Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway
Abstract
:1. Introduction
2. The Kynurenine System
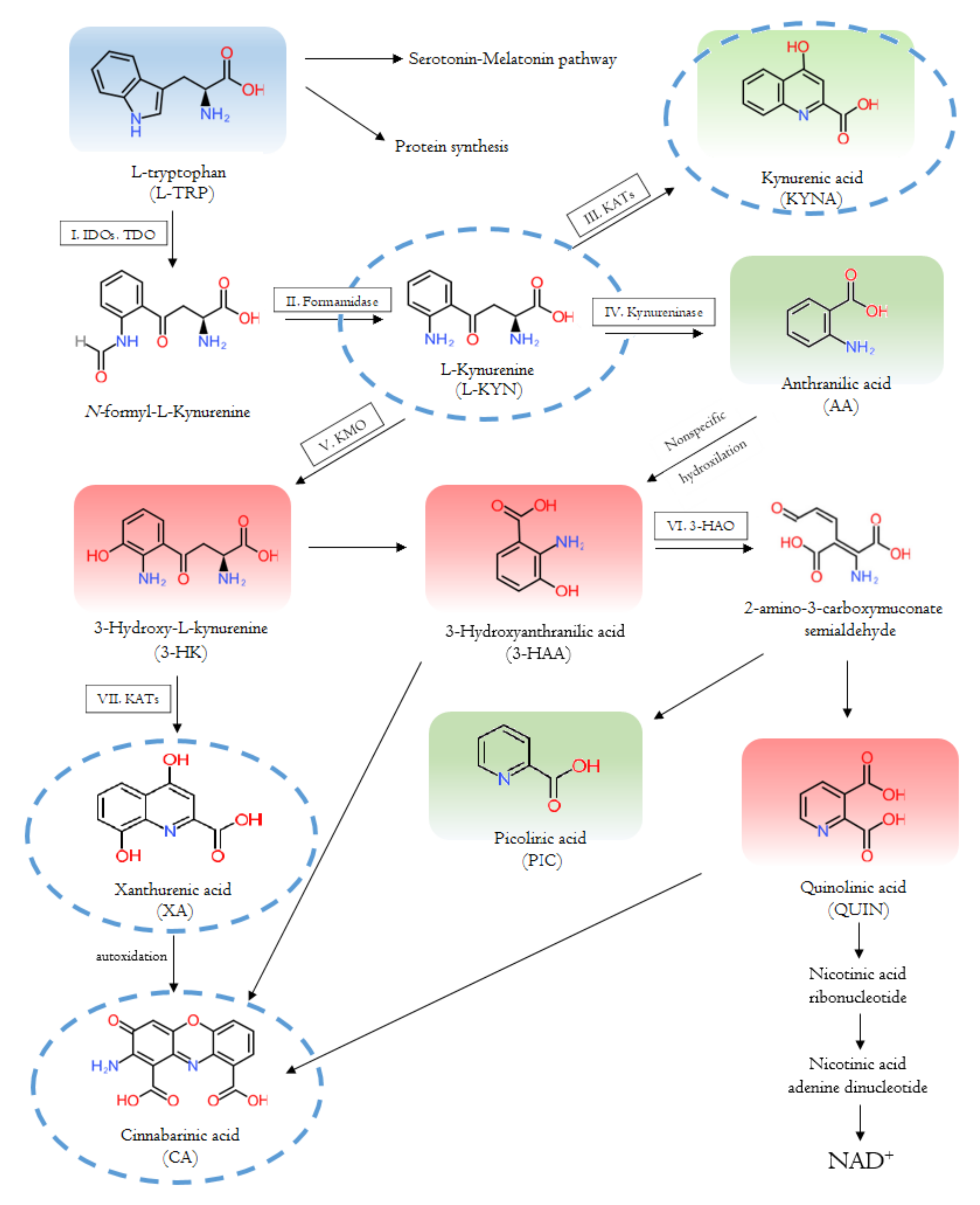
2.1. Enzymes and Metabolites of the Tryptophan-Kynurenine Pathway
2.1.1. The Interaction with the Immune System
2.1.2. Tryptophan 2,3-Dioxygenase
2.1.3. Indoleamine 2,3-Dioxygenase
2.1.4. Kynurenine Aminotransferases
2.1.5. Kynurenine 3-Monooxygenase
3. Symptoms, Inflammatory Status and Kynurenines in Psychiatric Disorders
3.1. Major Depressive Disorder
3.2. Bipolar Disorder
3.3. Generalized Anxiety Disorder
3.4. Substance Use Disorder
3.5. Post-Traumatic Stress Disorder
3.6. Schizophrenia
3.7. Autism Spectrum Disorder
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | anthranilic acid |
| AhR | aryl hydrocarbon receptor |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| APC | antigen-presenting cell |
| ASD | autism spectrum disorder |
| ATP | adenosine triphosphate |
| BBB | blood-brain barrier |
| BD | bipolar disorder |
| CA | cinnabarinic acid |
| CCBL | cysteine conjugate beta-lyase |
| CNS | central nervous system |
| CRP | C-reactive protein |
| CSF | cerebrospinal fluid |
| CUD | cocaine use disorder |
| DSM-5 | Diagnostic and Treatment Manual for Mental Disorders, Fifth Edition |
| EEG | electroencephalography |
| FEP | first-episode psychosis |
| GAD | generalized anxiety disorder |
| GPR35 | G-protein coupled receptor 35 |
| 3-HAA | 3-hydroxyanthranilic acid |
| 3-HK | 3-hydroxy-kynurenine |
| HD | Huntington’s disease |
| HRQL | Health-Related Quality of Life |
| 5-HT | serotonin, 5-hydroxytryptamine |
| IDO | indoleamine 2,3-dioxygenase |
| IFN | interferon |
| IL | interleukin |
| iNKT | invariant natural killer cell |
| KATs | kynurenine aminostransferase |
| KMO | kynurenine 3-monooxygenase |
| KP | kynurenine pathway |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| LGI | low-grade inflammation |
| MCP | monocyte chemoattractant protein |
| MDD | major depressive disorder |
| MS | multiple sclerosis |
| MT | melatonin |
| NAD+ | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenosine dinucleotide phosphate |
| NK | natural killer cell |
| PD | Parkinson’s disease |
| PIC | picolinic acid |
| PTSD | post-traumatic stress disorder |
| QUIN | quinolinic acid |
| ROS | reactive oxygen species |
| SANS | Scale for the Assessment of Negative Symptoms |
| SCZ | schizophrenia |
| sIL-2R | soluble IL-2 receptor |
| SjS | Sjögren’s syndrome |
| SLE | systemic lupus erythematosus |
| SUD | substance use disorder |
| SWS | slow wave sleep |
| TDO | tryptophan 2,3-dioxygenase |
| Th1 | T helper type 1 |
| Th17 | T helper type 17 |
| Th2 | T helper type 2 |
| TNF | tumor necrosis factor |
| TRP | tryptophan |
| XA | xanthurenic acid |
References
- Margină, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors. Int. J. Environ. Res. Public Health 2020, 17, 4135. [Google Scholar] [CrossRef] [PubMed]
- Dinh, K.M.; Kaspersen, K.A.; Mikkelsen, S.; Pedersen, O.B.; Petersen, M.S.; Thørner, L.W.; Hjalgrim, H.; Rostgaard, K.; Ullum, H.; Erikstrup, C. Low-grade inflammation is negatively associated with physi;cal Health-Related Quality of Life in healthy individuals: Results from The Danish Blood Donor Study (DBDS). PLoS ONE 2019, 14, e0214468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogovskii, V. Immune Tolerance as the Physiologic Counterpart of Chronic Inflammation. Front. Immunol. 2020, 11, 2061. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, K.W. Aryl hydrocarbon receptor (AHR)-mediated inflammation and resolution: Non-genomic and genomic signaling. Biochem. Pharmacol. 2020, 182, 114220. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Rossini, S.; Grohmann, U.; Mondanelli, G. Polyamines and Kynurenines at the Intersection of Immune Modulation. Trends Immunol. 2020, 41, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Dadvar, S.; Ferreira, D.; Cervenka, I.; Ruas, J.L. The weight of nutrients: Kynurenine metabolites in obesity and exercise. J. Intern. Med. 2018, 284, 519–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef]
- Joisten, N.; Walzik, D.; Metcalfe, A.J.; Bloch, W.; Zimmer, P. Physical Exercise as Kynurenine Pathway Modulator in Chronic Diseases: Implications for Immune and Energy Homeostasis. Int. J. Tryptophan Res. 2020, 8, 13. [Google Scholar] [CrossRef]
- Sundaram, G.; Lim, C.K.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway modulation reverses the experimental autoimmune encephalomyelitis mouse disease progression. J. Neuroinflammation 2020, 17, 176. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the Redox Status in Multiple Sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef]
- Solvang, S.H.; Nordrehaug, J.E.; Tell, G.S.; Nygård, O.; McCann, A.; Ueland, P.M.; Midttun, Ø.; Meyer, K.; Vedeler, C.A.; Aarsland, D.; et al. The kynurenine pathway and cognitive performance in community-dwelling older adults. The Hordaland Health Study. Brain Behav. Immun. 2019, 75, 155–162. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Török, N.; Vécsei, L. Novel Pharmaceutical Approaches in Dementia. In NeuroPsychopharmacotherapy; Riederer, P., Laux, G., Nagatsu, T., Le, W., Riederer, C., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Vécsei, L. Editorial: Are 5-HT1 receptor agonists effective anti-migraine drugs? Expert Opin. Pharmacother. 2021, 12, 1–5. [Google Scholar] [CrossRef]
