Mercury Hair Concentration among Primary School Children in Malaysia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sampling
2.2. Biological Sample Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Du, B.; Li, P.; Feng, X.; Qiu, G.; Zhou, J.; Maurice, L. Mercury exposure in Children of the Wanshan Mercury Mining Area, Guizhou, China. Int. J. Environ. Res. Public Health 2016, 13, 1107. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency; Integrated Risk Information System (IRIS); Environmental Protection Agency. Chemical Assessment Summary. Methylmercury (MeHg); CASRN 22967-92-6. 2001. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0073.htm (accessed on 18 October 2017).
- Poulin, J.; Gibb, H. Mercury: Assessing the Environmental Burden of Disease at National and Local Levels; WHO Environmental Burden of Disease Series No. 16; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Dunn, J.E.; Trachtenberg, F.L.; Barregard, L.; Bellinger, D.; McKinlay, S. Scalp Hair and Urine Mercury Content of Children in the Northeast United States: The New England Children’s Amalgam Trial. Environ. Res. 2008, 107, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Jeevanaraj, P.; Hashim, Z.; Elias, S.M.; Aris, A.Z. Mercury Accumulation in Marine Fish Most Favoured by Malaysian Women, the Predictors and the Potential Health Risk. Environ. Sci. Pollut. Res. 2016, 23, 23714–23729. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.J.; Thurston, S.W.; Pearson, A.T.; Davidson, P.W.; Cox, C.; Shamlaye, C.F.; Cernichiari, E.; Clarkson, T.W. Postnatal Exposure to Methyl Mercury from Fish Consumption: A Review and New Data from the Seychelles Child Development Study. Neurotoxicology 2009, 30, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Budtz-Jørgensen, E.; White, R.F.; Jørgensen, P.J.; Weihe, P.; Debes, F.; Keiding, N. Methylmercury Exposure Biomarkers as Indicators of Neurotoxicity in Children Aged 7 Years. Am. J. Epidemiol. 1999, 150, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Weihe, P.; White, R.F.; Debes, F. Cognitive Performance of Children Prenatally Exposed to “safe” levels of Methylmercury. Environ. Res. 1998, 77, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jordan, M.; Watkins, S.; Washam, R.; DuClos, C.; Jones, S.; Palcic, J.; Pawlowicz, M.; Blackmore, C. Fish Consumption and Hair Mercury Levels in Women of Childbearing Age, Martin County, Florida. Matern. Child Health J. 2014, 18, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Broussard, L.A.; Hammett-stabler, C.A.; Winecker, R.E.; Ropero-Miller, J.D. The Toxicity of Mercury. Lab. Med. 2002, 33, 614–625. [Google Scholar] [CrossRef]
- Fiedler, N.; Udasin, I.; Gochfeld, M.; Buckler, G.; Kelly-McNeil, K.; Kipen, H. Neuropsychological and Stress Evaluation of a Residential Mercury Exposure. Environ. Health Perspect. 1999, 107, 343–347. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Programme on Chemical Safety. Environmental Health Criteria 101: Methymercury; World Health Organization: Geneva, Switzerland, 1990; Available online: http://www.inchem.org/documents/ehc/ehc/ehc101.htm (accessed on 20 October 2017).
