Do Mothers Benefit from a Child-Focused Cognitive Behavioral Treatment (CBT) for Childhood Functional Abdominal Pain? A Randomized Controlled Pilot Trial
Abstract
:1. Introduction
2. Materials and Methods
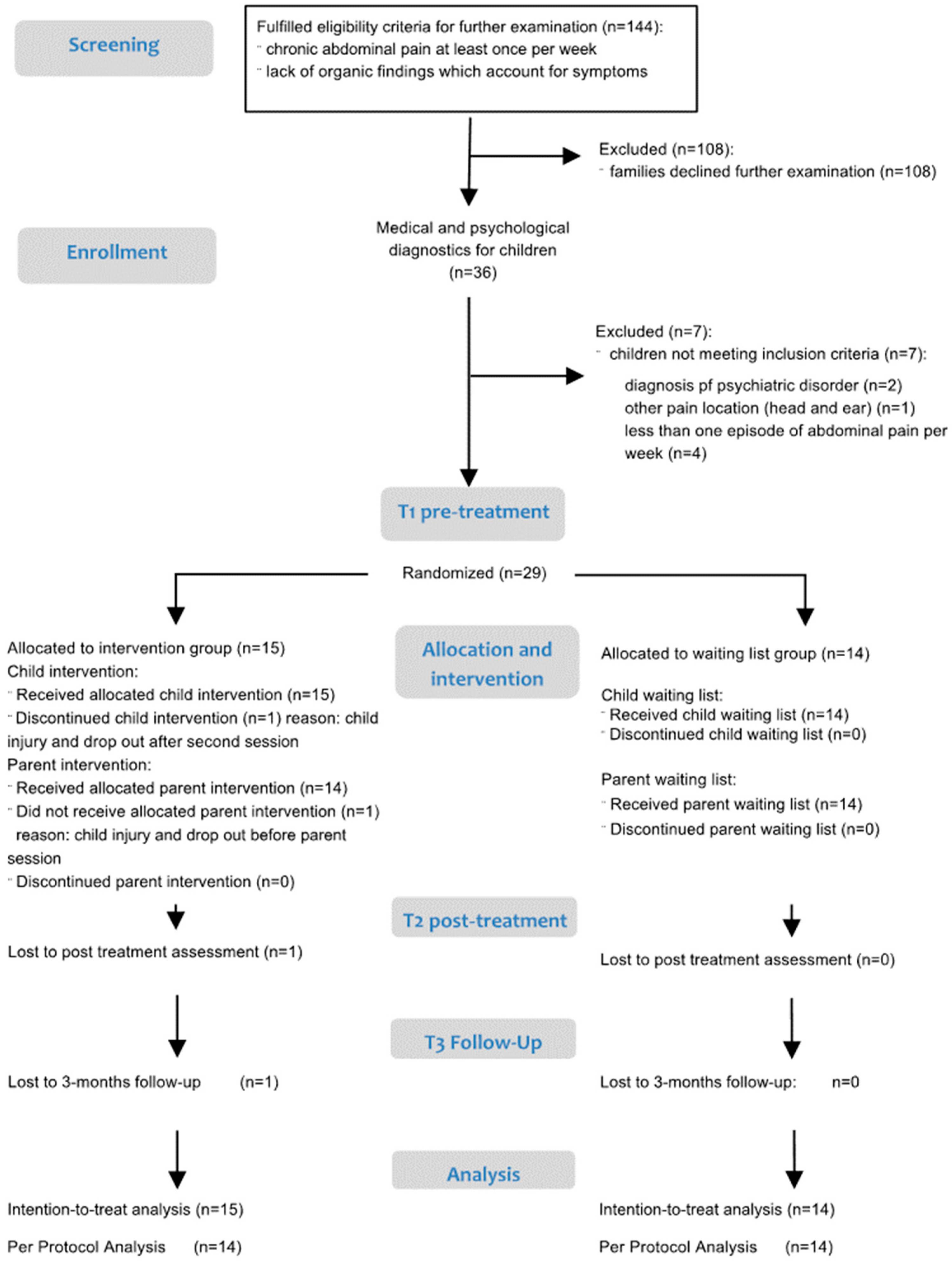
2.1. Study Design and Procedure
2.2. Intervention
2.3. Measures
2.3.1. Demographic and Socioeconomic Measures
2.3.2. Treatment Satisfaction
2.3.3. Parental Perceptions of Changes in Abdominal Pain
2.3.4. Parent-Reported Outcome Measures
- (1)
- Gastrointestinal Symptoms: Mothers were asked to report on their child’s GI symptoms in accordance with the current Rome-III classification (“My child was suffering during the last month from…”; six items: abdominal pain, diarrhea, constipation, nausea, vomiting, bloating; [33]). Items of this symptom list were answered on a five-point frequency scale (never, almost never, sometimes, often, almost always). We calculated a mean score as an indicator of GI symptom severity, which yielded an acceptable internal consistency (Cronbach’s alpha = 0.72).