- Morales-Puerto, N.; Giménez-Gómez, P.; Pérez-Hernández, M.; Abuin-Martínez, C.; Gil de Biedma-Elduayen, L.; Vidal, R.; Gutiérrez-López, M.D.; O’Shea, E.; Colado, M.I. Addiction and the kynurenine pathway: A new dancing couple? Pharmacol. Ther. 2021, 223, 107807. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Kang, Y.; Song, X. Tryptophan-kynurenine pathway as a novel link between gut microbiota and schizophrenia: A review. Trop. J. Pharm. Res. 2019, 18, 897–905. [Google Scholar] [CrossRef]
- Barroso, A.; Mahler, J.V.; Fonseca-Castro, P.H.; Quintana, F.J. The aryl hydrocarbon receptor and the gut-brain axis. Cell Mol. Immunol. 2021, 18, 259–268. [Google Scholar] [CrossRef]
- Encyclopedia. Available online: https://encyclopedia.pub/8633 (accessed on 21 June 2021).
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Heng, B.; Guillemin, G.J. The Gut Microbiota, Kynurenine Pathway, and Immune System Interaction in the Development of Brain Cancer. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Polyák, H.; Cseh, E.K.; Bohár, Z.; Rajda, C.; Zádori, D.; Klivényi, P.; Toldi, J.; Vécsei, L. Cuprizone markedly decreases kynurenic acid levels in the rodent brain tissue and plasma. Heliyon 2021, 7, e06124. [Google Scholar] [CrossRef]
- Németh, H.; Toldi, J.; Vécsei, L.; Kynurenines. Parkinson’s disease and other neurodegenerative disorders: Preclinical and clinical studies. J. Neural. Transm. 2006, 70, 285–304. [Google Scholar] [CrossRef]
- Muneer, A. Kynurenine Pathway of Tryptophan Metabolism in Neuropsychiatric Disorders: Pathophysiologic and Therapeutic Considerations. Clin. Psychopharmacol. Neurosci. 2020, 18, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- Boros, F.A.; Vécsei, L. Immunomodulatory Effects of Genetic Alterations Affecting the Kynurenine Pathway. Front. Immunol. 2019, 10, 2570. [Google Scholar] [CrossRef]
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vécsei, L. Kynurenines in the Pathogenesis of Multiple Sclerosis: Therapeutic Perspectives. Cells 2020, 9, 1564. [Google Scholar] [CrossRef]
- Mándi, Y.; Vécsei, L. The kynurenine system and immunoregulation. J. Neural. Transm. 2012, 11, 197–209. [Google Scholar] [CrossRef]
- Suhs, K.W.; Novoselova, N.; Kuhn, M.; Seegers, L.; Kaever, V.; Muller-Vahl, K.; Trebst, C.; Skripuletz, T.; Stangel, M.; Pessler, F. Kynurenine Is a Cerebrospinal Fluid Biomarker for Bacterial and Viral Central Nervous System Infections. J. Infect. Dis. 2019, 220, 127–138. [Google Scholar] [CrossRef]
- Furuzawa-Carballeda, J.; Hernandez-Molina, G.; Lima, G.; Rivera-Vicencio, Y.; Ferez-Blando, K.; Llorente, L. Peripheral regulatory cells immunophenotyping in primary Sjogren’s syndrome: A cross-sectional study. Arthritis Res. Ther. 2013, 15, R68. [Google Scholar] [CrossRef] [Green Version]
- Legany, N.; Berta, L.; Kovacs, L.; Balog, A.; Toldi, G. The role of B7 family costimulatory molecules and indoleamine 2,3-dioxygenase in primary Sjogren’s syndrome and systemic sclerosis. Immunol. Res. 2017, 65, 622–629. [Google Scholar] [CrossRef]
- Akesson, K.; Pettersson, S.; Stahl, S.; Surowiec, I.; Hedenstrom, M.; Eketjall, S.; Trygg, J.; Jakobsson, P.J.; Gunnarsson, I.; Svenungsson, E.; et al. Kynurenine pathway is altered in patients with SLE and associated with severe fatigue. Lupus Sci. Med. 2018, 5, e000254. [Google Scholar] [CrossRef] [Green Version]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-Induced Tryptophan Breakdown is Related with Anemia, Fatigue, and Depression in Cancer. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Sforzini, L.; Nettis, M.A.; Mondelli, V.; Pariante, C.M. Inflammation in cancer and depression: A starring role for the kynurenine pathway. Psychopharmacology 2019, 236, 2997–3011. [Google Scholar] [CrossRef] [Green Version]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-regulation and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Wichers, M.C.; Maes, M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J. Psychiatry Neurosci. 2004, 29, 11–17. [Google Scholar]
- Füvesi, J.; Rajda, C.; Bencsik, K.; Toldi, J.; Vécsei, L. The role of kynurenines in the pathomechanism of amyotrophic lateral sclerosis and multiple sclerosis: Therapeutic implications. J. Neural. Transm. 2012, 119, 225–234. [Google Scholar] [CrossRef]
- Zádori, D.; Klivényi, P.; Toldi, J.; Fülöp, F.; Vécsei, L. Kynurenines in Parkinson’s disease: Therapeutic perspectives. J. Neural Transm. 2012, 119, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Zádori, D.; Klivényi, P.; Vámos, E.; Fülöp, F.; Toldi, J.; Vécsei, L. Kynurenines in chronic neurodegenerative disorders: Future therapeutic strategies. J. Neural Transm. 2009, 116, 1403–1409. [Google Scholar] [CrossRef] [Green Version]
- Beal, M.F.; Matson, W.R.; Swartz, K.J.; Gamache, P.H.; Bird, E.D. Kynurenine Pathway Measurements in Huntington’s Disease Striatum: Evidence for Reduced Formation of Kynurenic Acid. J. Neurochem. 1990, 55, 1327–1339. [Google Scholar] [CrossRef]
