Rabini, R.A.; Galassi, R.; Fumelli, P.; Dousset, N.; Solera, M.L.; Valdiguie, P.; Curatola, G.; Ferretti, G.; Taus, M.; Mazzanti, L. Reduced Na(+)-K(+)-ATPase activity and plasma lysophosphatidylcholine concentrations in diabetic patients. Diabetes 1994, 43, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Okita, M.; Gaudette, D.C.; Mills, G.B.; Holub, B.J. Elevated levels and altered fatty acid composition of plasma lysophosphatidylcholine(lysoPC) in ovarian cancer patients. Int. J. Cancer 1997, 71, 31–34. [Google Scholar] [CrossRef]
- Sasagawa, T.; Suzuki, K.; Shiota, T.; Kondo, T.; Okita, M. The significance of plasma lysophospholipids in patients with renal failure on hemodialysis. J. Nutr. Sci. Vitam. 1998, 44, 809–818. [Google Scholar] [CrossRef]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Lee, A.S.; Lu, L.S.; Ke, L.Y.; Chen, W.Y.; Dong, J.W.; Lu, J.; Chen, Z.; Chu, C.S.; Chan, H.C.; et al. Human electronegative LDL induces mitochondrial dysfunction and premature senescence of vascular cells in vivo. Aging Cell 2018, 17, e12792. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Yang, J.H.; Burns, A.R.; Chen, H.H.; Tang, D.; Walterscheid, J.P.; Suzuki, S.; Yang, C.Y.; Sawamura, T.; Chen, C.H. Mediation of electronegative low-density lipoprotein signaling by LOX-1: A possible mechanism of endothelial apoptosis. Circ. Res. 2009, 104, 619–627. [Google Scholar] [CrossRef]
- Abe, Y.; Fornage, M.; Yang, C.Y.; Bui-Thanh, N.A.; Wise, V.; Chen, H.H.; Rangaraj, G.; Ballantyne, C.M. L5, the most electronegative subfraction of plasma LDL, induces endothelial vascular cell adhesion molecule 1 and CXC chemokines, which mediate mononuclear leukocyte adhesion. Atherosclerosis 2007, 192, 56–66. [Google Scholar] [CrossRef]
- Estruch, M.; Rajamaki, K.; Sanchez-Quesada, J.L.; Kovanen, P.T.; Oorni, K.; Benitez, S.; Ordonez-Llanos, J. Electronegative LDL induces priming and inflammasome activation leading to IL-1beta release in human monocytes and macrophages. Biochim. Biophys. Acta 2015, 1851, 1442–1449. [Google Scholar] [CrossRef]
- Chang, S.F.; Chang, P.Y.; Chou, Y.C.; Lu, S.C. Electronegative LDL Induces M1 Polarization of Human Macrophages Through a LOX-1-Dependent Pathway. Inflammation 2020, 43, 1524–1535. [Google Scholar] [CrossRef]
- Puig, N.; Montolio, L.; Camps-Renom, P.; Navarra, L.; Jimenez-Altayo, F.; Jimenez-Xarrie, E.; Sanchez-Quesada, J.L.; Benitez, S. Electronegative LDL Promotes Inflammation and Triglyceride Accumulation in Macrophages. Cells 2020, 9, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puig, N.; Estruch, M.; Jin, L.; Sanchez-Quesada, J.L.; Benitez, S. The Role of Distinctive Sphingolipids in the Inflammatory and Apoptotic Effects of Electronegative LDL on Monocytes. Biomolecules 2019, 9, 300. [Google Scholar] [CrossRef] [Green Version]
- Ligi, D.; Benitez, S.; Croce, L.; Rivas-Urbina, A.; Puig, N.; Ordonez-Llanos, J.; Mannello, F.; Sanchez-Quesada, J.L. Electronegative LDL induces MMP-9 and TIMP-1 release in monocytes through CD14 activation: Inhibitory effect of glycosaminoglycan sulodexide. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Wang, G.J.; Chan, H.C.; Chen, F.Y.; Chang, C.M.; Yang, C.Y.; Lee, Y.T.; Chang, K.C.; Chen, C.H. Electronegative low-density lipoprotein induces cardiomyocyte apoptosis indirectly through endothelial cell-released chemokines. Apoptosis 2012, 17, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Xi, Y.; Lai, C.H.; Chen, W.Y.; Peng, H.Y.; Chan, H.C.; Chen, C.H.; Chang, K.C. Human electronegative low-density lipoprotein modulates cardiac repolarization via LOX-1-mediated alteration of sarcolemmal ion channels. Sci. Rep. 2017, 7, 10889. [Google Scholar] [CrossRef]
