South Asian Children Have Increased Body Fat in Comparison to White Children at the Same Body Mass Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anthropometric Assessments
2.2. Physical Maturity (Biological Age)
2.3. Statistical Analysis
3. Results
3.1. Sample 1: Dependent Variable, Percent Body Fat
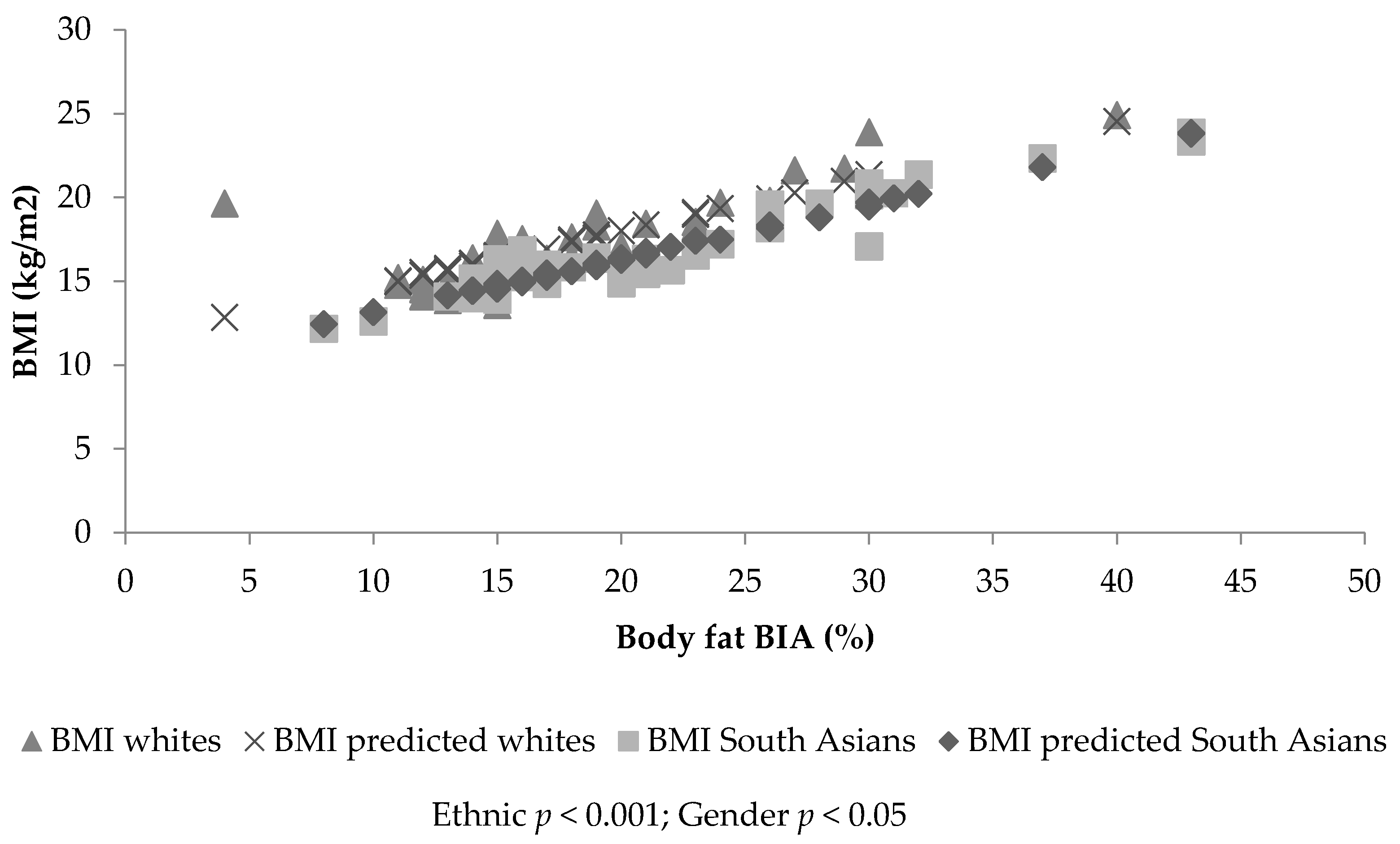
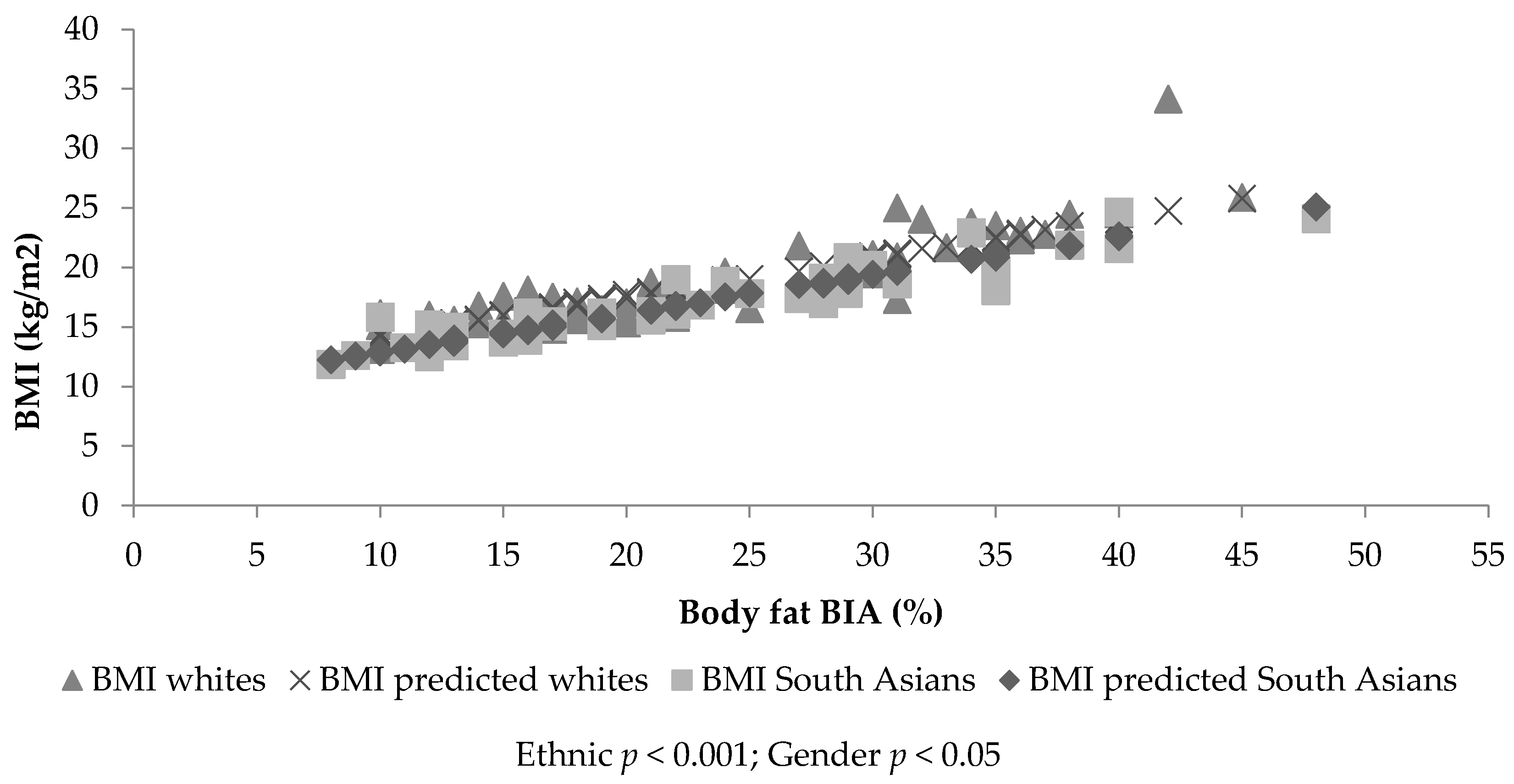
3.2. Sample 1: Dependent Variable Body Mass Index
3.3. Sample 2: Dependent Variable, Percent Body Fat
3.4. Sample 2: Dependent Variable Body Mass Index
4. Discussion
Author Contributions
Conflicts of Interest
References
- Brage, S.N.; Wedderkopp, U.; Ekelund, P.W.; Franks, A.J.; Wareham, N.J.; Andersen, L.B.; Froberg, K. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: The European Youth Heart Study (EYHS). Diabetes Care 2004, 27, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Ehtisham, S.; Hattersley, A.T.; Dunger, D.B.; Barrett, T.G. First UK survey of paediatric type 2 diabetes and MODY. Arch. Dis. Child. 2004, 89, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Whincup, P.H.; Gilg, J.A.; Papacosta, O.; Seymour, C.; Miller, G.J.; Alberti, K.G.M.M. Early evidence of ethnic differences in cardiovascular risk: Cross sectional study comparison of British South Asian and White Children. BMJ 2002, 324, 1–6. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Webster, P.; Sinclair, D. Trends in overweight and obesity among 5–7-year-old White and South Asian children born between 1991–1999. J. Public Health 2008, 30, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, C.M.; Rudnicka, A.R.; Owen, C.G.; Cook, D.G.; Whincup, P.H. Patterns of body size and adiposity in UK children of South Asian, Black African-Caribbean and white European origin, Child heart and health study in England (CHASE study). Int. J. Epidemiol. 2011, 40, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Lubree, H.G.; Rege, S.S.; Naik, S.S.; Deshpande, J.A.; Despande, S.S.; Joglekar, C.V.; Yudkin, J.S. Adiposity and hyper insulinemia in Indians are present at birth. J. Clin. Endorcrinol. Metab. 2002, 87, 5575–5580. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health and Care Excellence. Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults: NICE Clinical Guideline 189. Available online: http://www.nice.org.uk/Guidance/CG43 (accessed on 24 November 2014).