- Beal, M.F.; Matson, W.R.; Storey, E.; Milbury, P.; Ryan, E.A.; Ogawa, T.; Bird, E.D. Kynurenic acid concentrations are reduced in Huntington’s disease cerebral cortex. J. Neurol. Sci. 1992, 108, 80–87. [Google Scholar] [CrossRef]
- Jauch, D.; Urbańska, E.M.; Guidetti, P.; Bird, E.D.; Vonsattel, J.-P.G.; Whetsell, W.O.; Schwarcz, R. Dysfunction of brain kynurenic acid metabolism in Huntington’s disease: Focus on kynurenine aminotransferases. J. Neurol. Sci. 1995, 130, 39–47. [Google Scholar] [CrossRef]
- Iłżecka, J.; Kocki, T.; Stelmasiak, Z.; Turski, W.A. Endogenous protectant kynurenic acid in amyotrophic lateral sclerosis. Acta Neurol. Scand. 2003, 107, 412–418. [Google Scholar] [CrossRef]
- Erhardt, S.; Schwieler, L.; Nilsson, L.; Linderholm, K.; Engberg, G. The kynurenic acid hypothesis of schizophrenia. Physiol. Behav. 2007, 92, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Myint, A.M.; Schwarz, M.J. Kynurenine pathway in schizophrenia: Pathophysiological and therapeutic aspects. Cur. Pharm. Res. 2011, 17, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Schwarz, M. Schizophrenia as an inflammationmediated dysbalance of glutamatergic neurotransmission. Neurotox. Res. 2006, 10, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Lavebratt, C.; Olsson, S.; Backlund, L.; Frisén, L.; Sellgren, C.; Priebe, L.; Nikamo, P.; Träskman-Bendz, L.; Cichon, S.; Vawter, M.P.; et al. The KMO allele encoding Arg 452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol. Psychiatry 2014, 19, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Stephens, G.L.; Wang, Q.; Swerdlow, B.; Bhat, G.; Kolbeck, R.; Fung, M. Kynurenine 3-monooxygenase mediates inhibition of Th17 differentiation via catabolism of endogenous aryl hydrocarbon receptor ligands. Eur. J. Immunol. 2013, 43, 1727–1734. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef]
- Mándi, Y.; Endrész, V.; Mosolygó, T.; Burián, K.; Lantos, I.; Fülöp, F.; Szatmári, I.; Lőrinczi, B.; Balog, A.; Vécsei, L. The Opposite Effects of Kynurenic Acid and Different Kynurenic Acid Analogs on Tumor Necrosis Factor-α (TNF-α) Production and Tumor Necrosis Factor-Stimulated Gene-6 (TSG-6) Expression. Front. Immunol. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Savitz, J. The Kynurenine Pathway: A Finger in Every Pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Kanai, M.; Nakamura, T.; Funakoshi, H. Identification and characterization of novel variants of the tryptophan 2,3-dioxygenase gene: Differential regulation in the mouse nervous system during development. Neurosci. Res. 2009, 64, 111–117. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357. [Google Scholar] [CrossRef] [Green Version]
- Ogbechi, J.; Clanchy, F.I.; Huang, Y.-S.; Topping, L.M.; Stone, T.W.; Williams, R.O. IDO activation, inflammation and musculoskeletal disease. Exp. Gerontol. 2020, 131, 110820. [Google Scholar] [CrossRef]
- Wu, H.; Gong, J.; Liu, Y. Indoleamine 2, 3-dioxygenase regulation of immune response (Review). Mol. Med. Rep. 2018, 17, 4867–4873. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, G.C.; Malachowski, W.J.; Mondal, A.; Scherle, P.; Muller, A.J. Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer. Int. Rev. Cell Mol. Biol. 2018, 336, 175–203. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Smith, C.; Thomas, S.; Mandik-Nayak, L.; Laury-Kleintop, L.; Metz, R.; Muller, A.J. Indoleamine 2,3-dioxygenase pathways of pathgenic inflammation and immune escape in cancer. Cancer Immunol. Immunother. 2014, 63, 721–735. [Google Scholar] [CrossRef]
- Badawy, A.A.-B.; Guillemin, G. The Plasma [Kynurenine]/[Tryptophan] Ratio and Indoleamine 2,3-Dioxygenase: Time for Appraisal. Int. J. Tryptophan Res. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Guillemin, G.J.; Smythe, G.; Takikawa, O.; Brew, B.J. Expression of indoleamine 2, 3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 2005, 49, 15–23. [Google Scholar] [CrossRef]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Ciorba, M.A.; Bettonville, E.E.; McDonald, K.G.; Metz, R.; Prendergast, G.C.; Newberry, R.D.; Stenson, W.F. Induction of IDO-1 by Immunostimulatory DNA Limits Severity of Experimental Colitis. J. Immunol. 2010, 184, 3907–3916. [Google Scholar] [CrossRef]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2, 3-dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Park, H.; Kim, Y.S.; Kim, K.D.; Lee, H.K.; Cho, D.H.; Yang, J.W.; Hur, D.Y. L-kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species. Int. Immunopharmacol. 2011, 11, 932. [Google Scholar] [CrossRef]
- Molano, A.; Illarionov, P.; Besra, G.S.; Putterman, C.; Porcelli, S.A. Modulation of invariant natural killer T cell cytokine responses by indoleamine 2, 3-dioxygenase. Immunol. Lett. 2008, 117, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef] [Green Version]
- Guidetti, P.; Hoffman, G.E.; Melendez-Ferro, M.; Albuquerque, E.X.; Schwarcz, R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 2007, 55, 78–92. [Google Scholar] [CrossRef]