- Revuelta-Lopez, E.; Cal, R.; Julve, J.; Rull, A.; Martinez-Bujidos, M.; Perez-Cuellar, M.; Ordonez-Llanos, J.; Badimon, L.; Sanchez-Quesada, J.L.; Llorente-Cortes, V. Hypoxia worsens the impact of intracellular triglyceride accumulation promoted by electronegative low-density lipoprotein in cardiomyocytes by impairing perilipin 5 upregulation. Int. J. Biochem. Cell Biol. 2015, 65, 257–267. [Google Scholar] [CrossRef]
- Chang, K.C.; Lee, A.S.; Chen, W.Y.; Lin, Y.N.; Hsu, J.F.; Chan, H.C.; Chang, C.M.; Chang, S.S.; Pan, C.C.; Sawamura, T.; et al. Increased LDL electronegativity in chronic kidney disease disrupts calcium homeostasis resulting in cardiac dysfunction. J. Mol. Cell Cardiol. 2015, 84, 36–44. [Google Scholar] [CrossRef]
- Urata, J.; Ikeda, S.; Koga, S.; Nakata, T.; Yasunaga, T.; Sonoda, K.; Koide, Y.; Ashizawa, N.; Kohno, S.; Maemura, K. Negatively charged low-density lipoprotein is associated with atherogenic risk in hypertensive patients. Heart Vessel. 2012, 27, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Sawamura, T.; Kakino, A.; Fujita, Y. LOX-1: A multiligand receptor at the crossroads of response to danger signals. Curr. Opin. Lipidol. 2012, 23, 439–445. [Google Scholar] [CrossRef]
- Stancel, N.; Chen, C.C.; Ke, L.Y.; Chu, C.S.; Lu, J.; Sawamura, T.; Chen, C.H. Interplay between CRP, Atherogenic LDL, and LOX-1 and Its Potential Role in the Pathogenesis of Atherosclerosis. Clin. Chem. 2016, 62, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Raffioni, S.; Bradshaw, R.A. Activation of phosphatidylinositol 3-kinase by epidermal growth factor, basic fibroblast growth factor, and nerve growth factor in PC12 pheochromocytoma cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9121–9125. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Wu, S.Y.; Liao, C.Y.; Teng, C.M.; Wu, Y.C.; Kuo, S.C. The roles and mechanisms of PAR4 and P2Y12/phosphatidylinositol 3-kinase pathway in maintaining thrombin-induced platelet aggregation. Br. J. Pharm. 2010, 161, 643–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwali, M.R.; Hu, C.P.; Mohandas, B.; Dandapat, A.; Deonikar, P.; Chen, J.; Cawich, I.; Sawamura, T.; Kavdia, M.; Mehta, J.L. Modulation of ADP-induced platelet activation by aspirin and pravastatin: Role of lectin-like oxidized low-density lipoprotein receptor-1, nitric oxide, oxidative stress, and inside-out integrin signaling. J. Pharm. Exp. 2007, 322, 1324–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podrez, E.A.; Byzova, T.V. Prothrombotic lipoprotein patterns in stroke. Blood 2016, 127, 1221–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, T.C. Bad cholesterol breaking really bad. Blood 2013, 122, 3551–3553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundtman, C.; Wick, G. The autoimmune concept of atherosclerosis. Curr. Opin. Lipidol. 2011, 22, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Cinoku, I.I.; Mavragani, C.P.; Moutsopoulos, H.M. Atherosclerosis: Beyond the lipid storage hypothesis. The role of autoimmunity. Eur. J. Clin. Investig. 2020, 50, e13195. [Google Scholar]
