Ethoxyquin Inhibits the Progression of Murine Ehrlich Ascites Carcinoma through the Inhibition of Autophagy and LDH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Molecular Docking
2.2. Chemicals
2.3. Transplantation of Tumor Cells
2.4. Mice Experimental Design
2.5. Sampling
2.6. Cell Count and Viability
2.7. Lactate Dehydrogenase Activity
2.8. RNA Extraction from EAC Cells and Liver Tissue
2.9. qPCR Analysis
2.10. Detection of Autophagy by Flow Cytometry
2.11. Hematological and Biochemical Assays
2.12. Histopathological Investigation
2.13. Statistical Analysis
3. Results
3.1. Intensive Molecular Docking Analysis
3.2. The Effect of EAC and Treatments on Body Weight and Hematological Parmeters
3.3. The Anti-Tumor Effect of EQ against EAC
3.4. Effect of CIS and/or EQ on Biochemical Parameters of EAC-Bearing Mice
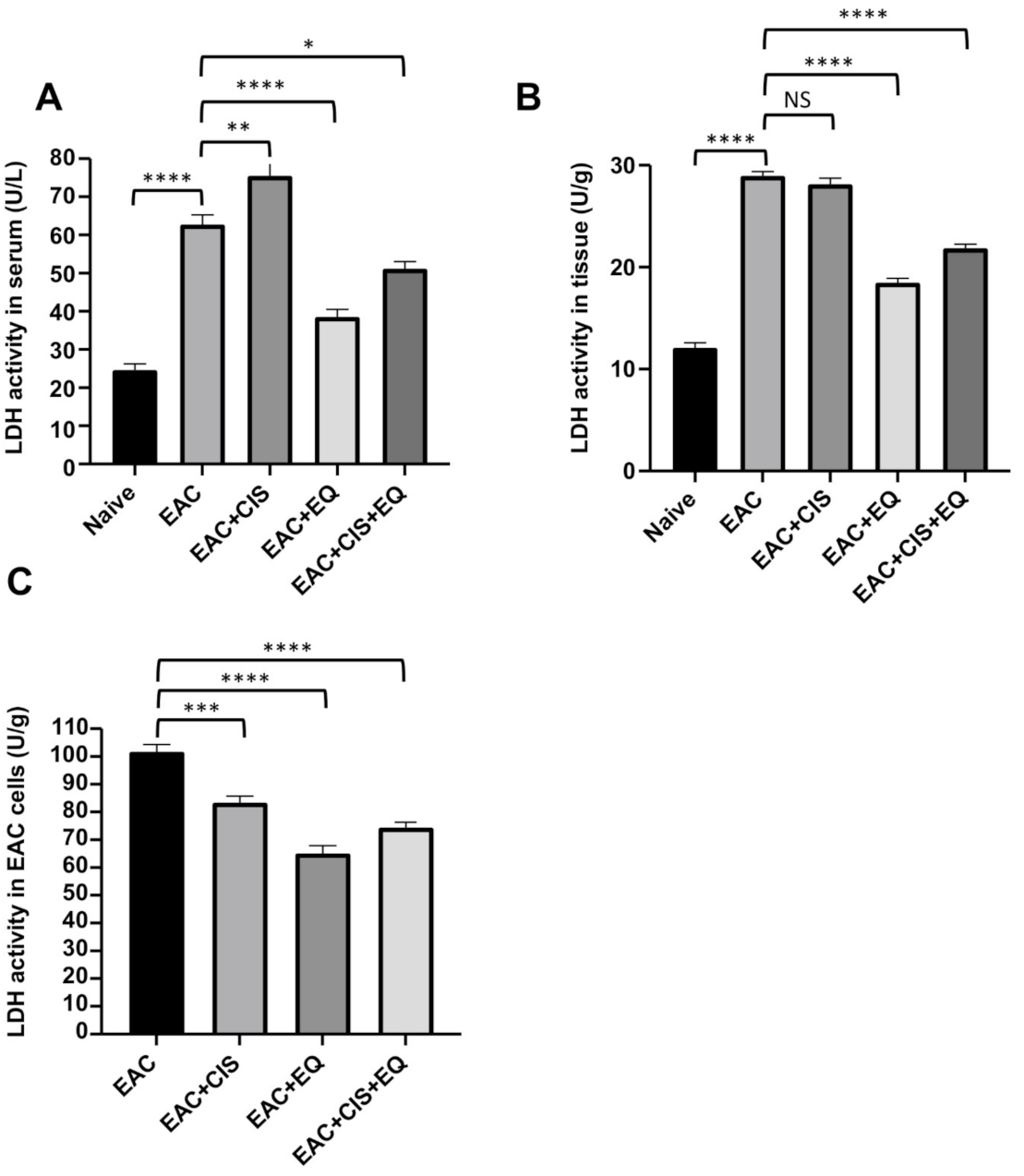
3.5. Effect of CIS and/or EQ on LDH Activity in Serum, Liver Tissue, and EAC Cells
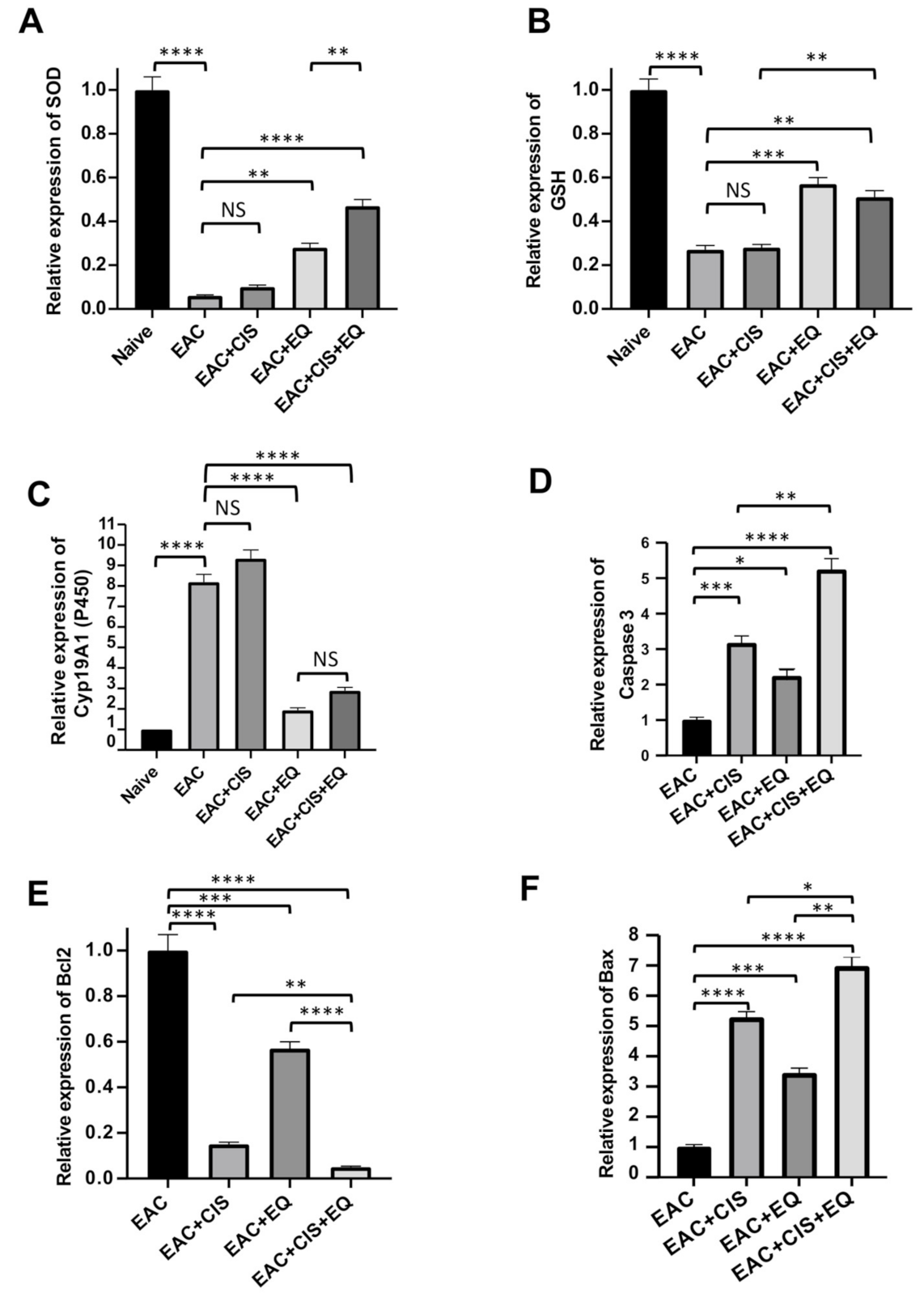
3.6. Effect of CIS and/or EQ on the Relative Expression of Antioxidant Genes (SOD, GSH, and CytP450) in Liver Tissue
3.7. Effect of CIS and/or EQ on the Relative Expression of Apoptotic Genes (Bcl-2, Caspase-3, and BAX Genes) in EAC Cells
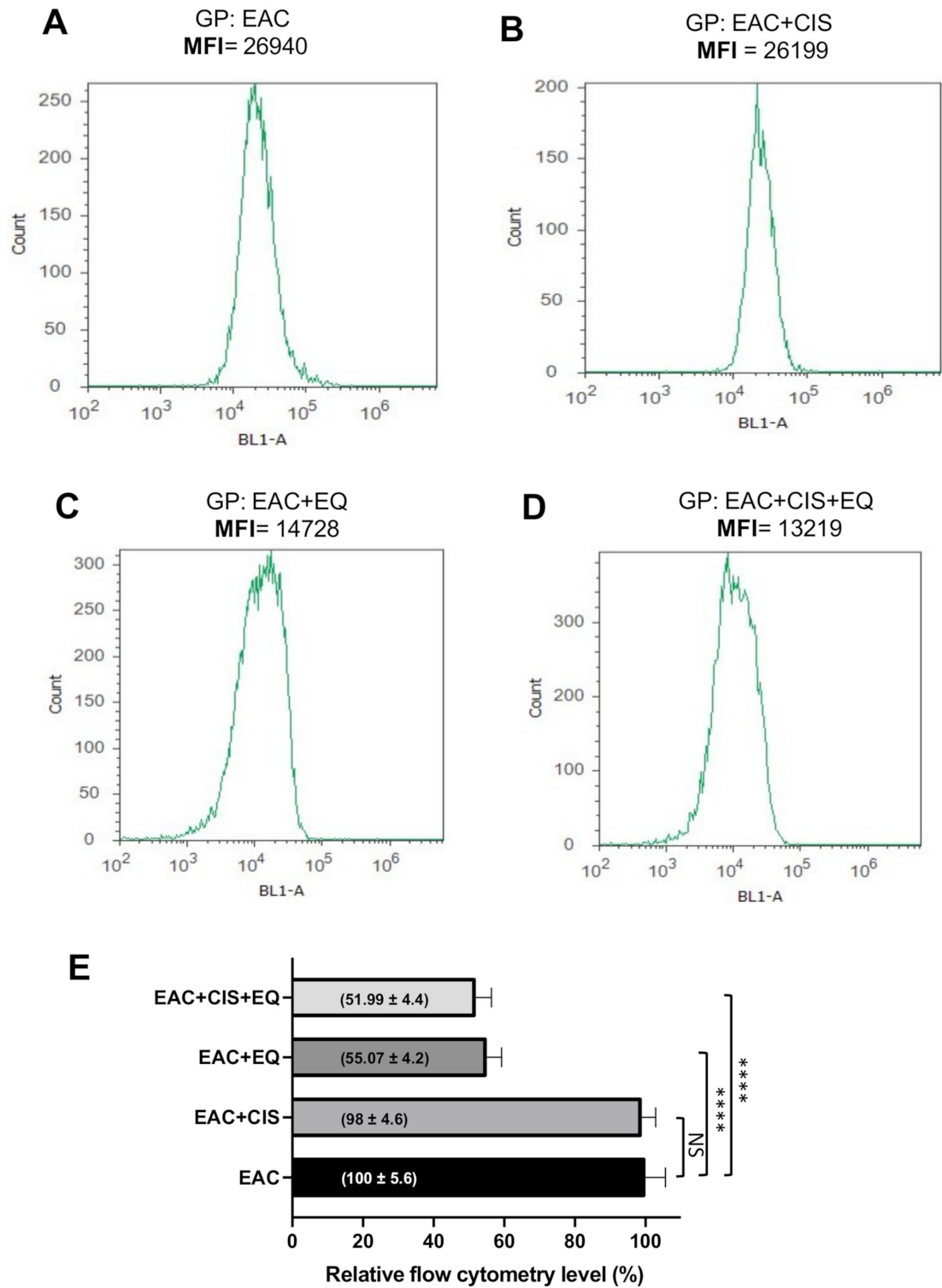
3.8. Effect of CIS and/or EQ on the Autophagic Activity of EAC Cells
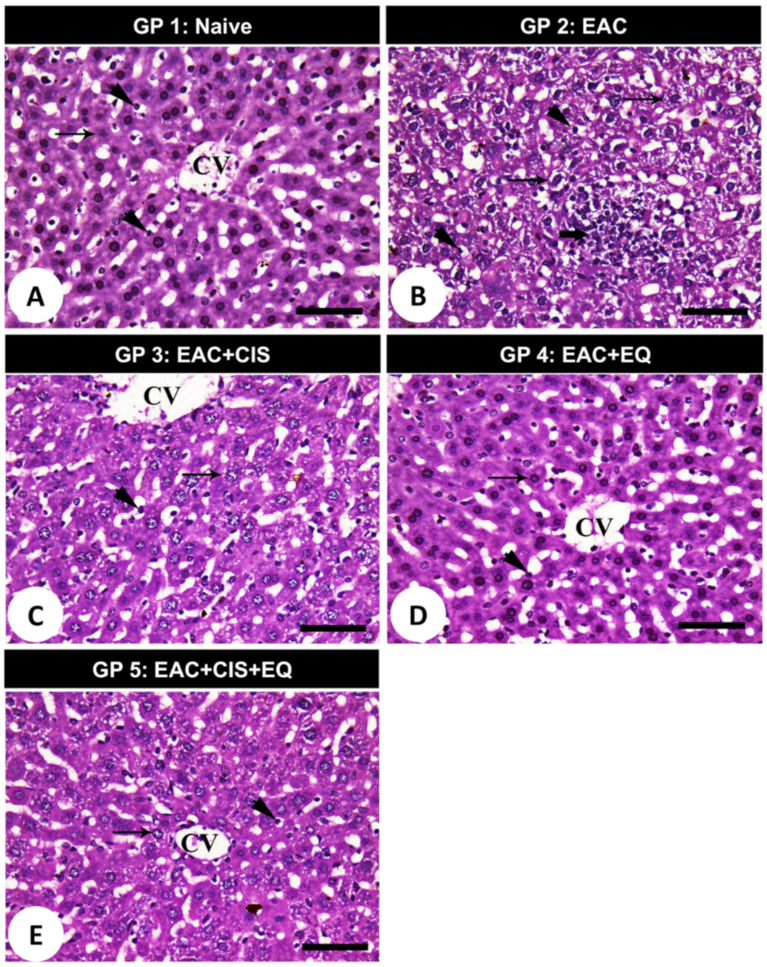
3.9. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arundhathi, J.R.D.; Mathur, S.R.; Gogia, A.; Deo, S.V.S.; Mohapatra, P.; Prasad, C.P. Metabolic changes in triple negative breast cancer-focus on aerobic glycolysis. Mol. Biol. Rep. 2021, 48, 4733–4745. [Google Scholar] [CrossRef]
- Cascardo, F.; Anselmino, N.; Páez, A.; Labanca, E.; Sanchis, P.; Antico-Arciuch, V.; Navone, N.; Gueron, G.; Vázquez, E.; Cotignola, J. HO-1 Modulates Aerobic Glycolysis through LDH in Prostate Cancer Cells. Antioxidants 2021, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.; McSheehy, P.M.; Griffiths, J.R.; Bashford, C.L. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today 2000, 6, 15–19. [Google Scholar] [CrossRef]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Reinfeld, B.I.; Rathmell, W.K.; Kim, T.K.; Rathmell, J.C. The therapeutic implications of immunosuppressive tumor aerobic glycolysis. Cell. Mol. Immunol. 2021. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Wu, L.; Yu, Q.; Ji, J.; Wu, J.; Dai, W.; Guo, C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 126. [Google Scholar] [CrossRef]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dhal, A.K.; Pani, A.; Mahapatra, R.K.; Yun, S.I. In-silico screening of small molecule inhibitors against Lactate Dehydrogenase (LDH) of Cryptosporidium parvum. Comput. Biol. Chem. 2018, 77, 44–51. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Kakouratos, C.; Kalamida, D.; Bampali, Z.; Mavropoulou, S.; Sivridis, E.; Giatromanolaki, A. Hypoxia-inducible proteins HIF1alpha and lactate dehydrogenase LDH5, key markers of anaerobic metabolism, relate with stem cell markers and poor post-radiotherapy outcome in bladder cancer. Int. J. Radiat. Biol. 2016, 92, 353–363. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Z.; Wang, Y.; Qi, B.; Zhang, W.; Ye, J.; Wu, H.; Jiang, H.; Song, L.N.; Yang, J.; et al. Overexpression of metabolic markers PKM2 and LDH5 correlates with aggressive clinicopathological features and adverse patient prognosis in tongue cancer. Histopathology 2014, 65, 595–605. [Google Scholar] [CrossRef]
- Qiao, T.; Xiong, Y.; Feng, Y.; Guo, W.; Zhou, Y.; Zhao, J.; Jiang, T.; Shi, C.; Han, Y. Inhibition of LDH-A by Oxamate Enhances the Efficacy of Anti-PD-1 Treatment in an NSCLC Humanized Mouse Model. Front. Oncol. 2021, 11, 632364. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Kim, J.H.; Baek, K.K.; Lim, T.; Lee, D.J.; Ahn, Y.C.; Kim, K.; Kim, S.J.; Ko, Y.H.; Kim, W.S. Elevated LDH and paranasal sinus involvement are risk factors for central nervous system involvement in patients with peripheral T-cell lymphoma. Ann. Oncol. 2011, 22, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Bougioukas, G.; Didilis, V.; Gatter, K.C.; Harris, A.L. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br. J. Cancer 2003, 89, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gao, J.; Tao, Y.L.; Xu, B.Q.; Tu, Z.W.; Liu, Z.G.; Zeng, M.S.; Xia, Y.F. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chin. J. Cancer 2012, 31, 197–206. [Google Scholar] [CrossRef] [Green Version]
- El-Gowily, A.H.; Loutfy, S.A.; Ali, E.M.; Mohamed, T.M.; Mansour, M.A. Tioconazole and Chloroquine Act Synergistically to Combat Doxorubicin-Induced Toxicity via Inactivation of PI3K/AKT/mTOR Signaling Mediated ROS-Dependent Apoptosis and Autophagic Flux Inhibition in MCF-7 Breast Cancer Cells. Pharmaceuticals 2021, 14, 254. [Google Scholar] [CrossRef]