- Boros, F.; Bohár, Z.; Vécsei, L. Genetic alterations affecting the kynurenine pathway and their association with diseases. Mut. Res. 2018, 776, 32–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nematollahi, A.; Sun, G.; Jayawickrama, G.S.; Church, W.B. Kynurenine Aminotransferase Isozyme Inhibitors: A Review. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood–Brain Barrier Transport of Kynurenines: Implications for Brain Synthesis and Metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Kerr, S.J.; Smythe, G.A.; Smith, D.G.; Kapoor, V.; Armati, P.J.; Croitoru, J.; Brew, B.J. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J. Neurochem. 2001, 78, 842–853. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Sellgren, C.; Kegel, M.; Bergen, S.; Ekman, C.; Olsson, S.; Larsson, M.; Vawter, M.; Backlund, L.; Sullivan, P.; Sklar, P.; et al. A genome-wide association study of kynurenic acid in cerebrospinal fluid: Implications for psychosis and cognitive impairment in bipolar disorder. Mol. Psychiatry 2014, 19, 334–341. [Google Scholar] [CrossRef]
- Stazka, J.; Luchowski, P.; Urbanska, E.M. Homocysteine, a risk factor for atherosclerosis, biphasically changes the endothelial production of kynurenic acid. Eur. J. Pharmacol. 2005, 517, 217–223. [Google Scholar] [CrossRef]
- Fallarini, S.; Magliulo, L.; Paoletti, T.; de Lalla, C.; Lombardi, G. Expression of functional GPR35 in human iNKT cells. Biochem. Biophys. Res. Commun. 2010, 398, 420–425. [Google Scholar] [CrossRef]
- Yamamura, T.; Sakuishi, K.; Illes, Z.; Miyake, S. Understanding the behavior of invariant NKT cells in autoimmune diseases. J. Neuroimmunol. 2007, 191, 8–15. [Google Scholar] [CrossRef]
- Hartai, Z.; Klivenyi, P.; Janaky, T.; Penke, B.; Dux, L.; Vecsei, L. Kynurenine metabolism in multiple sclerosis. Acta Neurol. Scand. 2005, 112, 93–96. [Google Scholar] [CrossRef]
- Bai, M.Y.; Lovejoy, D.B.; Guillemin, G.J.; Kozak, R.; Stone, T.W.; Koola, M.M. Galantamine-Memantine Combination and Kynurenine Pathway Enzyme Inhibitors in the Treatment of Neuropsychiatric Disorders. Complex Psychiatry 2021. [Google Scholar] [CrossRef]
- Coyle, J.T. Glial metabolites of tryptophan and excitotoxicity: Coming unglued. Exper. Neurol. 2006, 197, 4–7. [Google Scholar] [CrossRef]
- Schwarcz, R. The kynurenine pathway of tryptophan degradation as a drug target. Cur. Opin. Pharmacol. 2004, 4, 12–17. [Google Scholar] [CrossRef]
- Schwarcz, R.; Pellicciari, R. Manipulation of Brain Kynurenines: Glial Targets, Neuronal Effects, and Clinical Opportunities. J. Pharmacol. Exp. Ther. 2002, 303, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamos, E.; Pardutz, A.; Klivenyi, P.; Toldi, J.; Vecsei, L. The role of kynurenines in disorders of the central nervous system: Possibilities for neuroprotection. J. Neurol. Sci. Vasc. Dement. 2009, 283, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mazza, J.; Rossi, A.; Weinberg, J.M. Innovatives uses of tumor necrosis factor alpha inhibitors. Dermatol. Clin. 2010, 28, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Tiszlavicz, Z.; Németh, B.; Fülöp, F.; Vécsei, L.; Tápai, K.; Ocsovszky, I.; Mándi, Y. Different inhibitory effects of kynurenic acid and a novel kynurenic acid analogue on tumour necrosis factor-a (TNF-a) production by mononuclear cells, HMGB1 production by monocytes and HNP1-3 secretion by neutrophils. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 447–455. [Google Scholar] [CrossRef]
- Oztan, O.; Turksoy, V.A.; Daltaban, I.S.; Gunduzoz, M.; Tutkun, L.; Iritas, S.B.; Hakan, A.K. Pro-inflammatory cytokine and vascular adhesion molecule levels in manganese and lead-exposed workers. Int. J Immunother. Cancer Res. 2019, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Müller, N.; Schwarz, M.J. Immune system and schizophrenia. Cur. Immunol. Rev. 2010, 6, 213–220. [Google Scholar] [CrossRef]
- Raison, C.L.; Dantzer, R.; Kelley, K.W.; Lawson, M.A.; Woolwine, B.J.; Vogt, G.; Spivey, J.R.; Saito, K.; Miller, A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: Relationship to CNS immune responses and depression. Mol. Psychiatry 2010, 15, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Van Gool, A.R.; Verkerk, R.; Fekkes, D.; Bannink, M.; Sleijfer, S.; Kruit, W.H.J.; van der Holt, B.; Scharpe, S.; Eggermont, A.M.M.; Stoter, G.; et al. Neurotoxic and neuroprotective metabolites of kynurenine in patients with renal cell carcinoma treated with interferon-alpha: Course and relationship with psychiatric status. Psychiatry Clin. Neurosci. 2008, 62, 597–602. [Google Scholar] [CrossRef]
- Török, N.; Maszlag-Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single Nucleotide Polymorphisms of Indoleamine 2,3-Dioxygenase Influenced the Age Onset of Parkinson’s Disease. Preprints 2020, 2020100172. [Google Scholar] [CrossRef]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef]
- Mucci, F.; Marazziti, D.; Della Vecchia, A.; Baroni, S.; Morana, P.; Carpita, B.; Mangiapane, P.; Morana, F.; Morana, B.; Dell’Osso, L. State-of-the-Art: Inflammatory and Metabolic Markers in Mood Disorders. Life 2020, 10, 82. [Google Scholar] [CrossRef]
- Okamoto, H.; Hayaishi, O. Flavin adenine dinucleotide requirement for kynurenine hydroxylase of rat liver mitochondria. Biochem. Biophys. Res. Commun. 1967, 29, 394–399. [Google Scholar] [CrossRef]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine, an Endogenous Oxidative Stress Generator, Causes Neuronal Cell Death with Apoptotic Features and Region Selectivity. J. Neurochem. 1998, 70, 299–307. [Google Scholar] [CrossRef]
