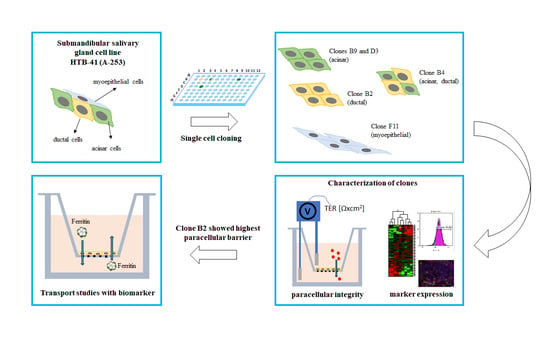
An In Vitro Barrier Model of the Human Submandibular Salivary Gland Epithelium Based on a Single Cell Clone of Cell Line HTB-41: Establishment and Application for Biomarker Transport Studies
Abstract
:1. Introduction
2. Experimental Section
2.1. Cell Culture
Single Cloning of HTB-41 Cells
2.2. Transwell Studies
2.3. Characterization of HTB-41 Clones with Cell Type-Specific Markers
2.3.1. Quantitative Real-Time PCR (qPCR)
2.3.2. Immunofluorescence Staining
2.4. Transport Studies with Ferritin
2.5. Statistical Analysis
3. Results
3.1. Establishment of Salivary Gland Epithelium Barrier Models
3.2. Salivary Gland Epithelium Marker Characterization of HTB-41 Clones
3.3. Transport Studies with Ferritin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desai, G.S. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J. Diabetes 2014, 5, 730. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.L.; Cooper-White, J.; Punyadeera, C.K. Saliva proteome research: Current status and future outlook. Crit. Rev. Biotechnol. 2013, 33, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Marti-Alamo, S.; Mancheno-Franch, A.; Marzal-Gamarra, C.; Carlos-Fabuel, L. Saliva as a diagnostic fluid. Literature review. J. Clin. Exp. Dent. 2012, 4, e237–e243. [Google Scholar] [CrossRef] [PubMed]
- Guillon, G.; Yearwood, G.; Snipes, C.; Boschi, D.; Reed, M.R. Human anti-HIV IgM detection by the OraQuick ADVANCE ® Rapid HIV 1/2 Antibody Test. PeerJ 2018, 6, e4430. [Google Scholar] [CrossRef] [Green Version]
- Bierbaumer, L.; Schwarze, U.Y.; Gruber, R.; Neuhaus, W. Cell culture models of oral mucosal barriers: A review with a focus on applications, culture conditions and barrier properties. Tissue Barriers 2018, 00, 1–42. [Google Scholar] [CrossRef]
- Pfaffe, T.; Cooper-White, J.; Beyerlein, P.; Kostner, K.; Punyadeera, C. Diagnostic potential of saliva: Current state and future applications. Clin. Chem. 2011, 57, 675–687. [Google Scholar] [CrossRef] [Green Version]
- Kouznetsova, I.; Gerlach, K.L.; Zahl, C. Expression Analysis of Human Salivary Glands by Laser Microdissection: Differences between Submandibular and Labial Glands. Cell. Physiol. Biochem. 2010, 375–382. [Google Scholar] [CrossRef]
- Savera, A.T.; Zarbo, R.J. Defining the role of myoepithelium in salivary gland neoplasia. Adv. Anat. Pathol. 2004, 11, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.K.; Mulla, A.F.; Mayank, M. Pathophysiology of myoepithelial cells in salivary glands. J. Oral Maxillofac. Pathol. 2016, 20, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Redman, R.S. Myoepithelium of salivary glands. Microsc. Res. Tech. 1994, 27, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Sato, E.; Inoue, R.; Oshiro, H.; Takahashi, R.H.; Nagai, T.; Yoshida, M.; Suzuki, F.; Obikane, H.; Yamashina, M.; et al. Immunohistochemical analysis of salivary gland tumors: Application for surgical pathology practice. Acta Histochem. Cytochem. 2012, 45, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namboodiripad, P.C.A. A review: Immunological markers for malignant salivary gland tumors. J. Oral Biol. Craniofacial Res. 2014, 4, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Schuerch, C.; Hunt, J. Review and Updates of Immunohistochemistry in Selected Salivary Gland and Head and Neck Tumors. Arch. Pathol. Lab. Med. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, R.S.; de Almeida, O.P.; Kowalski, L.P.; Pires, F.R. Comparative cytokeratin expression in the different cell types of salivary gland mucoepidermoid carcinoma. Head Neck Pathol. 2008, 2, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Li, L.; Su, Y.C.; Xiang, R.L.; Cong, X.; Yu, H.K.; Li, S.L.; Wu, L.L.; Yu, G.Y. Adiponectin Increases Secretion of Rat Submandibular Gland via Adiponectin Receptors-Mediated AMPK Signaling. PLoS ONE 2013, 8, e63878. [Google Scholar] [CrossRef]
- Mitsui, R.; Fujita-Yoshigaki, J.; Narita, T.; Matsuki-Fukushima, M.; Satoh, K.; Qi, B.; Guo, M.-Y.; Katsumata-Kato, O.; Sugiya, H. Maintenance of paracellular barrier function by insulin-like growth factor-I in submandibular gland cells. Arch. Oral Biol. 2010, 55, 963–969. [Google Scholar] [CrossRef]
- Mei, M.; Xiang, R.-L.; Cong, X.; Zhang, Y.; Li, J.; Yi, X.; Park, K.; Han, J.-Y.; Wu, L.-L.; Yu, G.-Y. Claudin-3 is required for modulation of paracellular permeability by TNF-alpha through ERK1/2/slug signaling axis in submandibular gland. Cell. Signal. 2015, 27, 1915–1927. [Google Scholar] [CrossRef]
- Bamforth, S.D.; Kniesel, U.; Wolburg, H.; Engelhardt, B.; Risau, W. A dominant mutant of occludin disrupts tight junction structure and function. J. Cell Sci. 1999, 112, 1879–1888. [Google Scholar]
- Hegyesi, O.; Földes, A.; Bori, E.; Németh, Z.; Barabás, J.; Steward, M.C.; Varga, G. Evidence for Active Electrolyte Transport by Two-Dimensional Monolayers of Human Salivary Epithelial Cells. Tissue Eng. Part C Methods 2015, 21, 1226–1236. [Google Scholar] [CrossRef]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.C.; Leitgeb, T.; Vladetic, A.; Friedl, H.-P.; Rhodes, N.; Rossi, A.; Roblegg, E.; Neuhaus, W. Optimization of an oral mucosa in vitro model based on cell line TR146. Tissue Barriers 2020, 8, 1748459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhaus, W.; Plattner, V.E.; Wirth, M.; Germann, B.; Lachmann, B.; Gabor, F.; Noe, C.R. Validation of In Vitro Cell Culture Models of the Blood–Brain Barrier: Tightness Characterization of Two Promising Cell Lines. J. Pharm. Sci. 2008, 97, 5158–5175. [Google Scholar] [CrossRef]
- Baker, O.J. Current trends in salivary gland tight junctions. Tissue Barriers 2016, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, S.D.; Wang, J.; Bandyopadhyay, B.C.; Redman, R.S.; Dutra, A.; Pak, E.; Swaim, W.D.; Gerstenhaber, J.A.; Bryant, J.M.; Zheng, C.; et al. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 2005, 11, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.M.; Kim, J.W.M.; Gerstenhaber, J.A.; Baum, B.J.; Tran, S.D. Distribution of tight junction proteins in adult human salivary glands. J. Histochem. Cytochem. 2008, 56, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Kusama, K.; Jiang, Y.; Ohno, J.; Shikata, H.; Ishikawa, F.; Taguchi, K.; Kikuchi, K.; Mori, K.; Sakashita, H.; Sakagami, H.; et al. Immunohistochemical detection of cytokeratin 18 and its neo-epitope in human salivary glands and pleomorphic adenomas. Anticancer Res. 2000, 20, 2485–2487. [Google Scholar]
- Draeger, A.; Nathrath, W.; Lane, E.; Sundström, B.; Stigbrand, T. Cytokeratins, smooth muscle actin and vimentin in human normal salivary gland and pleomorphic adenomas. APMIS 1991, 99, 405–415. [Google Scholar] [CrossRef]