- Mansour, M.A.; Ibrahim, W.M.; Salama, M.M.; Salama, A.F. Dual inhibition of glycolysis and autophagy as a therapeutic strategy in the treatment of Ehrlich ascites carcinoma. J. Biochem. Mol. Toxicol. 2020, 34, e22498. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; Ibrahim, W.M.; Shalaan, E.S.; Salama, A.F. Combination of arsenic trioxide and cisplatin synergistically inhibits both hexokinase activity and viability of Ehrlich ascites carcinoma cells. J. Biochem. Mol. Toxicol. 2019, 33, e22350. [Google Scholar] [CrossRef]
- Samudrala, P.K.; Augustine, B.B.; Kasala, E.R.; Bodduluru, L.N.; Barua, C.; Lahkar, M. Evaluation of antitumor activity and antioxidant status of Alternanthera brasiliana against Ehrlich ascites carcinoma in Swiss albino mice. Pharmacogn. Res. 2015, 7, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.A. SP3 is associated with migration, invasion, and Akt/PKB signalling in MDA-MB-231 breast cancer cells. J. Biochem. Mol. Toxicol. 2021, 35, e22657. [Google Scholar] [CrossRef]
- Román-Fernández, Á.; Roignot, J.; Sandilands, E.; Nacke, M.; Mansour, M.A.; McGarry, L.; Shanks, E.; Mostov, K.E.; Bryant, D.M. The phospholipid PI(3,4)P2 is an apical identity determinant. Nat. Commun. 2018, 9, 5041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, M.A.; Senga, T. HOXD8 exerts a tumor-suppressing role in colorectal cancer as an apoptotic inducer. Int. J. Biochem. Cell Biol. 2017, 88, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kurita, K.; Maeda, M.; Mansour, M.A.; Kokuryo, T.; Uehara, K.; Yokoyama, Y.; Nagino, M.; Hamaguchi, M.; Senga, T. TRIP13 is expressed in colorectal cancer and promotes cancer cell invasion. Oncol. Lett. 2016, 12, 5240–5246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akter, K.A.; Mansour, M.A.; Hyodo, T.; Senga, T. FAM98A associates with DDX1-C14orf166-FAM98B in a novel complex involved in colorectal cancer progression. Int. J. Biochem. Cell Biol. 2017, 84, 1–13. [Google Scholar] [CrossRef]
- Tousson, E.; Ali, E.M.; Ibrahim, W.; Mansour, M.A. Treatment with folic acid ameliorated the histopathological alterations caused by propylthiouracil-induced hypothyroid rat testes. Toxicol. Ind. Health. 2012, 28, 566–576. [Google Scholar] [CrossRef]