- (2)
- Parental pain-related behavior: A self-developed 24-item questionnaire was administered, covering different adaptive and maladaptive aspects of parental behavior and worries related to their child’s abdominal pain. Following the introductory sentence, “When my child has abdominal pain, …” mothers rated their reactions on a five-point Likert scale (never, seldom, sometimes, often, always). The inventory comprises the following six subscales: “protection” (five items, e.g., “let him/her stay home from school”; “allow things which are usually forbidden”; α = 0.77), “attention” (6 items, e.g., “I comfort my child”; α = 0.77), “medical help-seeking and home remedies” (4 items, e.g., ”I consult a doctor”; “I give him a hot water bottle”; α = 0.72), “support child’s coping” (3 items, e.g., “I support my child to distract on his own”; “I support my child to relax on his own”; α = 0.86) , and “worries” (6 items, e.g., “I am worried”; “I am concerned about my child’s future”; α = 0.83). Compilation of the items was based on literature of parent behavior [38,39] and pre-tested in another pilot-study [40]. Psychometric properties were sufficient with internal consistencies >0.70 for all scales and sufficient selectivity for all items (part-whole-correlations >0.30). As hypothesized, the scale “protection” showed significant positive correlations to attention (rT1 = 0.406, p = 0.029), medical help-seeking (rT1 = 0.404, p = 0.030) and worries (rT1 = 0.436, p = 0.018). The negative correlation to the scale “support child’s coping” was not statistically significant (rT1 = −0.189, p = 0.327).
- (3)
- Parental pain-related self-efficacy: We adapted a validated, illness-specific self-efficacy scale for parents with children suffering from atopic dermatitis or asthma [41,42] to measure pain-specific self-efficacy expectations. The pain-specific self-efficacy scale was preceded by a short introduction (“Dealing with abdominal pain challenges you. We would like to know how confident you are about dealing with those challenges.”). The nine-item scale covers self-efficacy expectations with respect to preventive actions (two items; e.g., “My child spontaneously wants to do something that could worsen the pain. I am able to refuse his/her wish.”), being consequent (two items; e.g., “My child is in pain in the morning and doesn’t want to go to school. I am able to encourage my child to go to school.”), as well as intervening actions when the child is in acute pain (Five items; e.g., “My child is in hard pain. I am able to distract my child.”). The items were answered on a six-point Likert scale (“very unsure”–“very sure”). Internal consistency of the total score for parental pain-specific self-efficacy was acceptable (Cronbach’s alpha = 0.72).
- (4)
- General self-efficacy: Self-efficacy was measured by the validated and broadly used General Self-Efficacy Scale (GSES; [43]). Schwarzer and Jerusalem [44] report internal consistencies from 0.82–0.93 and a 2-year retest-reliability of 0.47 (men) and 0.63 (women). The GSES total score is positively associated with self-esteem and optimism, and negatively correlated with anxiety and depression [43]. The GSES covers 10 items, enquiring about the subjective evaluation of one’s ability to cope with new or difficult demands in life (e.g., “When there are obstacles, I find my way to assert myself”; “I can find a solution for every problem”). Answer format was four-point (“not true”, “hardly true”, “somewhat true”, “exactly true”). Higher raw scores are associated with higher perceived self-efficacy. Internal consistency in our sample was excellent (Cronbach’s alpha = 0.92).
