The Capacity of APOB-Depleted Plasma in Inducing ATP-Binding Cassette A1/G1-Mediated Macrophage Cholesterol Efflux—But Not Gut Microbial-Derived Metabolites—Is Independently Associated with Mortality in Patients with ST-Segment Elevation Myocardial Infarction
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population and Data Collection
2.2. Blood Samples and Biochemical Measurements
2.3. Ex Vivo and In Vitro Cholesterol Efflux Capacity
2.4. Plasma TMAO, γBB and TML Determinations
2.5. Statistical Methods
3. Results
3.1. Study Subjects
3.2. ABCA1 and ABCG1-Mediated Macrophage Cholesterol Efflux to APOB-Fepleted Serum Ex Vivo Is Down Regulated in Deceased STEMI Patients
3.3. Circulating Levels of TMAO, γBB, and TML Are Increased in STEMI Patients Who Died during Follow-Up
3.4. ABCA1/G1-Mediated Macrophage Cholesterol Efflux Is Independently Associated with Mortality
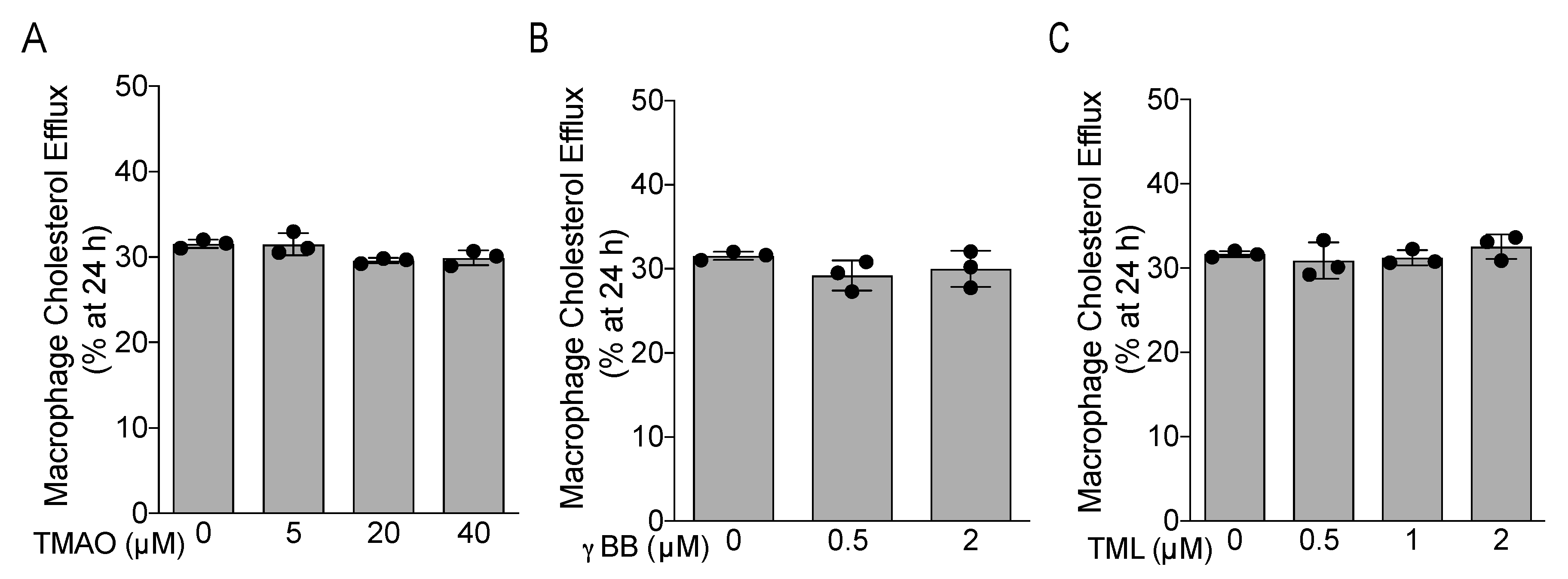
3.5. TMAO, γBB, and TML Did Not Affect APOB-Depleted Plasma-Mediated Macrophage Cholesterol Efflux In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.-A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef]
- Khera, A.V.; Cuchel, M.; De La Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-M.; Tang, W.H.W.; Mosior, M.K.; Huang, Y.; Wu, Y.; Matter, W.; Gao, V.; Schmitt, D.; DiDonato, J.A.; Fisher, E.; et al. Paradoxical Association of Enhanced Cholesterol Efflux with Increased Incident Cardiovascular Risks. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1696–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; De Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleheen, D.; Scott, R.; Javad, S.; Zhao, W.; Rodrigues, A.; Picataggi, A.; Lukmanova, D.; Mucksavage, M.L.; Luben, R.; Billheimer, J.; et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol. 2015, 3, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Ritsch, A.; Scharnagl, H.; Marz, W. HDL cholesterol efflux capacity and cardiovascular events. N. Engl. J. Med. 2015, 372, 1870–1871. [Google Scholar]
- Liu, C.; Zhang, Y.; Ding, D.; Li, X.; Yang, Y.; Li, Q.; Zheng, Y.; Wang, D.; Ling, W. Cholesterol efflux capacity is an independent predictor of all-cause and cardiovascular mortality in patients with coronary artery disease: A prospective cohort study. Atherosclerosis 2016, 249, 116–124. [Google Scholar] [CrossRef]
- Ritsch, A.; Duerr, A.; Kahler, P.; Hunjadi, M.; Stojakovic, T.; Silbernagel, G.; Scharnagl, H.; Kleber, M.E.; März, W. Cholesterol Efflux Capacity and Cardiovascular Disease: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Biomedicines 2020, 8, 524. [Google Scholar] [CrossRef]
- Guerin, M.; Silvain, J.; Gall, J.; Darabi, M.; Berthet, M.; Frisdal, E.; Hauguel-Moreau, M.; Zeitouni, M.; Kerneis, M.; Lattuca, B.; et al. Association of Serum Cholesterol Efflux Capacity with Mortality in Patients with ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 3259–3269. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Obeid, S.; Wang, Z.; Hazen, B.J.; Li, L.; Wu, Y.; Hurd, A.G.; Gu, X.; Pratt, A.; Levison, B.S.; et al. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur. Heart J. 2019, 40, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: Refining the gut hypothesis. J. Am. Coll. Cardiol. 2014, 64, 1908–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Heaney, L.M.; Bhandari, S.S.; Jones, D.; Ng, L. TrimethylamineN-oxide and prognosis in acute heart failure. Heart 2016, 102, 841–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N -Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Heaney, L.; Jones, D.; Ng, L. Trimethylamine N-oxide and Risk Stratification after Acute Myocardial Infarction. Clin. Chem. 2017, 63, 420–428. [Google Scholar] [CrossRef]
- Gencer, B.; Li, X.S.; Gurmu, Y.; Bonaca, M.P.; Morrow, D.A.; Cohen, M.; Bhatt, D.L.; Steg, P.G.; Storey, R.F.; Johanson, P.; et al. Gut Microbiota-Dependent Trimethylamine N-oxide and Cardiovascular Outcomes in Patients with Prior Myocardial Infarction: A Nested Case Control Study from the PEGASUS-TIMI 54 Trial. J. Am. Heart Assoc. 2020, 9, e015331. [Google Scholar] [CrossRef]
- Tan, Y.; Sheng, Z.; Zhou, P.; Liu, C.; Zhao, H.; Song, L.; Li, J.; Zhou, J.; Chen, Y.; Wang, L.; et al. Plasma Trimethylamine N-Oxide as a Novel Biomarker for Plaque Rupture in Patients With ST-Segment–Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2019, 12, e007281. [Google Scholar] [CrossRef]
- Skagen, K.; Trøseid, M.; Ueland, T.; Holm, S.; Abbas, A.; Gregersen, I.; Kummen, M.; Bjerkeli, V.; Reier-Nilsen, F.; Russell, D.; et al. The Carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis 2016, 247, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Bjørnestad, E.Ø.; Olset, H.; Dhar, I.; Løland, K.; Pedersen, E.K.R.; Svingen, G.F.; Svardal, A.; Berge, R.K.; Ueland, P.M.; Tell, G.S.; et al. Circulating trimethyllysine and risk of acute myocardial infarction in patients with suspected stable coronary heart disease. J. Intern. Med. 2020, 288, 446–456. [Google Scholar] [CrossRef]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Gholamhoseyniannajar, A.; Yaghoobi, M.M.; Jahani, Y.; Vahabzadeh, Z. Expression levels of heat shock protein 60 and glucose-regulated protein 78 in response to trimethylamine-N-oxide treatment in murine macrophage J774A.1 cell line. Cell. Mol. Boil. 2015, 61, 94–100. [Google Scholar]
- Collins, H.L.; Drazul-Schrader, D.; Sulpizio, A.C.; Koster, P.D.; Williamson, Y.; Adelman, S.J.; Owen, K.; Sanli, T.; Bellamine, A. L-carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in apoE(-/-) transgenic mice expressing CETP. Atherosclerosis 2016, 244, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third Universal Definition of Myocardial Infarction. Circulation 2012, 126, 2020–2035. [Google Scholar] [CrossRef] [Green Version]
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Lundqvist, C.B.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; Fernandez-Aviles, F.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef]
- A A Fox, K.; Fitzgerald, G.; Puymirat, E.; Huang, W.; Carruthers, K.F.; Simon, T.; Coste, P.; Monsegu, J.; Steg, P.G.; Danchin, N.; et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014, 4, e004425. [Google Scholar] [CrossRef]