- Batista, J.; Schuhmacher, M.; Domingo, J.L.; Corbella, J. Mercury in Hair for a Child Population from Tarragona Province, Spain. Sci. Total Environ. 1996, 193, 143–148. [Google Scholar] [CrossRef]
- Pirard, C.; Koppen, G.; De Cremer, K.; Van Overmeire, I.; Govarts, E.; Dewolf, M.C.; Van De Mieroop, E.; Aerts, D.; Biot, P.; Casteleyn, L.; et al. Hair Mercury and Urinary Cadmium Levels in Belgian Children and their Mothers within the Framework of the COPHES/DEMOCOPHES Projects. Sci. Total Environ. 2014, 472, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, D.S.; Moon, J.S.; Yang, W.H.; Son, B.S. Mercury Level in Hair of Primary School Children in Korea and China. Mol. Cell. Toxicol. 2008, 4, 235–245. [Google Scholar] [CrossRef]
- McDowell, M.A.; Dillon, C.F.; Osterloh, J.; Bolger, P.M.; Pellizzari, E.; Fernando, R.; Montes de Oca, R.; Schober, S.E.; Sinks, T.; Jones, R.L.; et al. Hair Mercury Levels in U.S. Children and Women of Childbearing Age: Reference Range Data from NHANES 1999–2000. Environ. Health Perspect. 2004, 112, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Pesch, A.; Wilhelm, M.; Rostek, U.; Schmitz, N.; Weishoff-Houben, M.; Ranft, U.; Idel, H. Mercury Concentrations in Urine, Scalp Hair, and Saliva in Children from Germany. J. Expo. Sci. Environ. Epidemiol. 2002, 12, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Jeon, C.K.; Paek, D.M. Hair Mercury Concentrations of Children and Mothers in Korea: Implication for Exposure and Evaluation. Sci. Total Environ. 2008, 402, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Díez, S.; Delgado, S.; Aguilera, I.; Astray, J.; Pérez-Gómez, B.; Torrent, M.; Sunyer, J.; Bayona, J.M. Prenatal and Early Childhood Exposure to Mercury and Methylmercury in Spain, a High-Fish Consumer Country. Arch. Environ. Contam. Toxicol. 2009, 56, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Sakamoto, M.; Nakai, K.; Weihe, P.; Dakeishi, M.; Iwata, T.; Liu, X.J.; Ohno, T.; Kurosawa, T.; Kamiya, K.; et al. Effects of Methylmercury on Neurodevelopment in Japanese Children in Relation to the Madeiran Study. Int. Arch. Occup. Environ. Health 2004, 77, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Marinho, J.S.; Lima, M.O.; Santos, E.D.O.C.; De Jesus, I.M.; Pinheiro, M.D.C.N.; Alves, C.N.; Muller, R.C.S. Mercury Speciation in Hair of Children in Three Communities of the Amazon, Brazil. Biomed. Res. Int. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Garí, M.; Grimalt, J.O.; Torrent, M.; Sunyer, J. Influence of Socio-Demographic and Diet Determinants on the Levels of Mercury in Preschool Children from a Mediterranean Island. Environ. Pollut. 2013, 182, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Wranová, K.; Čejchanová, M.; Spěváčková, V.; Korunová, V.; Vobecký, M.; Spěváček, V. Mercury and Methylmercury in Hair of Selected Groups of Czech Population. Cent. Eur. J. Public Health 2009, 17, 36–40. [Google Scholar] [PubMed]
- WWF Malaysia. Save Our Seafood (S.O.S) Campaign: Malaysia Sustainable Seafood Guide. 2013. Available online: http://www.wwf.org.my/media_and_information/publications_main/sustainable_seafood_guide/ (accessed on 22 December 2016).