- Sobenin, I.A.; Salonen, J.T.; Zhelankin, A.V.; Melnichenko, A.A.; Kaikkonen, J.; Bobryshev, Y.V.; Orekhov, A.N. Low density lipoprotein-containing circulating immune complexes: Role in atherosclerosis and diagnostic value. Biomed. Res. Int. 2014, 2014, 205697. [Google Scholar] [CrossRef]
- Benitez, S.; Camacho, M.; Bancells, C.; Vila, L.; Sanchez-Quesada, J.L.; Ordonez-Llanos, J. Wide proinflammatory effect of electronegative low-density lipoprotein on human endothelial cells assayed by a protein array. Biochim. Biophys. Acta 2006, 1761, 1014–1021. [Google Scholar] [CrossRef]
- Estruch, M.; Bancells, C.; Beloki, L.; Sanchez-Quesada, J.L.; Ordonez-Llanos, J.; Benitez, S. CD14 and TLR4 mediate cytokine release promoted by electronegative LDL in monocytes. Atherosclerosis 2013, 229, 356–362. [Google Scholar] [CrossRef]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.; Sameshima, M.; Hase, A.; Seto, Y.; Nagata, S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66, 233–243. [Google Scholar] [CrossRef]
- Klimov, A.N.; Denisenko, A.D.; Popov, A.V.; Nagornev, V.A.; Pleskov, V.M.; Vinogradov, A.G.; Denisenko, T.V.; Magracheva, E.; Kheifes, G.M.; Kuznetzov, A.S. Lipoprotein-antibody immune complexes. Their catabolism and role in foam cell formation. Atherosclerosis 1985, 58, 1–15. [Google Scholar] [CrossRef]
- Tertov, V.V.; Orekhov, A.N.; Sobenin, I.A.; Morrisett, J.D.; Gotto, A.M., Jr.; Guevara, J.G., Jr. Carbohydrate composition of protein and lipid components in sialic acid-rich and -poor low density lipoproteins from subjects with and without coronary artery disease. J. Lipid Res. 1993, 34, 365–375. [Google Scholar] [PubMed]
- Yang, T.C.; Chang, P.Y.; Kuo, T.L.; Lu, S.C. Electronegative L5-LDL induces the production of G-CSF and GM-CSF in human macrophages through LOX-1 involving NF-kappaB and ERK2 activation. Atherosclerosis 2017, 267, 1–9. [Google Scholar] [CrossRef]
- Leone, A.M.; Rutella, S.; Bonanno, G.; Contemi, A.M.; de Ritis, D.G.; Giannico, M.B.; Rebuzzi, A.G.; Leone, G.; Crea, F. Endogenous G-CSF and CD34+ cell mobilization after acute myocardial infarction. Int. J. Cardiol. 2006, 111, 202–208. [Google Scholar] [CrossRef]
- Cornish, A.L.; Campbell, I.K.; McKenzie, B.S.; Chatfield, S.; Wicks, I.P. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 554–559. [Google Scholar] [CrossRef]
- Yang, T.C.; Chang, P.Y.; Lu, S.C. L5-LDL from ST-elevation myocardial infarction patients induces IL-1beta production via LOX-1 and NLRP3 inflammasome activation in macrophages. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H265–H274. [Google Scholar] [CrossRef] [Green Version]
- Tai, M.H.; Kuo, S.M.; Liang, H.T.; Chiou, K.R.; Lam, H.C.; Hsu, C.M.; Pownall, H.J.; Chen, H.H.; Huang, M.T.; Yang, C.Y. Modulation of angiogenic processes in cultured endothelial cells by low density lipoproteins subfractions from patients with familial hypercholesterolemia. Atherosclerosis 2006, 186, 448–457. [Google Scholar] [CrossRef]