- (5)
- Parental quality of life: We used the “Ulm Quality of Life Inventory for Parents of Chronically Ill Children” (ULQUIE) [45] to assess mothers’ self-reported quality of life. The questionnaire was developed for parents of chronically ill children and has been shown to discriminate between clinical and non-clinical groups, as well as different stages of illness [45]. The authors had reported acceptable to good internal consistency for the total score in the validation study on 244 parents of chronically ill children [45]. The 29 items have a five-point answer format (never, seldom, sometimes, often, always) and refer to the last seven days. The wording was adapted to our study and “chronic illness” was replaced by “abdominal pain”. We used the total score as a global measure for mothers quality of life, which yielded excellent internal consistency in our sample (Cronbach’s α = 0.91).
2.4. Data Analysis
3. Results
3.1. Sample and Baseline Pain Characteristics
3.2. Initial Analyses
Treatment Satisfaction
3.3. Primary Outcomes
3.3.1. Maternal Perceptions of Changes in Pain
3.3.2. Gastrointestinal (GI) Symptoms
3.3.3. Pain-Related Self-Efficacy
3.3.4. Maternal Pain-Related Behavior
3.4. Secondary Outcomes
3.4.1. General Self-Efficacy
3.4.2. Maternal Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Korterink, J.J.; Diederen, K.; Benninga, M.A.; Tabbers, M.M. Epidemiology of pediatric functional abdominal pain disorders: A meta-analysis. PLoS ONE 2015, 10, e0126982. [Google Scholar] [CrossRef] [PubMed]
- Warschburger, P.; Hänig, J.; Friedt, M.; Posovszky, C.; Schier, M.; Calvano, C. Health-related quality of life in children with abdominal pain due to functional or organic gastrointestinal disorders. J. Pediatr. Psychol. 2014, 39, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lisman-van Leeuwen, Y.; Spee, L.A.A.; Benninga, M.A.; Bierma-Zeinstra, S.M.A.; Berger, M.Y. Prognosis of abdominal pain in children in primary care—A prospective cohort study. Ann. Fam. Med. 2013, 11, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Gulewitsch, M.D.; Enck, P.; Schwille-Kiuntke, J.; Weimer, K.; Schlarb, A.A. Rome III criteria in parents’ hands: Pain-related functional gastrointestinal disorders in community children and associations with somatic complaints and mental health. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Campo, J.V.; Bridge, J.; Ehmann, M.; Altman, S.; Lucas, A.; Birmaher, B.; Di Lorenzo, C.; Iyengar, S.; Brent, D.A. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics 2004, 113, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Hägglöf, B.L.; Bergström, E.I. Impaired health-related quality of life in children with recurrent pain. Pediatrics 2009, 124, e759–e767. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.W.; Bendo, C.B.; Nurko, S.; Shulman, R.J.; Self, M.M.; Franciosi, J.P.; Saps, M.; Pohl, J.F. Health-related quality of life in pediatric patients with functional and organic gastrointestinal diseases. J. Pediatr. 2015, 166, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Chambers, C.T. Parent and family factors in pediatric chronic pain and disability: An integrative approach. Pain 2005, 119, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Valrie, C.R.; Karlson, C.W. Family and parent influences on pediatric chronic pain: A developmental perspective. Am. Psychol. 2014, 69, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.; Claar, R.; Garber, J. Social consequences of children’s pain: When do they encourage symptom maintenance? J. Pediatr. Psychol. 