- Park, N.; Kim, S.; Kim, H.; Moon, S.; Kim, C.S.; Cho, S.; Kanai, Y.; Endou, H.; Kim, D.O.K. Characterization of Amino Acid Transport System L in HTB-41. Anticancer Res. 2008, 2656, 2649–2655. [Google Scholar]
- Reina, S.; Sterin-Borda, L.; Borda, E. Anti-M 3 peptide IgG from Sjögren’s syndrome triggers apoptosis in A253 cells. Cell. Immunol. 2012, 275, 33–41. [Google Scholar] [CrossRef]
- Andersson, C.; Zhang, A.L.; Roomans, G.M. CA2+ mobilization in the human submandibular duct cell line A253. Cell Biol. Int. 2000, 24, 273–277. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.L.; Spremulli, E.N.; Fligiel, Z.; Barbosa, J.A.; Vogel, R.; VanVoorhees, A.; Calabresi, P. Heterogeneity of cancer cells from a single human colon carcinoma. Am. J. Med. 1981, 71, 949–956. [Google Scholar] [CrossRef]
- Hastings, R.J.; Franks, L.M. Cellular heterogeneity in a tissue culture cell line derived from a human bladder carcinoma. Br. J. Cancer 1983, 47, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Abe, A.; Takano, K.; Kojima, T.; Nomura, K.; Kakuki, T.; Kaneko, Y.; Yamamoto, M.; Takahashi, H.; Himi, T. Interferon-gamma increased epithelial barrier function via upregulating claudin-7 expression in human submandibular gland duct epithelium. J. Mol. Histol. 2016, 47, 353–363. [Google Scholar] [CrossRef]
- Aframian, D.J.; Tran, S.D.; Cukierman, E.; Yamada, K.M.; Baum, B.J. Absence of Tight Junction Formation in an Allogeneic Graft Cell Line Used for Developing an Engineered Artificial Salivary Gland. Tissue Eng. 2002, 8, 871–878. [Google Scholar] [CrossRef]
- Burghartz, M.; Lennartz, S.; Schweinlin, M.; Hagen, R.; Kleinsasser, N.; Hackenberg, S.; Steussloff, G.; Scherzad, A.; Radeloff, K.; Ginzkey, C.; et al. Development of Human Salivary Gland-Like Tissue In Vitro. Tissue Eng. Part A 2018, 24, 301–309. [Google Scholar] [CrossRef]
- Nam, H.; Kim, J.H.; Hwang, J.Y.; Kim, G.H.; Kim, J.W.; Jang, M.; Lee, J.H.; Park, K.; Lee, G. Characterization of primary epithelial cells derived from human salivary gland contributing to in vivo formation of acini-like structures. Mol. Cells 2018, 41, 515–522. [Google Scholar] [CrossRef]
- Szlávik, V.; Szabó, B.; Vicsek, T.; Barabás, J.; Bogdán, S.; Gresz, V.; Varga, G.; O’Connell, B.; Vág, J. Differentiation of primary human submandibular gland cells cultured on basement membrane extract. Tissue Eng. Part A 2008, 14, 1915–1926. [Google Scholar] [CrossRef] [Green Version]
- Walters, G.O.; Miller, F.M.; Worwood, M. Serum ferritin concentration and iron stores in normal subjects. J. Clin. Pathol. 1973, 26, 770–772. [Google Scholar] [CrossRef] [Green Version]
- Jagannathan, N.; Thiruvengadam, C.; Ramani, P.; Premkumar, P.; Natesan, A.; Sherlin, H.J. Salivary ferritin as a predictive marker of iron deficiency anemia in children. J. Clin. Pediatr. Dent. 2012, 37, 25–30. [Google Scholar] [CrossRef]