- Tengku Hanidza, T.; Tunku Khalkausar, F.; Yasutake, A.; Sharifuddin, M.Z.; Hafizan, J.; Rosta, H. Hair Mercury Levels in Relation to Marine Fish Consumption among Adults in Malaysia. Environ. Asia 2010, 3, 175–185. [Google Scholar]
- Hajeb, P.; Jinap, S. Mercury Exposure through Fish and Seafood Consumption Inthe Rural and Urban Coastal Communities of Peninsular Malaysia. World J. Fish Mar. Sci. 2011, 3, 217–226. [Google Scholar]
- Hajeb, P.; Selamat, J.; Ismail, A.; Bakar, F.A.; Bakar, J.; Lioe, H.N. Hair Mercury Level of Coastal Communities in Malaysia: A Linkage with Fish Consumption. Eur. Food Res. Technol. 2008, 227, 1349–1355. [Google Scholar] [CrossRef] [Green Version]
- Sarmani, S.; Kiprawi, A.Z.; Ismail, R.B. Mercury Determination in Hair of Malaysian Fishermen by Neutron Activation Analysis. Biol. Trace Elem. Res. 1994, 43, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Nurul Izzah, A.; Mohd Fairulnizal, M.N.; Wan Rozita, W.M.; Hamdan, J.; Ismail, I.; Wan Nurul Farah, W.A.; Yuvaneswary, V.; Mohd Hairulhisam, H. Mercury Levels of Marine Fish Commonly Consumed in Peninsular Malaysia. Environ. Sci. Pollut. Res. Int. 2015, 22, 3672–3686. [Google Scholar]
- Agusa, T.; Kunito, T.; Sudaryanto, A.; Monirith, I.; Kan-Atireklap, S.; Iwata, H.; Ismail, A.; Sanguansin, J.; Muchtar, M.; Tana, T.S.; et al. Exposure Assessment for Trace Elements from Consumption of Marine Fish in Southeast Asia. Environ. Pollut. 2007, 145, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.C.; Ngim, C.H.; Phoon, W.O.; Lee, J. Mercury in Scalp Hair of Healthy Singapore Residents. Sci. Total Environ. 1988, 72, 113–122. [Google Scholar] [CrossRef]
- Foo, S.C.; Tan, T.C. Elements in the Hair of South-East Asian Islanders. Sci. Total Environ. 1998, 209, 185–192. [Google Scholar] [CrossRef]