2002, 27, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.L.; Whitehead, W.E.; Walker, L.S.; von Korff, M.; Feld, A.D.; Garner, M.; Christie, D. Increased somatic complaints and health-care utilization in children: Effects of parent IBS status and parent response to gastrointestinal symptoms. Am. J. Gastroenterol. 2004, 99, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.C.; Palermo, T.M. Parental reinforcement of recurrent pain: The moderating impact of child depression and anxiety on functional disability. J. Pediatr. Psychol. 2004, 29, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Levy, R.L.; Whitehead, W.E. Validation of a measure of protective parent responses to children’s pain. Clin. J. Pain 2006, 22, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Campo, J.V.; Bridge, J.; Lucas, A.; Savorelli, S.; Walker, L.; Di Lorenzo, C.; Iyengar, S.; Brent, D.A. Physical and emotional health of mothers of youth with functional abdominal pain. Arch. Pediatr. Adolesc. Med. 2007, 161, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Crombez, G.; Scotford, A.; Clinch, J.; Connell, H. Adolescent chronic pain: Patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain 2004, 108, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Lipani, T.A.; Walker, L.S. Children’s appraisal and coping with pain: relation to maternal ratings of worry and restrictions in family activities. J. Pediatr. Psychol. 2006, 31, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Eccleston, C.; Lewandowski, A.S.; Williams, A.C.; Morley, S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: An updated meta-analytic review. Pain 2010, 148, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, L.; Gerhards, F.; Goldbeck, L. Effects of psychological treatment on recurrent abdominal pain in children—A meta-analysis. Clin. Psychol. Rev. 2001, 31, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Palermo, T.M.; Williams, A.C.; Lewandowski Holley, A.; Morley, S.; Fisher, E.; Law, E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev. 2012, 12, CD003968. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Palermo, T.M.; Williams, A.C.; Lewandowski Holley, A.; Morley, S.; Fisher, E.; Law, E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev. 2014, 5, CD003968. [Google Scholar] [CrossRef] [Green Version]
- Eccleston, C.; Palermo, T.M.; Fisher, E.; Law, E. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst. Rev. 2012, 8, CD009660. [Google Scholar] [CrossRef] [Green Version]
- Eccleston, C.; Fisher, E.; Law, E.; Bartlett, J.; Palermo, T.M. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst. Rev. 2015, 4. [Google Scholar] [CrossRef]
- Levy, R.L.; Langer, S.L.; Walker, L.S.; Romano, J.M.; Christie, D.L.; Youssef, N.; DuPen, M.M.; Feld, A.D.; Ballard, S.A.; Welsh, E.M.; et al. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. Am. J. Gastroenterol. 2010, 105, 946–956. [Google Scholar] [CrossRef]
- Levy, R.L.; Langer, S.L.; Walker, L.S.; Romano, J.M.; Christie, D.L.; Youssef, N.; DuPen, M.M.; Ballard, S.A.; Labus, J.; Welsh, E.; et al. Twelve-month follow-up of cognitive behavioral therapy for children with functional abdominal pain. JAMA Pediatr. 2013, 167, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Law, E.F.; Fales, J.; Bromberg, M.H.; Jessen-Fiddick, T.; Tai, G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: A randomized controlled multicenter trial. Pain 2016, 157, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Self-Efficacy: The Exercise of Self-Control; W.H. Freeman: New York, NY, USA, 1997. [Google Scholar]