- Tang, D.; Lu, J.; Walterscheid, J.P.; Chen, H.H.; Engler, D.A.; Sawamura, T.; Chang, P.Y.; Safi, H.J.; Yang, C.Y.; Chen, C.H. Electronegative LDL circulating in smokers impairs endothelial progenitor cell differentiation by inhibiting Akt phosphorylation via LOX-1. J. Lipid Res. 2008, 49, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, L.B.; Kaplan, M.J. Update on cardiovascular disease in lupus. Curr. Opin. Rheumatol. 2016, 28, 468–476. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Kaplan, M.J. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu. Rev. Med. 2013, 64, 249–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asanuma, Y.; Oeser, A.; Shintani, A.K.; Turner, E.; Olsen, N.; Fazio, S.; Linton, M.F.; Raggi, P.; Stein, C.M. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 2003, 349, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Hegele, R.A.; Tsimikas, S. Lipid-Lowering Agents. Circ. Res. 2019, 124, 386–404. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Leibowitz, M.; Cohen-Stavi, C.; Basu, S.; Balicer, R.D. Targeting LDL Cholesterol: Beyond Absolute Goals Toward Personalized Risk. Curr. Cardiol. Rep. 2017, 19, 52. [Google Scholar] [CrossRef]
- Su, X.; Kong, Y.; Peng, D. Evidence for changing lipid management strategy to focus on non-high density lipoprotein cholesterol. Lipids Health Dis. 2019, 18, 134. [Google Scholar] [CrossRef] [Green Version]
- Nambi, V.; Bhatt, D.L. Primary Prevention of Atherosclerosis: Time to Take a Selfie? J. Am. Coll. Cardiol. 2017, 70, 2992–2994. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Khan, A.; Maki, K.C.; Brinton, E.A.; Cohen, J.D. Provider recommendations for patient-reported muscle symptoms on statin therapy: Insights from the Understanding Statin Use in America and Gaps in Patient Education survey. J. Clin. Lipidol. 2018, 12, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Matsunaga, A.; Rainwater, D.L.; Miura, S.; Noda, K.; Nishikawa, H.; Uehara, Y.; Shirai, K.; Ogawa, M.; Saku, K. Effects of rosuvastatin on electronegative LDL as characterized by capillary isotachophoresis: The ROSARY Study. J. Lipid Res. 2009, 50, 1832–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Miura, S.; Yanagi, D.; Noda, K.; Nishikawa, H.; Matsunaga, A.; Shirai, K.; Iwata, A.; Yoshinaga, K.; Adachi, H.; et al. Reduction of charge-modified LDL by statin therapy in patients with CHD or CHD risk factors and elevated LDL-C levels: The SPECIAL Study. Atherosclerosis 2008, 201, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Urbina, A.; Rull, A.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Electronegative LDL: An Active Player in Atherogenesis or a By-Product of Atherosclerosis? Curr. Med. Chem. 2019, 26, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Obama, T.; Koba, S.; Takaki, T.; Iwamoto, S.; Aiuchi, T.; Kato, R.; Kikuchi, M.; Hamazaki, Y.; Itabe, H. Circulating oxidized LDL, increased in patients with acute myocardial infarction, is accompanied by heavily modified HDL. J. Lipid Res. 2020, 61, 816–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulin, T.; Kazuma, S.M.; Tripodi, G.L.; Cavalcante, M.F.; Wakasuqui, F.; Oliveira, C.L.P.; Degenhardt, M.F.S.; Michaloski, J.; Giordano, R.J.; Ketelhuth, D.F.J.; et al. Proinflammatory Action of a New Electronegative Low-Density Lipoprotein Epitope. Biomolecules 2019, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.C.P.; Fernandez, D.G.E.; Cohen, D.; Figueiredo-Neto, A.M.; Maranhao, R.C.; Damasceno, N.R.T. Oxidized and electronegative low-density lipoprotein as potential biomarkers of cardiovascular risk in obese adolescents. Clinics 2018, 73, e189. [Google Scholar] [CrossRef]