- Cedó, L.; Fernández-Castillejo, S.; Rubió, L.; Metso, J.; Santos, D.; Muñoz-Aguayo, D.; Rivas-Urbina, A.; Tondo, M.; Méndez-Lara, K.A.; Farràs, M.; et al. Phenol-Enriched Virgin Olive Oil Promotes Macrophage-Specific Reverse Cholesterol Transport In Vivo. Biomedicines 2020, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Soria-Florido, M.T.; Schroder, H.; Grau, M.; Fitó, M.; Lassale, C. High density lipoprotein functionality and cardiovascular events and mortality: A systematic review and meta-analysis. Atherosclerosis 2020, 302, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Lee-Rueckert, M.; Escola-Gil, J.C.; Kovanen, P.T. HDL functionality in reverse cholesterol transport—Challenges in translating data emerging from mouse models to human disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Wang, Z.; Cajka, T.; Buffa, J.A.; Nemet, I.; Hurd, A.G.; Gu, X.; Skye, S.M.; Roberts, A.B.; Wu, Y.; et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Mueller, D.; Allenspach, M.; Othman, A.; Saely, C.H.; Muendlein, A.; Vonbank, A.; Drexel, H.; von Eckardstein, A. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis 2015, 243, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.-E.; Liao, P.-D.; Zhao, X.-J.; Wang, L. Trimethylamine-N-oxide has prognostic value in coronary heart disease: A meta-analysis and dose-response analysis. BMC Cardiovasc. Disord. 2020, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| UA n = 33 | STEMI Survivors n = 36 | STEMI Deceased n = 35 | p-Value | |
|---|---|---|---|---|
| Age (years) | 71.97 ± 3.45 | 72.06 ± 7.3 | 73.41 ± 9.86 | 0.6656 |
| Sex (M/F) | 17/16 | 20/16 | 19/15 | 0.923 |
| Body mass index (Kg/m2) | 26.38 ± 2.86 | 26.66 ± 2.06 | 27.07 ± 4.07 | 0.6774 |
| Hypertension (%) | 56 | 72 | 71 | 0.3525 |
| Diabetes mellitus (%) | 21 | 25 | 41 | 0.1903 |
| Smoking (%) | 17 | 25 | 35 | 0.0859 |
| Triglycerides (mmol/L) | 1.07 ± 0.47 | 0.97 ± 0.4 | 1.12 ± 0.73 | 0.5012 |
| HDL-C (mmol/L) | 1.63 ± 0.44 | 1.33 ± 0.34 ** | 1.19 ± 0.31 **** | <0.0001 |
| LDL-C (mmol/L) | 2.21 ± 0.64 | 2.81 ± 1.01 ** | 2.12 ± 0.89 †† | 0.0022 |
| Log10 C-reactive protein (mg/L) | 3.30 ± 0.63 | 3.78 ± 0.71 * | 4.42 ± 0.87 **** †† | <0.0001 |
| eGFR (mL/min/1.73 m2) | 77.74 ± 13.94 | 69.66 ± 18.21 | 51.11 ± 20.50 **** †††† | <0.0001 |
| GRACE 2.0 risk score | ND | 188.5 ± 44.23 | 251.6 ± 44.35 †††† | <0.0001 |
| Log10 hs-cTnT (ng/L) | 3.90 ± 0.17 | 5.36 ± 0.79 **** | 6.06 ± 0.72 †††† **** | <0.0001 |
| (a) | |||||
| Source | SS | Df | Mean Square | F | p Value |
| Corrected model | 2.610 * | 5 | 0.522 | 18.403 | 0.000 |
| Intercept | 2.287 | 1 | 2.287 | 80.636 | 0.000 |
| HDL-C | 0.776 | 1 | 0.776 | 27.371 | 0.000 |
| eGFR | 0.043 | 1 | 0.043 | 1.509 | 0.222 |
| TMAO | 0.000 | 1 | 0.000 | 0.011 | 0.917 |
| Death | 0.331 | 2 | 0.166 | 5.838 | 0.004 |
| Error | 2.666 | 94 | 0.028 | ||
| Total | 139.441 | 100 | |||
| Corrected total | 5.275 | 99 | |||
| * R-squared = 0.495 (adjusted R-squared = 0.468) | |||||
| (b) | |||||
| Source | SS | Df | Mean Square | F | p Value |