- Jones, V.; Whitehead, L.; Crowe, M.T. Self-efficacy in managing chronic respiratory disease: Parents’ experiences. Contemp. Nurse 2016, 52, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bursch, B.; Tsao, J.C.I.; Meldrum, M.; Zeltzer, L.K. Preliminary validation of a self-efficacy scale for child functioning despite chronic pain (child and parent versions). Pain 2006, 125, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Groß, M.; Warschburger, P. Chronische Bauchschmerzen im Kindesalter: Das “Stopp-den-Schmerz-mit-Happy-Pingu”-Programm; Hogrefe: Göttingen, Germany, 2011. [Google Scholar]
- Groß, M.; Warschburger, P. Chronic abdominal pain: Psychosocial strain and treatment-associated changes in coping. Verhaltenstherapie 2013, 23, 80–89. [Google Scholar] [CrossRef]
- Groß, M.; Warschburger, P. Evaluation of a cognitive-behavioral pain management program for children with chronic abdominal pain: A randomized controlled study. Int. J. Behav. Med. 2013, 20, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S. Kinder-DIPS: Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter, 2nd ed.; Springer: Heidelberg, Germany, 2009. [Google Scholar]
- Rasquin, A.; Di Lorenzo, C.; Forbes, D.; Guiraldes, E.; Hyams, J.S.; Staiano, A.; Walker, L.S. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2006, 130, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- SCAP. Chronic abdominal pain in children: Clinical Report. Pediatrics 2005, 115, 812–815. [Google Scholar]
- Woerner, W.; Becker, A.; Rothenberger, A. Normative data and scale properties of the German parent SDQ. Eur. Child. Adolesc. Psychiatry 2004, 13 (Suppl. 2), ii3–ii10. [Google Scholar] [CrossRef] [PubMed]
- Blossfeld, H.-P. Berufseintritt und Berufsverlauf: Eine Kohortenanalyse über die Bedeutung des ersten Berufs in der Erwerbsbiographie. Mitt. Arb.- Berufsforsch. 1985, 18, 177–197. [Google Scholar]
- Mattejat, F.; Remschmidt, H. Fragebögen zur Beurteilung der Behandlung (FBB); Hogrefe: Göttingen, Germany, 1999. [Google Scholar]
- Lohaus, A.; Klein-Heßling, J. Problemlösungen sind erlernbar. Zur Evaluation von Stressbewältigungs- und Entspannungstrainings für Kinder im Grundschulalter. Rep. Psychol. 2003, 28, 96–102. [Google Scholar]
- Sharrer, V.W.; Ryan-Wenger, N.M. Measurements of stress and coping among school-aged children with and without recurrent abdominal pain. J. Sch. Health 1991, 61, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Groß, M. Recurrent Abdominal Pain in Childhood. Development and Evaluation of Cognitive-Behavioral Pain Management Program—A Pilot Study; Diploma Psychology, University of Potsdam: Potsdam, Germany, May 2007. [Google Scholar]
- Buchholz, H.T.; Warschburger, P.; von Schwerin, A.-D.; Petermann, F. SEND: Eine Skala zur Erhebung der spezifischen Selbstwirksamkeit für Eltern neurodermitiskranker Vorschulkinder. Z. Med. Psychol. 2003, 12, 63–68. [Google Scholar]
- Warschburger, P.; von Schwerin, A.-D.; Buchholz, T.; Petermann, F. Eine Skala zur Erfassung von elterlichen Selbstwirksamkeitserwartungen im Umgang mit dem Asthma ihres Kindes. Z. Klin. Psychol. Psychother. 2003, 32, 184–190. [Google Scholar] [CrossRef]
- Schwarzer, R.; Jerusalem, M. Skalen zur Erfassung von Lehrer- und Schülermerkmalen: Dokumentation der Psychometrischen Verfahren im Rahmen der Wissenschaftlichen Begleitung des Modellversuchs Selbstwirksame Schulen; Freie Universität Berlin: Berlin, Germany, 1999. [Google Scholar]