- Bonsignore, M.; Andolfi, N.; Barra, M.; Madeddu, A.; Tisano, F.; Ingallinella, V.; Castorina, M.; Sprovieri, M. Assessment of Mercury Exposure in Human Populations: A Status Report from Augusta Bay (Southern Italy). Environ. Res. 2016, 150, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Hrubá, F.; Strömberg, U.; Černá, M.; Chen, C.; Harari, F.; Harari, R.; Horvat, M.; Koppová, K.; Kos, A.; Krsková, A.; et al. Blood Cadmium, Mercury, and Lead in Children: An International Comparison of Cities in Six European Countries, and China, Ecuador, and Morocco. Environ. Int. 2012, 41, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Aldroobi, K.S.A.; Shukri, A.; Bauk, S.; Munem, E.M.A.; Abuarra, A.M.A. Determination of Arsenic and Mercury Level in Scalp Hair from a Selected Population in Penang, Malaysia Using XRF Technique. Radiat. Phys. Chem. 2013, 91, 9–14. [Google Scholar] [CrossRef]
- Jeevanaraj, P.; Hashim, Z.; Mohd, S.; Zaharin, A. Total Mercury (THg), Lead (Pb), Cadmium (Cd) and Arsenic (As) in Hair Samples: Method Validation and Quantification among Women at Reproductive Age in Selangor. Int. J. Sci. Basic Appl. Res. 2015, 24, 332–347. [Google Scholar]
- Bobakera, A.M.; Alakili, I.; Elkhidirb, E.E.; Sarmani, S.B. Predict and Describe Relationships between the Levels of Methylmercury in Hair Samples of Kuala Lumpur Residents and Their Social Characteristics. Asian J. Microbiol. Biotechnol. Environ. Sci. 2008, 10, 749–752. [Google Scholar]
- Barbosa, A.C.; Jardim, W.; Dórea, J.G.; Fosberg, B.; Souza, J. Hair Mercury Speciation as a Function of Gender, Age, and Body Mass Index in Inhabitants of the Negro River Basin, Amazon, Brazil. Arch. Environ. Contam. Toxicol. 2001, 40, 439–444. [Google Scholar] [PubMed]
- Jedrychowski, W.; Perera, F.; Rauh, V.; Flak, E.; Mróz, E.; Pac, A.; Skolicki, Z.; Kaim, I. Fish Intake during Pregnancy and Mercury Level in Cord and Maternal Blood at Delivery: An Environmental Study in Poland. Int. J. Occup. Med. Environ. Health 2007, 20, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Farida el Baz, M.; Eman Ahmed, Z.; Adel Bassuoni, E.; Reham Mohameed, E.; Sally Soliman, Z.; Waleed Salah, E.; Walaa Yousef, Y.; Rania Abdelmgeed, K.; Azza Mohamed, Y. Assessment of Hair Aluminum, Lead, and Mercury in a Sample of Autistic Egyptian Children: Environmental Risk Factors of Heavy Metals in Autism. Behav. Neurol. 2015, 2015, 545674. [Google Scholar]