- Ibrahim, W.; Tousson, E.; Ali, E.M.; Mansour, M.A. Folic acid alleviates oxidative stress and hyperhomocysteinemia involved in testicular dysfunction of hypothyroid rats. Gen. Comp. Endocrinol. 2011, 174, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Tousson, E.; Ali, E.M.; Ibrahim, W.; Mansour, M.A. Proliferating cell nuclear antigen as a molecular biomarker for spermatogenesis in PTU-induced hypothyroidism of rats. Reprod. Sci. 2011, 18, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; Hyodo, T.; Akter, K.A.; Kokuryo, T.; Uehara, K.; Nagino, M.; Senga, T. SATB1 and SATB2 play opposing roles in c-Myc expression and progression of colorectal cancer. Oncotarget 2016, 7, 4993–5006. [Google Scholar] [CrossRef] [Green Version]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R. Lactate dehydrogenase 5: An old friend and a new hope in the war on cancer. Cancer Lett. 2015, 358, 1–7. [Google Scholar] [CrossRef]
- Lu, R.; Jiang, M.; Chen, Z.; Xu, X.; Hu, H.; Zhao, X.; Gao, X.; Guo, L. Lactate dehydrogenase 5 expression in Non-Hodgkin lymphoma is associated with the induced hypoxia regulated protein and poor prognosis. PLoS ONE 2013, 8, e74853. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Simopoulos, C.; Polychronidis, A.; Sivridis, E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin. Exp. Metastasis 2005, 22, 25–30. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Liu, A.; Fang, C.; Hao, J.; Wang, Z. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget 2015, 6, 19456–19468. [Google Scholar] [CrossRef]
- Zhuang, L.; Scolyer, R.A.; Murali, R.; McCarthy, S.W.; Zhang, X.D.; Thompson, J.F.; Hersey, P. Lactate dehydrogenase 5 expression in melanoma increases with disease progression and is associated with expression of Bcl-XL and Mcl-1, but not Bcl-2 proteins. Mod. Pathol. 2010, 23, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Shi, M.; Xie, D.; Wei, D.; Jia, Z.; Zheng, S.; Gao, Y.; Huang, S.; Xie, K. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin. Cancer Res. 2014, 20, 2595–2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Hanai, J.I.; Ren, J.G.; Kats, L.; Burgess, K.; Bhargava, P.; Signoretti, S.; Billiard, J.; Duffy, K.J.; Grant, A.; et al. Targeting lactate dehydrogenase—A inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014, 19, 795–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, Z.Y.; Liu, J.M.; Chen, Q.K.; Chen, H.Z.; Ye, C.J.; Xue, J.; Yang, H.Q.; Li, J.L.; Liu, X.F.; Kuang, S.J. Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumour. Biol. 2015, 36, 8093–8100. [Google Scholar] [CrossRef] [Green Version]