- Schwarzer, R.; Jerusalem, M. Generalized Self-Efficacy scale. In Measures in Health Psychology: A User’s Portfolio; Weinman, J., Wright, S., Johnston, M., Eds.; NFER-NELSON: Windsor, UK, 1995; pp. 35–37. [Google Scholar]
- Goldbeck, L.; Storck, M. ULQIE: A quality-of-life inventory for parents of chronically ill children. Z. Klin. Psychol. Psychother. 2002, 31, 31–39. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using SPSS (and Sex and Drugs and Rock ‘n’ Roll), 3rd ed.; SAGE Publications: Los Angeles, SC, USA, 2009. [Google Scholar]
- Fisher, E.; Heathcote, L.; Palermo, T.M.; Williams, A.C.; Lau, J.; Ellert, U.; Neuhauser, H.; Roth-Isigkeit, A. Systematic review and meta-analysis of psychological therapies for children with chronic pain. J. Pediatr. Psychol. 2014, 39, 763–782. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Ceballos, A.A.; Logan, S.; Bennett, C.; Macarthur, C. Psychosocial interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst. Rev. 2008, CD003014. [Google Scholar] [CrossRef]
- Sanders, M.R.; Shepherd, R.W.; Cleghorn, G.; Woolford, H. The treatment of recurrent abdominal pain in children: A controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J. Consult. Clin. Psychol. 1994, 62, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Robins, P.M.; Smith, S.M.; Glutting, J.J.; Bishop, C.T. A randomized controlled trial of a cognitive-behavioral family intervention for pediatric recurrent abdominal pain. J. Pediatr. Psychol. 2005, 30, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Frerker, M.; Hechler, T.; Schmidt, P.; Zernikow, B. [Pain-related parental behavior: Maternal and paternal responses to chronic pain of their child and modifications following inpatient interdisciplinary pain treatment]. Schmerz 2016, 30, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Crushell, E.; Rowland, M.; Doherty, M.; Gormally, S.; Harty, S.; Bourke, B.; Drumm, B. Importance of parental conceptual model of illness in severe recurrent abdominal pain. Pediatrics 2003, 112, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Law, E.F.; Fisher, E.; Fales, J.; Noel, M.; Eccleston, C. Systematic review and meta-analysis of parent and family-based interventions for children and adolescents with chronic medical conditions. J. Pediatr. Psychol. 2014, 39, 866–886. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Lewandowski Holley, A. The importance of the family environment in pediatric chronic pain. JAMA Pediatr. 2013, 167, 93–94. [Google Scholar] [CrossRef]
- Levy, R.L.; Langer, S.L.; Romano, J.M.; Labus, J.; Walker, L.S.; Murphy, T.B.; van Tilburg, M.A.L.; Feld, L.D.; Christie, D.L.; Whitehead, W.E. Cognitive mediators of treatment outcomes in pediatric functional abdominal pain. Clin. J. Pain 2014, 30, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Schumacher, J.; Albani, C.; Schmid, G.; Brähler, E. Bevölkerungsrepräsentative Normierung der Skala zur Allgemeinen Selbstwirksamkeit. Diagnostica 2006, 52, 26–32. [Google Scholar] [CrossRef]
- Romppel, M.; Herrmann-Lingen, C.; Wachter, R.; Edelmann, F.; Düngen, H.D.; Pieske, B.; Grande, G.A. Short form of the General Self-Efficacy Scale (GSE-6): Development, psychometric properties and validity in an intercultural non-clinical sample and a sample of patients at risk for heart failure. GMS Psychosoc. Med. 2013, 10, Doc01. [Google Scholar] [PubMed]