- Moroni, F. Tryptophan metabolism and brain function: Focus on kynurenine and other indole metabolites. Eur. J. Pharmacol. 1999, 375, 87–100. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nakahama, T.; Le, D.H.; Van Son, L.; Chu, H.H.; Kishimoto, T. Aryl hydrocarbon receptor and kynurenine: Recent advances in autoimmune disease research. Front. Immunol. 2014, 5, 551. [Google Scholar] [CrossRef] [Green Version]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolusic, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.J.; et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef] [Green Version]
- Andiné, P.; Lehmann, A.; Ellrén, K.; Wennberg, E.; Kjellmer, I.; Nielsen, T.; Hagberg, H. The excitatory amino acid antagonist kynurenic acid administered after hypoxic-ischemia in neonatal rats offers neuroprotection. Neurosci. Lett. 1988, 90, 208–212. [Google Scholar] [CrossRef]
- Foster, A.C.; Vezzani, A.; French, E.D.; Schwarcz, R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 1984, 48, 273–278. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic acid: The Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 2017, 8, 1957. [Google Scholar] [CrossRef] [Green Version]
- Connor, T.J.; Starr, N.; O’Sullivan, J.B.; Harkin, A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: A role for IFN-γ? Neurosci. Lett. 2008, 441, 29–34. [Google Scholar] [CrossRef]
- Jacobs, K.R.; Castellano-Gonzalez, G.; Guillemin, G.J.; Lovejoy, D.B. Major Developments in the Design of Inhibitors along the Kynurenine Pathway. Cur. Med. Chem. 2017, 24, 2471–2495. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [Green Version]
- Kubo, H.; Hoshi, M.; Mouri, A.; Tashita, C.; Yamamoto, Y.; Nabeshima, T.; Saito, K. Absence of kynurenine 3-monooxygenase reduces mortality of acute viral myocarditis in mice. Immunol. Lett. 2016, 181, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef]
- Ibos, K.E.; Bodnár, É.; Bagosi, Z.; Bozsó, Z.; Tóth, G.; Szabó, G.; Csabafi, K. Kisspeptin-8 Induces Anxiety-Like Behavior and Hypolocomotion by Activating the HPA Axis and Increasing GABA Release in the Nucleus Accumbens in Rats. Biomedicines 2021, 9, 112. [Google Scholar] [CrossRef]
- Muntsant, A.; Jiménez-Altayó, F.; Puertas-Umbert, L.; Jiménez-Xarrie, E.; Vila, E.; Giménez-Llort, L. Sex-Dependent End-of-Life Mental and Vascular Scenarios for Compensatory Mechanisms in Mice with Normal and AD-Neurodegenerative Aging. Biomedicines 2021, 9, 111. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Marin-Pardo, D.; Marazuela, P.; Hernández-Guillamón, M. Survival Bias and Crosstalk between Chronological and Behavioral Age: Age- and Genotype-Sensitivity Tests Define Behavioral Signatures in Middle-Aged, Old, and Long-Lived Mice with Normal and AD-Associated Aging. Biomedicines 2021, 9, 636. [Google Scholar] [CrossRef]
- Cantón-Habas, V.; Rich-Ruiz, M.; Romero-Saldaña, M.; Carrera-González, M. Depression as a Risk Factor for Dementia and Alzheimer’s Disease. Biomedicines 2020, 8, 457. [Google Scholar] [CrossRef]
- Kowalska, K.; Krzywoszański, Ł.; Droś, J.; Pasińska, P.; Wilk, A.; Klimkowicz-Mrowiec, A. Early Depression Independently of Other Neuropsychiatric Conditions, Influences Disability and Mortality after Stroke (Research Study-Part of PROPOLIS Study). Biomedicines 2020, 8, 509. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef]
- Park, S.; Bak, A.; Kim, S.; Nam, Y.; Kim, H.S.; Yoo, D.H.; Moon, M. Animal-Assisted and Pet-Robot Interventions for Ameliorating Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis. Biomedicines 2020, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.; Schatzberg, A. Prevalence of depressive episodes with psychotic features in the general population. Am. J. Psychiatry 2002, 159, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Galynker, I.; Cohen, L.; Cai, J. Negative symptoms in patients with major depressive disorder: A preliminary report. Neuropsychiatry. Neuropsychol. Behav. Neurol. 2000, 13, 171–176. [Google Scholar]
- McIntyre, R.; Cha, D.; Soczynska, J. Cognitive deficits and functional outcomes in major depressive disorder: Determinants, substrates, and treatment interventions. Depress. Anxiety 2013, 30, 515–527. [Google Scholar] [CrossRef]
- Ballenger, J. Anxiety and depression: Optimizing treatments. Prim Care Companion. J. Clin. Psychiatry 2000, 2, 71–79. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Liu, Y.; Ho, R.C.; Mak, A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef]
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013, 150, 736–744. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Hiles, S.A.; Baker, A.L.; de Malmanche, T.; Attia, J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: Exploring the causes of heterogeneity. Brain Behav. Immun. 2012, 26, 1180–1188. [Google Scholar] [CrossRef]