- Kolev, Y.; Uetake, H.; Takagi, Y.; Sugihara, K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: Association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann. Surg. Oncol. 2008, 15, 2336–2344. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Gatter, K.C.; Turley, H.; Harris, A.L.; Koukourakis, M.I. Lactate dehydrogenase 5 (LDH-5) expression in endometrial cancer relates to the activated VEGF/VEGFR2(KDR) pathway and prognosis. Gynecol. Oncol. 2006, 103, 912–918. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 2007, 405, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shim, H.; Dolde, C.; Lewis, B.C.; Wu, C.S.; Dang, G.; Jungmann, R.A.; Dalla-Favera, R.; Dang, C.V. c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA 1997, 94, 6658–6663. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L.; Jiang, B.H.; Leung, S.W.; Passantino, R.; Concordet, J.P.; Maire, P.; Giallongo, A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 1996, 271, 32529–32537. [Google Scholar] [CrossRef] [Green Version]
- Serganova, I.; Rizwan, A.; Ni, X.; Thakur, S.B.; Vider, J.; Russell, J.; Blasberg, R.; Koutcher, J.A. Metabolic imaging: A link between lactate dehydrogenase A, lactate, and tumor phenotype. Clin. Cancer Res. 2011, 17, 6250–6261. [Google Scholar] [CrossRef] [Green Version]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Valera, V.A.; Merino, M.J.; Amato, A.M.; Signoretti, S.; Linehan, W.M.; Sukhatme, V.P.; Seth, P. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol. Cancer Ther. 2009, 8, 626–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Liu, H.; Liu, Z.; Ding, Y.; LeDoux, S.P.; Wilson, G.L.; Voellmy, R.; Lin, Y.; Lin, W.; Nahta, R.; et al. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011, 71, 4585–4597. [Google Scholar] [CrossRef] [Green Version]
- Gomez, M.S.; Piper, R.C.; Hunsaker, L.A.; Royer, R.E.; Deck, L.M.; Makler, M.T.; Vander Jagt, D.L. Substrate and cofactor specificity and selective inhibition of lactate dehydrogenase from the malarial parasite P. falciparum. Mol. Biochem. Parasitol. 1997, 90, 235–246. [Google Scholar] [CrossRef]