- Lederman, S.A.; Jones, R.L.; Caldwell, K.L.; Rauh, V.; Sheets, S.E.; Tang, D.; Viswanathan, S.; Becker, M.; Stein, J.L.; Wang, R.Y.; et al. Relation between Cord Blood Mercury Levels and Early Child Development in a World Trade Center Cohort. Environ. Health Perspect. 2008, 116, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Boucher, O.; Jacobson, S.W.; Plusquellec, P.; Dewailly, É.; Ayotte, P.; Forget-Dubois, N.; Jacobson, J.L.; Muckle, G. Prenatal Methylmercury, Postnatal Lead Exposure, and Evidence of Attention Deficit/hyperactivity Disorder among Inuit Children in Arctic Québec. Environ. Health Perspect. 2012, 120, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Debes, F.; Budtz-Jørgensen, E.; Weihe, P.; White, R.F.; Grandjean, P. Impact of Prenatal Methylmercury Exposure on Neurobehavioral Function at Age 14 Years. Neurotoxicol. Teratol. 2006, 28, 363–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandjean, P.; Weihe, P.; White, R.; Debes, F.; Araki, S.; Yokoyama, K.; Murata, K.; Sorensen, N.; Dahl, R.; Jorgensen, P. Cognitive Deficit in 7 Year Old Children with Prenatal Exposure to Methylmercury. Neurotoxicol. Teratol. 1997, 19, 417–428. [Google Scholar] [CrossRef]
- Golding, J.; Gregory, S.; Emond, A.; Iles-Caven, Y.; Hibbeln, J.; Taylor, C.M. Prenatal Mercury Exposure and Offspring Behaviour in Childhood and Adolescence. Neurotoxicology 2016, 57, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Bernardi, J.V.E.; Dórea, J.G.; Brandão, K.G.; Bueno, L.; Leão, R.S.; Malm, O. Fish Consumption during Pregnancy, Mercury Transfer, and Birth Weight along the Madeira River Basin in Amazonia. Int. J. Environ. Res. Public Health 2013, 10, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Radesky, J.S.; Wright, R.O.; Bellinger, D.C.; Amarasiriwardena, C.J.; Kleinman, K.P.; Hu, H.; Gillman, M.W. Maternal Fish Intake during Pregnancy, Blood Mercury Levels, and Child Cognition at Age 3 Years in a US Cohort. Am. J. Epidemiol. 2008, 167, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Leino, O.; Karjalainen, A.K.; Tuomisto, J.T. Effects of Docosahexaenoic Acid and Methylmercury on Child’s Brain Development due to Consumption of Fish by Finnish Mother during Pregnancy: A Probabilistic Modeling Approach. Food Chem. Toxicol. 