- Wang, A.K.; Miller, B.J. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr. Bull. 2018, 44, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, S.; Fujii, T.; Koga, N.; Hori, H.; Teraishi, T.; Hattori, K.; Noda, T.; Higuchi, T.; Motohashi, N.; Kunugi, H. Plasma L-tryptophan concentration in major depressive disorder: New data and meta-analysis. J. Clin. Psychiatry 2014, 75, e906–e915. [Google Scholar] [CrossRef]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci, Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef]
- Réus, G.; Jansen, K.; Titus, S.; Carvalho, A.F.; Gabbay, V.; Quevedo, J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015, 68, 316–328. [Google Scholar] [CrossRef] [Green Version]
- Dunayevich, E.; Keck, P.E., Jr. Prevalence and description of psychotic features in bipolar mania. Cur. Psychiatry Rep. 2000, 2, 286–290. [Google Scholar] [CrossRef]
- Ameen, S.; Ram, D. Negative symptoms in the remission phase of bipolar disorder. Ger. J. Psychiatry 2007, 10, 1–7. [Google Scholar]
- Malhi, G.S.; Ivanovski, B.; Hadzi-Pavlovic, D.; Mitchell, P.B.; Vieta, E.; Sachdev, P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. 2007, 9, 114–125. [Google Scholar] [CrossRef]
- Martínez-Arán, A.; Vieta, E.; Colom, F.; Torrent, C.; Sánchez-Moreno, J.; Reinares, M.; Benabarre, A.; Goikolea, J.M.; Brugué, E.; Daban, C.; et al. Cognitive impairment in euthymic bipolar patients: Implications for clinical and functional outcome. Bipolar Disord. 2004, 6, 224–232. [Google Scholar] [CrossRef]
- Das, A. Anxiety disorders in bipolar I mania: Prevalence, effect on illness severity, and treatment implications. Indian J. Psychol. Med. 2013, 35, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Modabbernia, A.; Taslimi, S.; Brietzke, E.; Ashrafi, M. Cytokine alterations in bipolar disorder: A meta-analysis of 30 studies. Biol. Psychiatry 2013, 74, 15–25. [Google Scholar] [CrossRef]
- Munkholm, K.; Braüner, J.V.; Kessing, L.V.; Vinberg, M. Cytokines in bipolar disorder vs. healthy control subjects: A systematic review and meta-analysis. J.; Psychiatr, Res. 2013, 47, 1119–1133. [Google Scholar] [CrossRef]
- Munkholm, K.; Vinberg, M.; Vedel Kessing, L. Cytokines in bipolar disorder: A systematic review and meta-analysis. J. Affect. Disord. 2013, 144, 16–27. [Google Scholar] [CrossRef]
- Birner, A.; Platzer, M.; Bengesser, S.A.; Dalkner, N.; Fellendorf, F.T.; Queissner, R.; Pilz, R.; Rauch, P.; Maget, A.; Hamm, C.; et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS ONE 2017, 12, e0172699. [Google Scholar] [CrossRef]
- Arnone, D.; Saraykar, S.; Salem, H.; Teixeira, A.L.; Dantzer, R.; Selvaraj, S. Role of Kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci. Biobehav. Rev. 2018, 92, 477–485. [Google Scholar] [CrossRef]
- Hartley, S.; Barrowclough, C.; Haddock, G. Anxiety and depression in psychosis: A systematic review of associations with positive psychotic symptoms. Acta Psychiatr. Scand. 2013, 128, 327–346. [Google Scholar] [CrossRef]
- Wigman, J.T.; van Nierop, M.; Vollebergh, W.A.; Lieb, R.; Beesdo-Baum, K.; Wittchen, H.U.; van Os, J. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity—Implications for diagnosis and ultra-high risk research. Schizophr. Bull. 2012, 38, 247–257. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zhu, Y.; Dai, Y.; Liu, T.; Wang, Y. Cognitive impairment in generalized anxiety disorder revealed by event-related potential N270. Neuropsychiatr. Dis. Treat. 2015, 11, 1405–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, H.; Gould, R.L.; Abrol, E.; Howard, R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalized anxiety disorder. BMJ Open 2019, 9, e027925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, R.; Garner, M.; Holmes, C.; Osmond, C.; Teeling, J.; Lau, L.; Baldwin, D.S. Peripheral inflammatory cytokines and immune balance in Generalised Anxiety Disorder: Case-controlled study. Brain Behav. Immun. 2017, 62, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Orlikov, A.B.; Prakhye, I.B.; Ryzov, I.V. Kynurenine in blood plasma and DST in patients with endogenous anxiety and endogenous depression. Biol. Psychiatry 1994, 36, 97–102. [Google Scholar] [CrossRef]
- Altmaier, E.; Emeny, R.T.; Krumsiek, J.; Lacruz, M.E.; Lukaschek, K.; Häfner, S.; Kastenmüller, G.; Römisch-Margl, W.; Prehn, C.; Mohney, R.P.; et al. Metabolomic profiles in individuals with negative affectivity and social inhibition: A population-based study of Type D personality. Psychoneuroendocrinology 2013, 38, 1299–1309. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse. Advancing Addiction Science. Available online: https://www.drugabuse.gov/publications/media-guide/science-drug-use-addiction-basics (accessed on 28 April 2021).
- UpToDate. Co-Occurring Schizophrenia and Substance Use Disorder: Epidemiology, Pathogenesis, Clinical Manifestations, Course, Assessment and Diagnosis. Available online: https://www.uptodate.com/contents/co-occurring-schizophrenia-and-substance-use-disorder-epidemiology-pathogenesis-clinical-manifestations-course-assessment-and-diagnosis (accessed on 28 April 2021).
- Connor, J.P.; Stjepanović, D.; Le Foll, B.; Hoch, E.; Budney, A.J.; Hall, W.D. Cannabis use and cannabis use disorder. Nat. Rev. Dis. Primers 2021, 7, 16. [Google Scholar] [CrossRef]
- Addiction Center. Schizophrenia and Addiction. Available online: https://www.addictioncenter.com/addiction/schizophrenia/ (accessed on 28 April 2021).