- Zhang, S.L.; He, Y.; Tam, K.Y. Targeting cancer metabolism to develop human lactate dehydrogenase (hLDH)5 inhibitors. Drug Discov. Today 2018, 23, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Beeler, A.B.; Pradhan, A.; Watkins, E.B.; Rimoldi, J.M.; Tekwani, B.; Avery, M.A. Generation of oxamic acid libraries: Antimalarials and inhibitors of Plasmodium falciparum lactate dehydrogenase. J. Comb. Chem. 2007, 9, 292–300. [Google Scholar] [CrossRef]
- Di Bussolo, V.; Calvaresi, E.C.; Granchi, C.; Del Bino, L.; Frau, I.; Lang, M.C.D.; Tuccinardi, T.; Macchia, M.; Martinelli, A.; Hergenrother, P.J. Synthesis and biological evaluation of non-glucose glycoconjugated N-hydroyxindole class LDH inhibitors as anticancer agents. RSC Adv. 2015, 5, 19944–19954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorhouse, A.D.; Spiteri, C.; Sharma, P.; Zloh, M.; Moses, J.E. Targeting glycolysis: A fragment based approach towards bifunctional inhibitors of hLDH-5. Chem. Commun. 2011, 47, 230–232. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Taeb, S.; Haghi-Aminjan, H.; Afrashi, S.; Moloudi, K.; Musa, A.E.; Najafi, M.; Farhood, B. Resveratrol as an Enhancer of Apoptosis in Cancer: A Mechanistic Review. Anti-Cancer Agents Med. Chem. 2020. [Google Scholar] [CrossRef]
- Xing, H.; Tan, J.; Miao, Y.; Lv, Y.; Zhang, Q. Crosstalk between exosomes and autophagy: A review of molecular mechanisms and therapies. J. Cell Mol. Med. 2021, 25, 2297–2308. [Google Scholar] [CrossRef]
- Chen, C.; Gao, H.; Su, X. Autophagy-related signaling pathways are involved in cancer (Review). Exp. Ther. Med. 2021, 22, 710. [Google Scholar] [CrossRef] [PubMed]

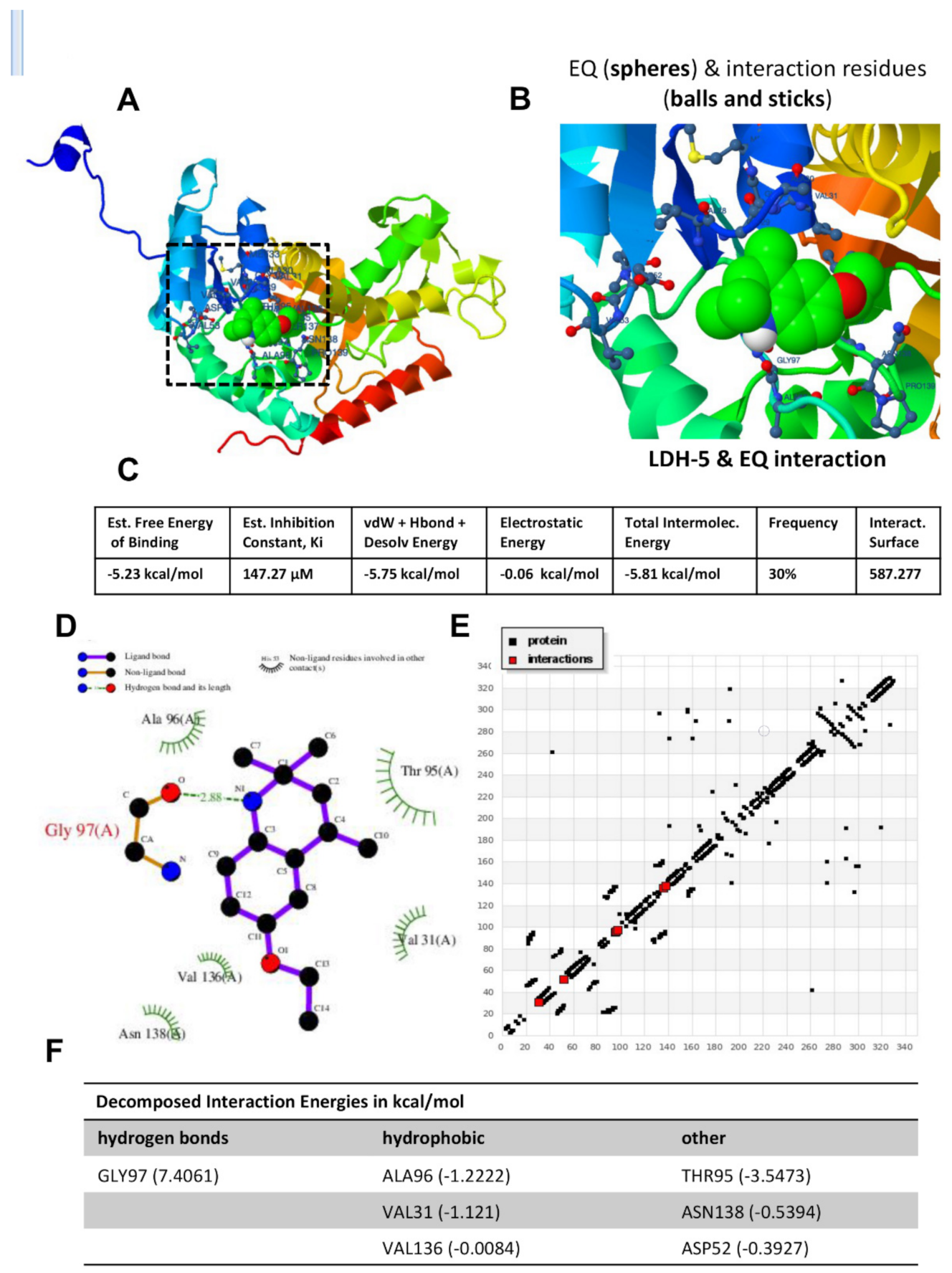
| Compound | Est. Free Energy of Binding (Kcal/mol) | Est. Inhibition Constant, Ki (µM) | VdW + Hbond + Desolv Energy (Kcal/mol) | Electrostatic Energy (Kcal/mol) | Total Intermolec. Energy (Kcal/mol) | Frequency (%) | Interact. Surface |
|---|---|---|---|---|---|---|---|
| Ethoxyquin (1,2-dihydro-2,2,4-trimethyl-6-Ethoxyquinoline) | −5.23 | 147.27 | −5.75 | −0.06 | −5.81 | 30% | 587.277 |
| Ethyl 4-hydroxy-7-trifllouromethyl-3-quinolinecarboxylate | −5.13 | 174.28 | −5.97 | −0.24 | −6.21 | 20% | 670.941 |
| 2,2,4-Trimethyl-6-Trityl-1,2-Dihydro-quinoline | −5.09 | 185.74 | −5.63 | −0.02 | −5.65 | 40% | 597.442 |