- Costa, E.M.; de Azevedo, J.A.P.; Martins, R.F.M.; Rodrigues, V.P.; Alves, C.M.C.; Ribeiro, C.C.C.; Thomaz, E.B.A.F. Salivary Iron (Fe) Ion Levels, Serum Markers of Anemia and Caries Activity in Pregnant Women. Rev. Bras. Ginecol. Obstet. 2017, 39, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.-N.; Yang, Y.-Z.; Feng, Y.-Z. Serum and salivary ferritin and Hepcidin levels in patients with chronic periodontitis and type 2 diabetes mellitus. BMC Oral Health 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta 2010, 1800, 760–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkstra, A.; van den Hurk, K.; Bilo, H.J.G.; Slingerland, R.J.; Vos, M.J. Repeat whole blood donors with a ferritin level of 30 μg/L or less show functional iron depletion. Transfusion 2019, 59, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez-Ortegón, M.F.; McLachlan, S.; Price, A.H.; Fernández-Balsells, M.; Franch-Nadal, J.; Mata-Cases, M.; Barrot-de la Puente, J.; Mundet-Tudurí, X.; Mauricio, D.; Ricart, W.; et al. Decreased iron stores are associated with cardiovascular disease in patients with type 2 diabetes both cross-sectionally and longitudinally. Atherosclerosis 2018, 272, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Björkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Marker for | References | |
|---|---|---|
| α-Amylase | Acinar cells | Nagao et al. (2012) [11] |
| Namboodiripad et al. (2014) [12] | ||
| CK7 | Ductal cells | Zhu et al. (2005) [13] |
| Dreager et al. (1991) [28] | ||
| Intercalated, striated ducts | Azevedo et al. (2008) [14] | |
| CK14 | Myoepithelial cells | Nagao et al. (2012) [11] |
| Azevedo et al. (2008) [14] | ||
| CK18 | Acinar and ductal cells | Namboodiripad et al. (2014) [12] |
| Azevedo et al. (2008) [14] | ||
| Kusama et al. (2000) [27] | ||
| CLDN1 | SMG | Baker et al. (2016) [24] |
| Intercalated/striated duct cells, weak in serous cells | Maria et al. (2008) [26] | |
| E-cadherin | SMG | Baker et al. (2016) [24] |
| Tran et al. (2005) [25] | ||
| S100 | Myoepithelial cells, Intercalated duct cells | Zhu et al. (2005) [13] |
| Vimentin | Myoepithelial cells | Nagao et al. (2012) [11] |
| Namboodiripad et al. (2014) [12] | ||
| Zhu et al. (2005) [13] | ||
| ZO-1 | SMG | Baker et al. (2016) [24] |
| Tran et al. (2005) [25] | ||
| Maria et al. (2008) [26] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.C.; Smajlhodzic, M.; Bandian, A.-M.; Friedl, H.-P.; Leitgeb, T.; Oerter, S.; Stadler, K.; Giese, U.; Peham, J.R.; Bingle, L.; et al. An In Vitro Barrier Model of the Human Submandibular Salivary Gland Epithelium Based on a Single Cell Clone of Cell Line HTB-41: Establishment and Application for Biomarker Transport Studies. Biomedicines 2020, 8, 302. https://doi.org/10.3390/biomedicines8090302
Lin GC, Smajlhodzic M, Bandian A-M, Friedl H-P, Leitgeb T, Oerter S, Stadler K, Giese U, Peham JR, Bingle L, et al. An In Vitro Barrier Model of the Human Submandibular Salivary Gland Epithelium Based on a Single Cell Clone of Cell Line HTB-41: Establishment and Application for Biomarker Transport Studies. Biomedicines. 2020; 8(9):302. https://doi.org/10.3390/biomedicines8090302
Chicago/Turabian StyleLin, Grace C., Merima Smajlhodzic, Anna-Maria Bandian, Heinz-Peter Friedl, Tamara Leitgeb, Sabrina Oerter, Kerstin Stadler, Ulrich Giese, Johannes R. Peham, Lynne Bingle, and et al. 2020. "An In Vitro Barrier Model of the Human Submandibular Salivary Gland Epithelium Based on a Single Cell Clone of Cell Line HTB-41: Establishment and Application for Biomarker Transport Studies" Biomedicines 8, no. 9: 302. https://doi.org/10.3390/biomedicines8090302