- Simon, N.; Belzeaux, R.; Adida, M.; Azorin, J.M. Negative symptoms in schizophrenia and substance-related disorders. L’Encephale 2015, 41 Suppl. S1, 6S27–6S31. [Google Scholar] [CrossRef]
- Whiting, D.; Lichtenstein, P.; Fazel, S. Violence and mental disorders: A structured review of associations by individual diagnoses, risk factors, and risk assessment. Lancet Psychiatry 2021, 8, 150–161. [Google Scholar] [CrossRef]
- Bruijnen, C.; Dijkstra, B.; Walvoort, S.; Markus, W.; van der Nagel, J.; Kessels, R.; De Jong, C. Prevalence of cognitive impairment in patients with substance use disorder. Drug Alcohol Rev. 2019, 38, 435–442. [Google Scholar] [CrossRef]
- Elmquist, J.; Shorey, R.C.; Anderson, S.E.; Stuart, G.L. The Relationship Between Generalized Anxiety Symptoms and Treatment Dropout Among Women in Residential Treatment for Substance Use Disorders. Subst. Use Misuse 2016, 51, 835–839. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.T.; Levis, S.C.; O’Neill, C.E.; Northcutt, A.L.; Fabisiak, T.J.; Watkins, L.R.; Bachtell, R.K. Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain Behav. Immune 2018, 67, 130–138. [Google Scholar] [CrossRef]
- Kohno, M.; Link, J.; Dennis, L.E.; McCready, H.; Huckans, M.; Hoffman, W.F.; Loftis, J.M. Neuroinflammation in addiction: A review of neuroimaging studies and potential immunotherapies. Pharmacol. Biochem. Behav. 2019, 179, 34–42. [Google Scholar] [CrossRef]
- Fonseca, F.; Mestre-Pintó, J.I.; Gómez-Gómez, À.; Martinez-Sanvisens, D.; Rodríguez-Minguela, R.; Papaseit, E.; Pérez-Mañá, C.; Langohr, K.; Valverde, O.; Pozo, Ó.J.; et al. On Behalf of Neurodep Group the Tryptophan System in Cocaine-Induced Depression. J. Clin. Med. 2020, 9, 4103. [Google Scholar] [CrossRef]
- Bedard-Gilligan, M.; Zoellner, L.A.; Feeny, N.C. Is Trauma Memory Special? Trauma Narrative Fragmentation in PTSD: Effects of Treatment and Response. Clin. Psychol. Sci. 2017, 5, 212–225. [Google Scholar] [CrossRef]
- De Boer, M.; Nijdam, M.J.; Jongedijk, R.A.; Bangel, K.A.; Olff, M.; Hofman, W.F.; Talamini, L.M. The spectral fingerprint of sleep problems in post-traumatic stress disorder. Sleep 2020, 43, zsz269. [Google Scholar] [CrossRef] [Green Version]
- Compean, E.; Hamner, M. Posttraumatic stress disorder with secondary psychotic features (PTSD-SP): Diagnostic and treatment challenges. Prog Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Maeng, L.Y.; Milad, M.R. Post-Traumatic Stress Disorder: The Relationship Between the Fear Response and Chronic Stress. Chronic Stress 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.P.; Vanelzakker, M.B.; Shin, L.M. Emotion and cognition interactions in PTSD: A review of neurocognitive and neuroimaging studies. Front. Integr. Nneurosci. 2012, 6, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, M.; Legrand, A.C.; Brier, Z.; Hébert-Dufresne, L. The symptoms at the center: Examining the comorbidity of posttraumatic stress disorder, generalized anxiety disorder, and depression with network analysis. J. Psychiatr. Res. 2019, 109, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Lee, S.; Yoon, S. Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective. Antioxidants 2020, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Amidfar, M.; Won, E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 91, 103–112. [Google Scholar] [CrossRef]
- Cohen, M.; Meir, T.; Klein, E.; Volpin, G.; Assaf, M.; Pollack, S. Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int. J. Psychiatry Med. 2011, 42, 117–131. [Google Scholar] [CrossRef]
- Berkwits, M.; Porter, R.; Jones, T.V.; Fletcher, A.J.; Beers, M.H. The Merck Manual of Medical Information; Merck & Co. Inc.: New York, NY, USA, 2003. [Google Scholar]
- Kiran, C.; Chaudhury, S. Prevalence of comorbid anxiety disorders in schizophrenia. Indian J. Psychiatry 2016, 25, 35–40. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Frydecka, D.; Krzystek-Korpacka, M.; Lubeiro, A.; Stramecki, F.; Stańczykiewicz, B.; Beszłej, J.A.; Piotrowski, P.; Kotowicz, K.; Szewczuk-Bogusławska, M.; Pawlak-Adamska, E.; et al. Profiling inflammatory signatures of schizophrenia: A cross-sectional and meta-analysis study. Brain Behav. Immunity 2018, 71, 28–36. [Google Scholar] [CrossRef]
- Gallego, J.A.; Blanco, E.A.; Husain-Krautter, S.; Madeline Fagen, E.; Moreno-Merino, P.; Del Ojo-Jiménez, J.A.; Ahmed, A.; Rothstein, T.L.; Lencz, T.; Malhotra, A.K. Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: New data and an updated meta-analysis. Schizophr. Res. 2018, 202, 64–71. [Google Scholar] [CrossRef]
- Koola, M.M.; Raines, J.K.; Hamilton, R.G.; McMahon, R.P. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Cur. Psychiatry 2016, 15, 52–57. [Google Scholar]
- Okusaga, O.; Fuchs, D.; Reeves, G.; Giegling, I.; Hartmann, A.M.; Konte, B.; Friedl, M.; Groer, M.; Cook, T.B.; Stearns-Yoder, K.A.; et al. Kynurenine and Tryptophan Levels in Patients with Schizophrenia and Elevated Antigliadin Immunoglobulin G Antibodies. Psychosom. Med. 2016, 78, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Plitman, E.; Iwata, Y.; Caravaggio, F.; Nakajima, S.; Chung, J.K.; Gerretsen, P.; Kim, J.; Takeuchi, H.; Chakravarty, M.M.; Remington, G.; et al. Kynurenic acid in schizophrenia: A systematic review and meta-analysis. Schizophr. Bull. 2017, 43, 764–777. [Google Scholar] [CrossRef]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Koola, M.M.; Praharaj, S.K.; Pillai, A. Galantamine-Memantine Combination as an Antioxidant Treatment for Schizophrenia. Cur. Behav. Neurosci. Rep. 2019, 6, 37–50. [Google Scholar] [CrossRef]