| 5-chloro-7-iodo-8-quinolinol | −4.97 | 226.28 | −5.30 | 0.03 | −5.27 | 30% | 439.625 |
| 6-Methoxy-2,2,4-Trimethyl-8-Trityl-1,2-Dihydro-Quinoline | −4.42 | 577.84 | −5.96 | −0.90 | −6.85 | 10% | 779.012 |
| Parameters | Gp 1 (Naive) | Gp 2 (EAC) | Gp 3 (EAC + CIS) | Gp 4 (EAC + EQ) | Gp 5 (EAC + CIS + EQ) |
|---|---|---|---|---|---|
| Body weight (g) | 19.8 ± 0.58 | 31.0 ± 0.71 *** | 23.0 ± 0.71 #### | 23.2 ± 0.86 #### | 20.4 ± 0.81 #### |
| Liver weight (g) | 1.64 ± 0.09 | 2.38 ± 0.12 *** | 1.7 ± 0.07 ## | 1.74 ± 0.12 ## | 1.46 ± 0.07 #### |
| Relative liver weight (g/100 g body weight) | 8.28 ± 0.34 | 5.74 ± 0.13 **** | 7.39 ± 0.26 ## | 8.02 ± 0.36 #### | 7.16 ± 0.22 ### |
| Hb% (g/dL) | 12.02 ± 0.19 | 7.14 ± 0.22 **** | 8.49 ± 0.32 ## | 10.16 ± 0.41 #### | 10.62 ± 0.3 #### |
| WBCs (103/µL) | 7.69 ± 0.58 | 16.66 ± 0.70 **** | 10.62 ± 0.64 #### | 11.02 ± 0.50 #### | 13.12 ± 0.44 ## |
| RBCs (106/µL) | 7.76 ± 0.1 | 5.0 ± 0.23 **** | 5.88 ± 0.16 ## | 6.34 ± 0.29 # | 6.65 ± 0.17 #### |
| Platelets (106/µL) | 0.43 ± 0.02 | 0.86 ± 0.03 **** | 0.5 ± 0.03 #### | 0.48 ± 0.03 #### | 0.52 ± 0.01 #### |
| Plasma AST (U/L) | 37.32 ± 1.07 | 85.31 ± 1.24 **** | 70.78 ± 1.27 #### | 50.88 ± 1.20 #### | 45.23 ± 1.66 #### |
| Plasma ALT (U/L) | 26.96 ± 0.95 | 60.90 ± 1.21 **** | 44.86 ± 0.91 #### | 41.32 ± 0.56 #### | 37.27 ± 0.63 #### |
| Plasma total protein (g/dL) | 5.30 ± 0.17 | 3.32 ± 0.17 **** | 6.48 ± 0.13 #### | 5.35 ± 0.2 #### | 5.44 ± 0.21 #### |
| Plasma albumin (U/L) | 3.49 ± 0.19 | 2.48 ± 0.14 *** | 3.02 ± 0.08 # | 3.03 ± 0.07 # | 3.13 ± 0.1 # |
| Plasma GGT (U/L) | 18.93 ± 0.33 | 46.52 ± 0.88 **** | 36.29 ± 0.54 #### | 25.12 ± 0.70 #### | 33.36 ± 0.37 #### |
| Parameters | Gp 1 (Naive) | Gp 2 (EAC) | Gp 3 (EAC + CIS) | Gp 4 (EAC + EQ) | Gp 5 (EAC + CIS + EQ) |
|---|---|---|---|---|---|
| Tumour width (cm) | - | 5.6 ± 0.29 | 4.0 ± 0.18 ### | 3.8 ± 0.3 ### | 3.6 ± 0.19 #### |
| Tumour length (cm) | - | 5.29 ± 0.19 | 3.95 ± 0.20 ### | 3.9 ± 0.19 ### | 3.82 ± 0.13 #### |
| Fluid volume (mL) | - | 1.59 ± 0.12 | 0.56 ± 0.05 #### | 0.83 ± 0.07 #### | 0.66 ± 0.04 #### |
| Tumour volume (cm3) | - | 83.58 ± 8.99 | 31.35 ± 1.59 #### | 28.99 ± 5.07 #### | 25.16 ± 3.02 #### |
| Total no. of cells (million/mouse) | - | 75.92 ± 5.17 | 43.08 ± 3.56 ### | 60.14 ± 3.61 NS | 17.68 ± 2.33 #### |
| Viable cells (%) | - | 85.33 ± 3.89 | 68.15 ± 1.23 ### | 73.5 ± 1.77 ## | 65.27 ± 0.96 #### |