| Corrected model | 0.755 * | 5 | 0.151 | 4.652 | 0.001 |
| Intercept | 0.119 | 1 | 0.119 | 3.674 | 0.060 |
| hs-cTnT | 0.034 | 1 | 0.034 | 1.054 | 0.309 |
| GRACE score | 0.007 | 1 | 0.007 | 0.213 | 0.646 |
| HDL-C | 0.440 | 1 | 0.440 | 13.553 | 0.000 |
| CRP | 0.000 | 1 | 0.000 | 0.008 | 0.931 |
| Death | 0.188 | 1 | 0.188 | 5.804 | 0.019 |
| Error | 2.012 | 62 | 0.032 | ||
| Total | 81.888 | 68 | |||
| Corrected total | 2.767 | 67 | |||
| * R-squared = 0.114 (adjusted R-squared = 0.058) |
| (a) | |||||
| Source | SS | df | Mean Square | F | p Value |
| Corrected model | 7.423 * | 4 | 1.856 | 10.054 | 0.000 |
| Intercept | 4.155 | 1 | 4.155 | 22.512 | 0.000 |
| eGFR | 1.415 | 1 | 1.415 | 7.665 | 0.007 |
| Normalized ABCA1/G1-mediated efflux | 0.002 | 1 | 0.002 | 0.011 | 0.917 |
| Death | 1.934 | 2 | 0.967 | 5.239 | 0.007 |
| Error | 17.535 | 95 | 0.185 | ||
| Total | 103.990 | 100 | |||
| Corrected total | 24.958 | 99 | |||
| * R Squared = 0.297 (Adjusted R Squared = 0.268) | |||||
| (b) | |||||
| Source | SS | df | Mean Square | F | p Value |
| Corrected model | 5.948 * | 4 | 1.487 | 5.554 | 0.001 |
| Intercept | 0.051 | 1 | 0.051 | 0.191 | 0.663 |
| hs-cTnT | 0.005 | 1 | 0.005 | 0.018 | 0.894 |
| GRACE score | 1.733 | 1 | 1.733 | 6.471 | 0.013 |
| CRP | 1.455 × 10−5 | 1 | 1.455 × 10−5 | 0.000 | 0.994 |
| Death | 0.596 | 1 | 0.596 | 2.224 | 0.141 |
| Error | 17.137 | 64 | 0.268 | ||
| Total | 94.710 | 69 | |||
| Corrected total | 23.086 | 68 | |||
| * R-squared = 0.258 (adjusted R-squared = 0.211) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canyelles, M.; García-Osuna, Á.; Junza, A.; Yanes, O.; Puig, N.; Ordóñez-Llanos, J.; Sionis, A.; Sans-Roselló, J.; Alquézar-Arbé, A.; Santos, D.; et al. The Capacity of APOB-Depleted Plasma in Inducing ATP-Binding Cassette A1/G1-Mediated Macrophage Cholesterol Efflux—But Not Gut Microbial-Derived Metabolites—Is Independently Associated with Mortality in Patients with ST-Segment Elevation Myocardial Infarction. Biomedicines 2021, 9, 1336. https://doi.org/10.3390/biomedicines9101336
Canyelles M, García-Osuna Á, Junza A, Yanes O, Puig N, Ordóñez-Llanos J, Sionis A, Sans-Roselló J, Alquézar-Arbé A, Santos D, et al. The Capacity of APOB-Depleted Plasma in Inducing ATP-Binding Cassette A1/G1-Mediated Macrophage Cholesterol Efflux—But Not Gut Microbial-Derived Metabolites—Is Independently Associated with Mortality in Patients with ST-Segment Elevation Myocardial Infarction. Biomedicines. 2021; 9(10):1336. https://doi.org/10.3390/biomedicines9101336
Chicago/Turabian StyleCanyelles, Marina, Álvaro García-Osuna, Alexandra Junza, Oscar Yanes, Núria Puig, Jordi Ordóñez-Llanos, Alessandro Sionis, Jordi Sans-Roselló, Aitor Alquézar-Arbé, David Santos, and et al. 2021. "The Capacity of APOB-Depleted Plasma in Inducing ATP-Binding Cassette A1/G1-Mediated Macrophage Cholesterol Efflux—But Not Gut Microbial-Derived Metabolites—Is Independently Associated with Mortality in Patients with ST-Segment Elevation Myocardial Infarction" Biomedicines 9, no. 10: 1336. https://doi.org/10.3390/biomedicines9101336