- Lacasse, A.; Bourgault, P.; Tousignant-Laflamme, Y.; Courtemanche-Harel, R.; Choiniere, M. Development and validation of the French-Canadian Chronic Pain Self-efficacy Scale. Pain Res. Manag. 2015, 20, 75–83. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, L.A.; Kowal, J.; Wilson, K.G. Development and evaluation of short forms of the Pain Catastrophizing Scale and the Pain Self-efficacy Questionnaire. Eur. J. Pain 2015, 19, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Warschburger, P.; von Schwerin, A.D.; Buchholz, H.T.; Petermann, F. An educational program for parents of asthmatic preschool children: Short- and medium-term effects. Patient Educ. Couns. 2003, 51, 83–91. [Google Scholar] [CrossRef]
- Goldbeck, L. The impact of newly diagnosed chronic paediatric conditions on parental quality of life. Qual. Life Res. 2006, 15, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- West, C.A.; Besier, T.; Borth-Bruhns, T.; Goldbeck, L. Effectiveness of a family-oriented rehabilitation program on the quality of life of parents of chronically ill children. Klin. Padiatr. 2009, 221, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Greenley, R.N.; Cunningham, C. Parent quality of life in the context of pediatric inflammatory bowel disease. J. Pediatr. Psychol. 2009, 34, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Calvano, C.; Warschburger, P. Chronic abdominal pain in children and adolescents: Parental threat perception plays a major role in seeking medical consultations. Pain Res. Manag. 2016, 2016, 3183562. [Google Scholar] [CrossRef] [PubMed]
- Warschburger, P.; Calvano, C.; Becker, S.; Friedt, M.; Hudert, C.; Posovszky, C.; Schier, M.; Wegscheider, K. Stop the pain: Study protocol for a randomized-controlled trial. Trials 2014, 15, 357. [Google Scholar] [CrossRef]
| Variables | IG (n = 15) | WLC (n = 14) | p-Value |
|---|---|---|---|
| Single parent | 0.280 | ||
| yes | 14 | 11 | |
| no | 1 | 3 | |
| Educational level mother | 0.362 | ||
| ≤10 years | 5 | 7 | |
| >10 years | 10 | 7 | |
| Educational level father | 0.909 | ||
| ≤10 years | 4 | 4 | |
| >10 years | 11 | 10 | |
| Job situation mother | |||
| not employed | 1 | 1 | 0.960 |
| (self-) employed | 14 | 13 | |
| Job situation father | |||
| not employed | 0 | 0 | NA |
| (self-) employed | 15 | 14 | |
| Number of siblings | |||
| M (SD) | 1.79 (1.67) | 1.50 (0.76) | 0.566 |
| range | 0–7 | 1–3 | |
| Psychological distress of the child 1 | |||
| Total problem score | |||
| Normal | 8 | 11 | 0.415 |
| Borderline | 5 | 2 | |
| Abnormal | 1 | 1 | |
| Abdominal pain intensity (last 7 days) | |||
| M (SD) | 3.00 (1.00) | 2.64 (1.28) | 0.407 |
| range | 1–4 | 1–5 | |
| Abdominal pain frequency * | |||
| M | 9.13 | 11.61 | 0.396 |
| SD | (4.91) | (9.55) | |
| range | 2–20 | 3–30 | |
| Abdominal pain duration ** | |||
| M (SD) | 57.13 (34.41) | 61.79 (73.00) | 0.826 |
| range | 15–120 | 10–300 |
| IG n = 15 | WLC n = 14 | Main Effect Time | Main Effect Group | Group × Time Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p a/d a | Post Hoc Test b | |||||||||
| M | SD | M | SD | p/d | p/d | F | p | d | ||
| Gastrointestinal Symptoms | ||||||||||
| Baseline | 32.22 | 11.72 | 26.49 | 15.90 | <0.001/1.36 | 0.492/0.27 | 0.003/0.97 | 1.722 (1–2) | 0.201 (1–2) | 0.51 (1–2) |
| Post | 18.61 | 11.12 | 20.24 | 12.43 | 5.390 (2–3) | 0.028 (2–3) | 0.89 (2–3) | |||
| Follow-up | 11.67 | 9.61 | 23.81 | 16.24 | 13.128 (1–3) | 0.001 (1–3) | 1.39 (1–3) | |||