2013, 54, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, L.; Abbott, L.C. Effects of Methyl Mercury Exposure on Pancreatic Beta Cell Development and Function. J. Appl. Toxicol. 2017, 37, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Benefice, E.; Monrroy, S.J.L.; Rodriguez, R.W.L. A Nutritional Dilemma: Fish Consumption, Mercury Exposure and Growth of Children in Amazonian Bolivia. Int. J. Environ. Health Res. 2008, 18, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Hajeb, P.; Jinap, S.; Ahmad, I. Biomagnifications of Mercury and Methylmercury in Tuna and Mackerel. Environ. Monit. Assess. 2010, 171, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Junqué, E.; Garí, M.; Arce, A.; Torrent, M.; Sunyer, J.; Grimalt, J.O. Integrated Assessment of Infant Exposure to Persistent Organic Pollutants and Mercury via Dietary Intake in a Central Western Mediterranean Site (Menorca Island). Environ. Res. 2017, 156, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Llull, R.M.; Garí, M.; Canals, M.; Rey-Maquieira, T.; Grimalt, J.O. Mercury Concentrations in Lean Fish from the Western Mediterranean Sea: Dietary Exposure and Risk Assessment in the Population of the Balearic Islands. Environ. Res. 2017, 158, 16–23. [Google Scholar] [CrossRef] [PubMed]
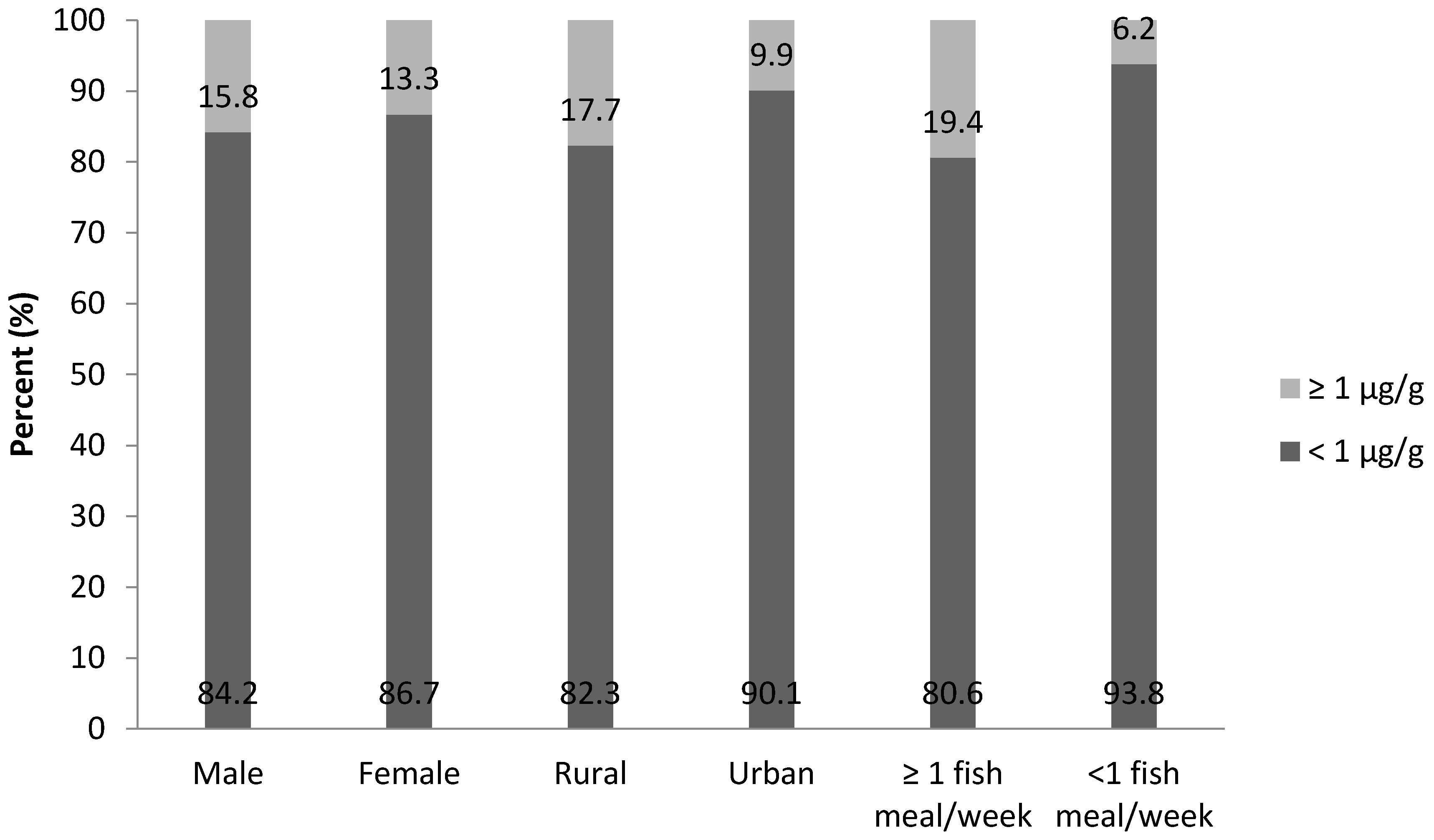
| Characteristics | N (%) | Total Hg, µg/g GM (Range) a | p-Value |
|---|---|---|---|
| Total | 215 (100) | 0.47 (0.01–4.67) | |
| Gender | |||
| Male | 95 (44) | 0.46 (0.01–4.67) | 0.867 |
| Female | 120 (56) | 0.47 (0.03–3.97) | |
| Location | |||
| Urban | 91 (42) | 0.41 (0.01–3.97) | 0.001 * |
| Rural | 124 (58) | 0.55 (0.03–4.67) | |
| Race | |||
| Bumiputera | 205 (95) | 0.47 (0.01–4.67) | 0.458 |
| Non-Bumiputera | 10 (5) | 0.57 (0.12–3.97) | |
| Fish consumption | |||
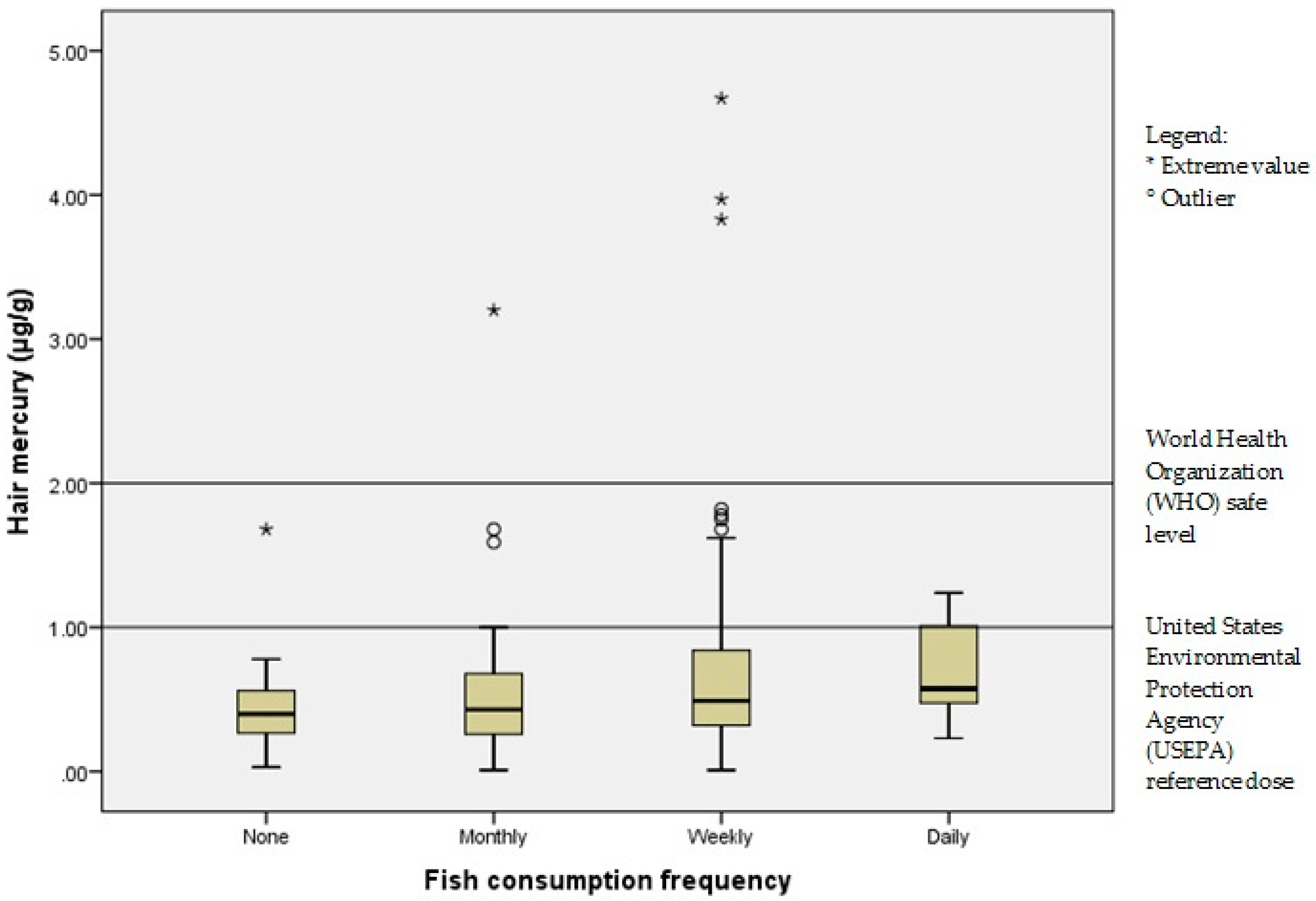
| Never | 11 (5) | 0.33 (0.03–1.68) | 0.047 *,+ |
| Monthly | 70 (33) | 0.40 (0.01–3.20) | |
| Weekly | 122 (57) | 0.51 (0.01–4.67) | |