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut-offs to define thinness in children and adolescents: International survey. BMJ 2007, 335, 194–202. [Google Scholar] [CrossRef] [PubMed]
- WHO. Multicentre Growth Reference Study Group. In WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Cole, T.J.; Freeman, J.V.; Preece, M.A. Body mass index reference curves for the UK, 1990. Arch. Dis. Child. 1995, 73, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Methven, E.; McDowell, Z.C.; Hacking, B.; Alexander, D.; Stewart, L.; Kelnar, C.J. Health consequences of obesity. Arch. Dis. Child. 2003, 88, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Sherry, B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009, 124, S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Information Standards Board; Department for Education and Skills. Ethnicity Type Controlled List. Available online: https://data.gov.uk/education-standards/cl/ethnicity-type-controlled-list (accessed on 24 January 2012).
- Cole, T.J.; Pan, H.; LMS Growth. An Excel Add-in to Convert Measurements to z-Scores. Centre for Epidemiology and Biostatistics. Available online: http://www.healthforallchildren.co.uk (accessed on 24 January 2012).
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Van Loan, M.D.; Bemben, D.A. Skinfold equations for estimation of body fatness in children and youths. Hum. Biol. 1988, 60, 709–723. [Google Scholar] [PubMed]
- Mirwald, R.L.; Baxter-Jones, A.D.; Baily, D.A.; Beunen, G.P. An assessment of maturity from anthropometric measurements. Med. Sci. Sports Exerc. 2002, 34, 689–694. [Google Scholar] [PubMed]
- Harding, J.E. The nutritional basis of fetal origins of adult disease. Int. J. Epidemiol. 2001, 30, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Neel, J.V. Diabetes mellitus: A ‘thrifty’ genotype rendered detrimental by ‘progress’? Am. J. Hum. Genet. 1962, 4, 352–353. [Google Scholar]
- Liu, J.H.; Jones, S.J.; Sun, H.; Probst, J.C.; Merchant, A.T.; Cavicchia, P. Diet, physical activity, and sedentary behaviours as risk factors for childhood obesity: An urban and rural comparison. Child. Obes. 2012, 8, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Fagot-Campagna, A.; Narayan, K. Type 2 diabetes in children. BMJ 2001, 22, 377–387. [Google Scholar] [CrossRef]
- Zimmet, P. Globalization, coco-colonization and the chronic disease epidemic: Can the Doomsday scenario be adverted? J. Intern. Med. 2000, 247, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Eyre, E.L.J. Physical Activity and Body Composition Differences between White EU and South Asian Primary School Children. Master’s Thesis, University of Warwick, Coventry, UK, 2012. [Google Scholar]
- Owens, C.G.; Nightingale, C.M.; Rudnicka, A.R.; Cook, D.G.; Ekelund, U.; Whincup, P.H. Ethnic and gender differences in physical activity levels among 9–10 year-old children of white European, South Asian and African-Caribbean origin. The child heart health study in England (CHASE). Int. J. Epidemiol. 2009, 1, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Eyre, E.L.J.; Duncan, M.J.; Smith, E.; Matyka, K.A. Objectively measured patterns of physical activity in primary school children in Coventry, the influence of ethnicity. Diabet. Med. 2013, 30, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Eyre, E.L.J.; Cox, V.M.; Birch, S.L.; Duncan, M.J. An integrated curriculum approach to increasing habitual physical activity in deprived South Asian children. Eur. J. Sport Sci. 2016, 16, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Pallan, M.; Parry, J.; Adab, P. Contextual influences on the development of obesity in children: A Case study of UK South Asian Communities. Prev. Med. 2012, 54, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.; Drew, M.K. What is the most accurate and reliable methodological approach for predicting peak height velocity in adolescents? A systematic review. J. Sci. Med. Sports 2017, 20, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Nevill, A.M.; Duncan, M.J.; Lahart, I.; Davies, P.; Ramirez-Velez, R.; Sandercock, G. Scaling children’s waist circumference for differences in body size. Am. J. Hum. Biol. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eyre, E.L.J.; Duncan, M.J.; Nevill, A. South Asian Children Have Increased Body Fat in Comparison to White Children at the Same Body Mass Index. Children 2017, 4, 102. https://doi.org/10.3390/children4110102
Eyre ELJ, Duncan MJ, Nevill A. South Asian Children Have Increased Body Fat in Comparison to White Children at the Same Body Mass Index. Children. 2017; 4(11):102. https://doi.org/10.3390/children4110102
Chicago/Turabian StyleEyre, Emma L. J., Michael J. Duncan, and Alan Nevill. 2017. "South Asian Children Have Increased Body Fat in Comparison to White Children at the Same Body Mass Index" Children 4, no. 11: 102. https://doi.org/10.3390/children4110102