- Lobo, J.; Santos, F.; Grosso, D.; Lima, R.; Barreira, A.L.; Leite, M., Jr.; Mafra, D.; Abdalla, D.S. Electronegative LDL and lipid abnormalities in patients undergoing hemodialysis and peritoneal dialysis. Nephron Clin. Pract. 2008, 108, c298–c304. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Sevanian, A.; Rodrigues, R.J.; Apolinario, E.; Abdalla, D.S. Minimally modified electronegative LDL and its autoantibodies in acute and chronic coronary syndromes. Clin. Biochem. 2006, 39, 708–714. [Google Scholar] [CrossRef]

| Publications | Sci Rep [7] | JCEM [9] | Blood [10] | Blood [11] | JCM [13] | AR [12] |
|---|---|---|---|---|---|---|
| Subjects | HLP | MetS | STEMI | stroke | RA | SLE |
| n | 35 | 29 | 30 | 35 | 30 | 45 |
| T-CHOL | 235.9 ± 36.6 | 232.9 ± 31.6 a | 179.1 ± 33.9 | 151.4 ± 34.3 | 219 (193–245) c | NA |
| TG | 164.5 ± 90.6 | 259.6 ± 209.1 a | 119.6 ± 65.6 | 123.8 ± 72.5 | 123 (87–170) c | NA |
| HDL-C | 53.1 ± 16.4 | 45.4 ± 9.7 a,** | 38.5 ± 8.6 *** | 32.7 ± 6.6 | 58.5 (48–66) c | 48.9 ± 17.5 ** |
| LDL-C | 146.0 ± 34.9 *** | 142.2 ± 41.8 a | 116.7 ± 32.4 | 105.4 ± 34.5 | 142 (111–168) c | 105.1 ± 32.3 * |
| L5% | 2.3 ± 1.3 *** | 5.3 ± 6.9 a,*** | 15.4 ± 14.5 *** | 19.1 ± 10.6 *** | 2.0 (1.3–4.5) c,*** | 2.4 ± 1.3 *** |
| [L5] | 3.2 ± 2.0 *** | 7.3 ± 9.8 a,** | 18.9 ± 21.0 *** | 20.6 ± 13.5 *** | 2.9 (1.7–5.7) c,*** | 2.4 ± 1.3 *** |
| Controls | NHC | None-MetS | NHC | NHC | NHC | NHC |
| n | 35 | 29 | 30 | 25 | 12 | 37 |
| T-CHOL | 173.4 ± 32.8 | 215.3 ± 50.8 b | 179.3 ± 32.9 | 150.8 ± 32.9 | 208 (201–231) c | NA |
| TG | 79.7 ± 56.1 | 91.6 ± 47.5 b | 78.6 ± 59.8 | 109 ± 38.5 | 90 (72.8–126) c | NA |
| HDL-C | 54.4 ± 14.0 | 56.5 ± 17.4 b | 55.6 ± 14.2 | 41.8 ± 12.1 | 59 (46–78) c | 58 ± 16 |
| LDL-C | 103.3 ± 27.6 | 140.9 ± 44.5 b | 108.1 ± 28.4 | 92.6 ± 33.5 | 131 (120–155) c | 118.2 ± 23.3 |
| L5% | 1.3 ± 0.7 | 2.1 ± 1.4 b | 1.5 ± 1.1 | 0.5 ± 0.3 | 0.9 (0.6–1.1) c | 0.7 ± 0.3 |
| [L5] | 1.3 ± 0.7 | 3.0 ± 2.0 b | 1.7 ± 1.5 | 0.5 ± 0.4 | 1.3 (0.8–1.5) c | 0.8 ± 0.4 |
| L5% [P’t-NHC] | 1.0 ± 0.2 | 3.2 ± 1.3 | 13.9 ± 2.7 | 18.6 ± 1.8 | NA | 1.7 ± 0.2 |
| [L5] [P’t-NHC] | 1.9 ± 0.4 | 4.3 ± 1.9 | 17.2 ± 3.8 | 20.1 ± 2.3 | NA | 1.6 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.-S.; Law, S.H.; Lenzen, D.; Tan, Y.-H.; Weng, S.-F.; Ito, E.; Wu, J.-C.; Chen, C.-H.; Chan, H.-C.; Ke, L.-Y. Clinical Significance of Electronegative Low-Density Lipoprotein Cholesterol in Atherothrombosis. Biomedicines 2020, 8, 254. https://doi.org/10.3390/biomedicines8080254
Chu C-S, Law SH, Lenzen D, Tan Y-H, Weng S-F, Ito E, Wu J-C, Chen C-H, Chan H-C, Ke L-Y. Clinical Significance of Electronegative Low-Density Lipoprotein Cholesterol in Atherothrombosis. Biomedicines. 2020; 8(8):254. https://doi.org/10.3390/biomedicines8080254
Chicago/Turabian StyleChu, Chih-Sheng, Shi Hui Law, David Lenzen, Yong-Hong Tan, Shih-Feng Weng, Etsuro Ito, Jung-Chou Wu, Chu-Huang Chen, Hua-Chen Chan, and Liang-Yin Ke. 2020. "Clinical Significance of Electronegative Low-Density Lipoprotein Cholesterol in Atherothrombosis" Biomedicines 8, no. 8: 254. https://doi.org/10.3390/biomedicines8080254