| Dead cells (%) | - | 14.67 ± 2.1 | 31.85 ± 4.48 # | 26.5 ± 3.61 # | 35.74 ± 2.66 ## |
| Mean survival time (MST) | 59.5 | 15 | 33 | 38 | 43.4 |
| Increase in life span (ILs) | 296.67 | 0 | 120 | 153.3 | 190 |
| Parameters | Gp 1 (Naive) | Gp 2 (EAC) | Gp 3 (EAC + CIS) | Gp 4 (EAC + EQ) | Gp 5 (EAC + CIS + EQ) |
|---|---|---|---|---|---|
| Hepatic catalase (mmole/min/mg tissue) | 85.74 ± 1.91 | 35.27 ± 0.62 **** | 47.05 ± 1.01 #### | 77.22 ± 1.19 #### | 59.91 ± 1.07 #### |
| Hepatic GST (µmole/min/mg tissue) | 2.33 ± 0.07 | 1.09 ± 0.02 **** | 1.38 ± 0.03 ## | 2.16 ± 0.05 #### | 2.23 ± 0.04 #### |
| Hepatic MDA (nmole/g tissue) | 3.37 ± 0.29 | 23.58 ± 0.76 **** | 17.35 ± 0.39 #### | 9.76 ± 0.56 #### | 13.43 ± 0.25 #### |
| Hepatic total protein (U/g tissue) | 4.35 ± 0.09 | 2.12 ± 0.04 **** | 2.58 ± 0.07 #### | 3.1 ± 0.06 #### | 2.85 ± 0.11 #### |
| Hepatic TAC (µmole/g tissue) | 6.51 ± 0.22 | 3.47 ± 0.17 **** | 4.75 ± 0.16 #### | 5.39 ± 0.12 #### | 6.23 ± 0.05 #### |
| EAC catalase (mmole/min/mg protein) | - | 14.8 ± 0.25 | 22.28 ± 0.32 ## | 31.26 ± 2.55 #### | 26.0 ± 0.34 #### |
| EAC GST (μmole/min/mg protein) | - | 1.13 ± 0.02 | 1.62 ± 0.06 #### | 2.25 ± 0.03 #### | 1.84 ± 0.05 #### |
| EAC MDA (nmole/mL) | - | 46.35 ± 1.27 | 33.96 ± 0.45 #### | 25.09 ± 0.34 #### | 28.77 ± 0.17 #### |
| EAC total protein (µg/mL) | - | 1.86 ± 0.1 | 2.2 ± 0.09 # | 3.92 ± 0.05 #### | 3.01 ± 0.05 #### |
| EAC TAC (µmole/mL) | - | 0.19 ± 0.02 | 0.71 ± 0.02 #### | 0.89 ± 0.02 #### | 0.82 ± 0.02 #### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayel, F.; Mahfouz, M.E.; Salama, A.F.; Mansour, M.A. Ethoxyquin Inhibits the Progression of Murine Ehrlich Ascites Carcinoma through the Inhibition of Autophagy and LDH. Biomedicines 2021, 9, 1526. https://doi.org/10.3390/biomedicines9111526
Tayel F, Mahfouz ME, Salama AF, Mansour MA. Ethoxyquin Inhibits the Progression of Murine Ehrlich Ascites Carcinoma through the Inhibition of Autophagy and LDH. Biomedicines. 2021; 9(11):1526. https://doi.org/10.3390/biomedicines9111526
Chicago/Turabian StyleTayel, Fekria, Magdy E. Mahfouz, Afrah F. Salama, and Mohammed A. Mansour. 2021. "Ethoxyquin Inhibits the Progression of Murine Ehrlich Ascites Carcinoma through the Inhibition of Autophagy and LDH" Biomedicines 9, no. 11: 1526. https://doi.org/10.3390/biomedicines9111526