- Koola, M.M.; Sklar, J.; Davis, W.; Nikiforuk, A.; Meissen, J.K.; Sawant-Basak, A.; Aaronson, S.T.; Kozak, R. Kynurenine pathway in schizophrenia: Galantamine-memantine combination for cognitive impairments. Schizophr. Res. 2018, 193, 459–460. [Google Scholar] [CrossRef]
- Bell, V.; Dunne, H.; Zacharia, T.; Brooker, K.; Shergill, S. A symptom-based approach to treatment of psychosis in autism spectrum disorder [corrected]. BJPsych. Open 2018, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Leekam, S. Social cognitive impairment and autism: What are we trying to explain? Philos Trans. R. Soc. 2016, 371, 20150082. [Google Scholar] [CrossRef] [Green Version]
- Van Steensel, F.J.A.; Heeman, E.J. Anxiety Levels in Children with Autism Spectrum Disorder: A Meta-Analysis. J. Child Fam. Stud. 2017, 26, 1753–1767. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of co-occurring mental health diagnoses in the autism population: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Ataeinia, B.; Keynejad, K.; Abdolalizadeh, A.; Hirbod-Mobarakeh, A.; Rezaei, N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J. Psychiatr. Res. 2019, 115, 90–102. [Google Scholar] [CrossRef]
- Molloy, C.A.; Morrow, A.L.; Meinzen-Derr, J.; Schleifer, K.; Dienger, K.; Manning-Courtney, P.; Altaye, M.; Wills-Karp, M. Elevated cytokine levels in children with autism spectrum disorder. J. Neuroimmunol. 2006, 172, 198–205. [Google Scholar] [CrossRef]
- Bryn, V.; Verkerk, R.; Skjeldal, O.H.; Saugstad, O.D.; Ormstad, H. Kynurenine Pathway in Autism Spectrum Disorders in Children. Neuropsychobiology 2017, 76, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Essa, M.M.; de Paula Martins, R.; Lovejoy, D.B.; Bilgin, A.A.; Waly, M.I.; Al-Farsi, Y.M.; Al-Sharbati, M.; Al-Shaffae, M.A.; Guillemin, G.J. Altered kynurenine pathway metabolism in autism: Implication for immune-induced glutamatergic activity. Autism Res. 2016, 9, 621–631. [Google Scholar] [CrossRef]
- Kordestani Moghadam, P.; Nouriyengejeh, S.; Seyedhoseini, B.; Pourabbasi, A. The Study of Relationship between Nutritional Behaviors and Metabolic Indices: A Systematic Review. Adv. Biomed. Res. 2020, 9, 66. [Google Scholar] [CrossRef]
- Kordestani-Moghadam, P.; Assari, S.; Nouriyengejeh, S.; Mohammadipour, F.; Pourabbasi, A. Cognitive Impairments and Associated Structural Brain Changes in Metabolic Syndrome and Implications of Neurocognitive Intervention. J. Obes. Metab. Syndr. 2020, 29, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kordestani Moghadam, P.; Nasehi, M.; Khodagholi, F.; Reza Zarrindast, M. Vulnerability of Left Amygdala to Total Sleep Deprivation and Reversed Circadian Rhythm in Molecular Level: Glut1 as a Metabolic Biomarker. Galen. Med. J. 2019, 8, 970. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.K. Crosstalk between Depression and Dementia with Resting-State fMRI Studies and Its Relationship with Cognitive Functioning. Biomedicines 2021, 9, 82. [Google Scholar] [CrossRef]
- Komatsu, H.; Watanabe, E.; Fukuchi, M. Psychiatric Neural Networks and Precision Therapeutics by Machine Learning. Biomedicines 2021, 9, 403. [Google Scholar] [CrossRef]


| Enzymes | Substrates | Products | Diseases |
|---|---|---|---|
| TDO | Tryptophan | L-kynurenine | Human brain tumors [34] Other tumor types [35,36] |
| IDO | Tryptophan | L-kynurenine | Tumors [36,37] Neurological and neurodegenerative diseases [25] Depression [38] Systemic lupus erythematosus [33] Sjörgen’s syndrome [31,32] |
| KAT | L-kynurenine 3-hydroxy-L-kynurenine | Kynurenic acid Xanthurenic acid | Multiple sclerosis [39] Parkinson’s disease [40] Alzheimer’s disease [41] Huntington’s disease [42,43,44] Epilepsy [25] Amyotrophic lateral sclerosis [45] Schizophrenia [46,47,48] |
| KMO | L-kynurenine | 3-hydroxy-L-kynurenine | Bipolar disorder [49] Autoimmunity related diseases [50] |
| Major Depressive Disorder | Bipolar Disorder | Generalized Anxiety Disorder | Substance Use Disorder | Post-Traumatic Stress Disorder | Schizophrenia | Autism Spectrum Disorder | ||
|---|---|---|---|---|---|---|---|---|
| Symptoms | Positive | + | ++ | ++ | ++ | ++ | ++ | + |
| Negative | ++ | ++ | ++ | + | ++ | ++ | + | |
| Cognitive | + | + | ++ | ++ | ++ | - | + | |
| Anxiety | ++ | ++ | ++ | ++ | ++ | ++ | ++ | |
| Inflammatory factors | Proinflammatory | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Anti-inflammatory | ↑ | ↑ | ↓ | ↓ | ↓,? | ↑ | ↑ | |
| Kynurenines | Toxic | ↑ | ? | ↑ | ? | ? | ↑ | ↑ |
| Protective | ↓ | ? (serum), ↑ (CSF) | ? | ? | ? | ↑,? | ↓ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. https://doi.org/10.3390/biomedicines9070734
Tanaka M, Tóth F, Polyák H, Szabó Á, Mándi Y, Vécsei L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines. 2021; 9(7):734. https://doi.org/10.3390/biomedicines9070734
Chicago/Turabian StyleTanaka, Masaru, Fanni Tóth, Helga Polyák, Ágnes Szabó, Yvette Mándi, and László Vécsei. 2021. "Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway" Biomedicines 9, no. 7: 734. https://doi.org/10.3390/biomedicines9070734