| Daily | 12 (6) | 0.64 (0.23–1.24) | |
| Smoking status | |||
| Non-smoker | 121 (56) | 0.43 (0.01–1.82) | 0.072 |
| Passive smoker | 94 (44) | 0.53 (0.09–4.67) | |
| Amalgam filling | |||
| No | 162 (75) | 0.44 (0.01–4.67) | 0.033 * |
| Yes | 53 (25) | 0.55 (0.14–1.82) |
| Location | AM a (µg/g) | GM b (µg/g) | Range | N | Remarks |
|---|---|---|---|---|---|
| Malaysia (this study) | 0.63 | 0.47 | 0.01–4.67 | 215 | 11 years old |
| Tarragona, Spain [13] | - | 0.77 | 0.30–2.44 | 233 | 6–16 years old |
| Belgium [14] | - | 0.20 | <LOQ d–1.99 | 129 | 6–11 years old |
| China [15] | 0.11 | 0.10 | 0.03–0.52 | 184 | 10–13 years old |
| United States [16] | 0.22 | 0.12 | 0.18–0.25 | 838 | 1–5 years old |
| German [17] | 0.23 | 0.18 | 0.06–1.70 | 245 | 8–10 years old |
| Korea [18] | 0.74 | 0.62 | 0.12–3.46 | 112 | <15 years old |
| Korea [15] | 0.76 | 0.69 | 0.27–2.24 | 54 | 10–12 years old |
| Spain [19] | 0.94 | - | 0.19–5.63 | 136 | Pre school |
| Japan [20] | 1.65 c | - | 0.27–6.32 | 327 | 7 years old |
| Maranhao, Brazil [21] | 2.27 | - | 0.13–9.54 | 139 | <12 years old |
| Spain [22] | 1.4 | 0.99 | 0.04–10.0 | 302 | 4 years old |
| Variable | B | S.E. | Odds Ratio (95% CI) | p Value |
|---|---|---|---|---|
| Location (rural) | 0.40 | 0.44 | 1.50 (0.63–3.55) | 0.359 |
| BMI for age | 0.05 | 0.04 | 1.06 (0.98–1.14) | 0.169 |
| Fish consumption once a week | 1.31 | 0.53 | 3.69 (1.31–10.36) | 0.013 * |
| Mom ate fish during pregnancy | 0.65 | 0.49 | 1.92 (0.73–5.02) | 0.186 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Samad, N.I.; Md Isa, Z.; Hod, R. Mercury Hair Concentration among Primary School Children in Malaysia. Children 2017, 4, 109. https://doi.org/10.3390/children4120109
Abdul Samad NI, Md Isa Z, Hod R. Mercury Hair Concentration among Primary School Children in Malaysia. Children. 2017; 4(12):109. https://doi.org/10.3390/children4120109
Chicago/Turabian StyleAbdul Samad, Nurul Izzah, Zaleha Md Isa, and Rozita Hod. 2017. "Mercury Hair Concentration among Primary School Children in Malaysia" Children 4, no. 12: 109. https://doi.org/10.3390/children4120109