| Pain-related self-efficacy | ||||||||||
| Baseline | 65.33 | 12.79 | 72.22 | 7.98 | 0.059/0.70 | 0.730/0.13 | 0.011/0.92 | 5.483 (1–2) | 0.027 (1–2) | 0.90 (1–2) |
| Post | 73.63 | 11.93 | 70.00 | 9.58 | 1.101 (2–3) | 0.303 (2–3) | 0.40 (2–3) | |||
| Follow-up | 77.63 | 13.92 | 70.79 | 10.38 | 7.496 (1–3) | 0.011 (1–3) | 1.05 (1–3) | |||
| General self-efficacy | ||||||||||
| Baseline | 70.44 | 15.78 | 69.74 | 13.84 | 0.030/0.76 | 0.705/0.16 | 0.818/0.18 | NA | - | - |
| Post | 73.33 | 12.60 | 70.25 | 13.43 | - | - | - | |||
| Follow-up | 76.22 | 15.47 | 74.10 | 16.79 | - | - | - | |||
| Quality of life | ||||||||||
| Baseline | 76.03 | 6.73 | 67.86 | 12.70 | 0.278/0.43 | 0.886/0.06 | 0.487/0.28 | - | - | - |
| Post | 71.55 | 11.40 | 65.70 | 10.96 | - | - | - | |||
| Follow-up | 77.87 | 11.25 | 72.29 | 14.78 | - | - | - | |||
| IG n = 15 | WLC n = 14 | Main Effect Time | Main Effect Group | Group × Time Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p a/d a | Post Hoc Test b | |||||||||
| M | SD | M | SD | p/d | p/d | F | p | d | ||
| Attention | ||||||||||
| Baseline | 51.39 | 19.71 | 57.14 | 12.17 | 0.001/1.04 | <0.001/1.59 | 0.004/0.95 | 9.746 (1–2) | 0.004 (1–2) | 1.20 (1–2) |
| Post | 34.72 | 12.27 | 58.93 | 16.00 | 0.044 (2–3) | 0.835 (2–3) | 0.09 (2–3) | |||
| Follow-up | 31.39 | 13.81 | 54.46 | 12.71 | 7.320 (1–3) | 0.012 (1–3) | 1.04 (1–3) | |||
| Protection | ||||||||||
| Baseline | 17.67 | 17.61 | 28.93 | 18.52 | 0.109/0.59 | 0.0004/1.21 | 0.466/0.34 | NA | – | – |
| Post | 12.33 | 15.34 | 29.29 | 17.41 | – | – | – | |||
| Follow-up | 8.00 | 9.60 | 26.07 | 16.66 | – | – | – | |||
| Support coping | ||||||||||
| Baseline | 51.67 | 19.21 | 60.12 | 11.40 | <0.001/1.17 | 0.0342/0.37 | 0.002/1.03 | 8.147 (1–2) | 0.008 (1–2) | 1.10 (1–2) |
| Post | 69.44 | 17.16 | 59.52 | 15.28 | 0.276 (2–3) | 0.603 (2–3) | 0.20 (2–3) | |||
| Follow-up | 75.56 | 17.10 | 62.50 | 17.30 | 11.994 (1–3) | 0.002 (1–3) | 1.33 (1–3) | |||
| Help-seeking | ||||||||||
| Baseline | 28.75 | 15.99 | 30.80 | 15.97 | <0.001/1.46 | 0.032/0.87 | 0.005/0.92 | 5.891 (1–2) | 0.022 (1–2) | 0.93 (1–2) |
| Post | 12.08 | 10.94 | 25.00 | 13.31 | 1.100 (2–3) | 0.304 (2–3) | 0.40 (2–3) | |||
| Follow-up | 11.67 | 11.54 | 28.57 | 16.39 | 8.108 (1–3) | 0.008 (1–3) | 1.10 (1–3) | |||
| Worries | ||||||||||
| Baseline | 41.11 | 19.79 | 36.01 | 20.39 | <0.001/1.69 | 0.222/0.48 | 0.002/1.03 | 7.133 (1–2) | 0.013 (1–2) | 1.03 (1–2) |
| Post | 21.11 | 17.21 | 32.44 | 16.19 | 0.444 (2–3) | 0.511 (2–3) | 0.26 (2–3) | |||
| Follow-up | 14.72 | 13.90 | 28.87 | 13.02 | 10.120 (1–3) | 0.004 (1–3) | 1.23 (1–3) | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvano, C.; Groß, M.; Warschburger, P. Do Mothers Benefit from a Child-Focused Cognitive Behavioral Treatment (CBT) for Childhood Functional Abdominal Pain? A Randomized Controlled Pilot Trial. Children 2017, 4, 13. https://doi.org/10.3390/children4020013
Calvano C, Groß M, Warschburger P. Do Mothers Benefit from a Child-Focused Cognitive Behavioral Treatment (CBT) for Childhood Functional Abdominal Pain? A Randomized Controlled Pilot Trial. Children. 2017; 4(2):13. https://doi.org/10.3390/children4020013
Chicago/Turabian StyleCalvano, Claudia, Martina Groß, and Petra Warschburger. 2017. "Do Mothers Benefit from a Child-Focused Cognitive Behavioral Treatment (CBT) for Childhood Functional Abdominal Pain? A Randomized Controlled Pilot Trial" Children 4, no. 2: 13. https://doi.org/10.3390/children4020013






