Neonatal and Maternal 25-OH Vitamin D Serum Levels in Neonates with Early-Onset Sepsis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Controls
2.3. Laboratory Investigations
2.4. Statistical Analysis
3. Results
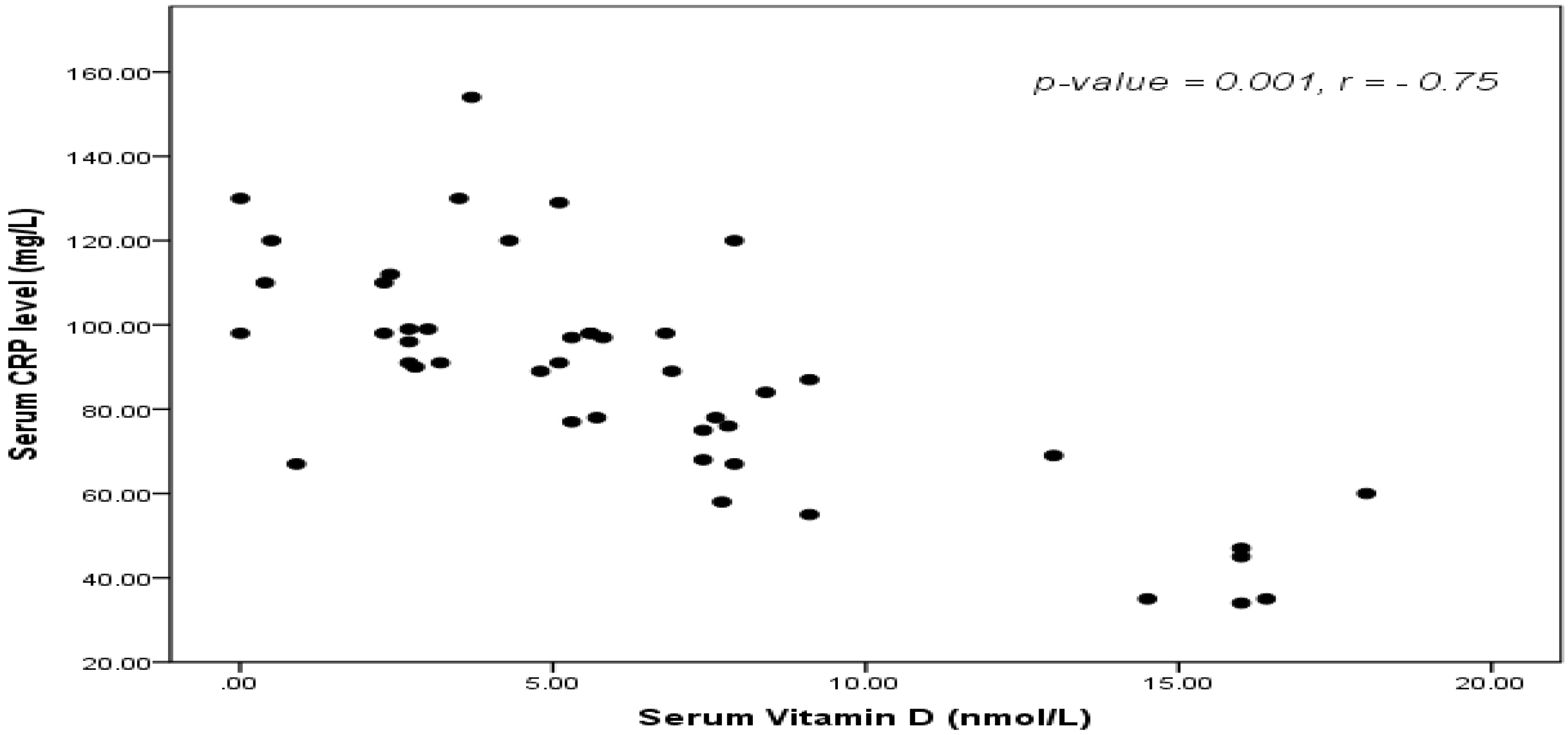
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Satar, M.; Ozlü, J. Neonatal sepsis: A continuing disease burden. Pediatr. 2012, 54, 449–457. [Google Scholar]
- Dollner, H.; Vatten, L.; Austgalen, R. Early diagnostic markers for neonatal sepsis: Comparing C-reactive protein, interleukin-6, soluble tumor necrosis factor receptors and soluble adhesion molecules. J. Clin. Epidemiol. 2001, 54, 1251–1257. [Google Scholar] [CrossRef]
- Cizmeci, M.N.; Kara, S.; Kanburoglu, M.K.; Simavli, S.; Duvan, C.I.; Tatli, M.M. Detection of cord blood hepcidin levels as a biomarker for early-onset neonatal sepsis. Med. Hypotheses 2014, 82, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. 25 OH Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Kempker, J.A.; Han, J.E.; Tangpricha, V.; Ziegler, T.R.; Martin, G.S. 25-OH Vitamin D and sepsis: An emerging relationship. Dermato-endocrinol. 2012, 4, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, I.M. Neonatal sepsis. Biochemia. Medica. 2011, 21, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Tappero, E.; Johnson, P. Laboratory Evaluation of Neonatal Sepsis. Newborn Infant Nurs. Rev. 2010, 10, 209–217. [Google Scholar] [CrossRef]
- Burris, H.H.; Van Marter, L.J.; McElrath, T.F.; Tabatabai, P.; Litonjua, A.A.; Weiss, S.T.; Christou, H. Vitamin D status among preterm and full-term infants at birth. Pediatr. Res. 2014, 75, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Farrell, C.L.; Pusceddu, I.; Fabregat-Cabello, N.; Cavalier, E. Assessment of vitamin D status — a changing landscape. Clin. Chem. Lab. Med. 2017, 55, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Snellman, G.; Melhus, H.; Gedeborg, R.; Byberg, L.; Berglund, L.; Wernroth, L.; Michaëlsson, K. Determining Vitamin D Status: A Comparison between Commercially Available Assays. PLoS ONE 2010, 5, e11555. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Brannon, P.M.; Rosen, C.J.; Taylor, C.L. Vitamin D Deficiency — Is There Really a Pandemic? N. Engl. J. Med. 2016, 375, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef] [PubMed]
- Jeng, L.; Judd, S.E.; Blumberg, H.M.; Martin, G.S.; Ziegler, T.R.; Tangpricha, V. Alterations in 25-OH Vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 2009, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Rech, M.A.; Hunsaker, T.; Rodriguez, J. Deficiency in 25-hydroxy Vitamin D and 30-day mortality in patients with severe sepsis and septic shock. Am. J. Crit. Care. 2014, 23, e72–e79. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Litonjua, A.A.; Moromizato, T.; Quraishi, S.A.; Gibbons, F.K.; Pieber, T.R.; Camargo, C.A., Jr.; Giovannucci, E.; Christopher, K.B. Increases in pre-hospitalization serum 25(OH) D concentrations are associated with improved 30-day mortality after hospital admission: A cohort study. Clin. Nutr. 2016, 35, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Camargo, C.A.J.; Shapiro, N.I. 25-OH Vitamin D insufficiency and sepsis severity in emergency department patients with suspected infection. Acad. Emerg. Med. 2011, 18, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Yu, J.; Kim, K.W.; Ahn, K.; Hong, S.A.; Lee, E.; Yang, S.I.; Jung, Y.H.; Kim, H.Y.; Seo, J.H.; et al. Association between cord blood 25-hydroxy Vitamin D concentrations and respiratory tract infections in the first 6 months of age in a Korean population: a birth cohort study (COCOA). Korean J. Pediatr. 2013, 56, 439–445. [Google Scholar]
- Braun, A.; Chang., D.; Mahadevappa, K.; Gibbons, F.K.; Liu, Y.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxy Vitamin D levels and mortality in the critically ill. Crit. Care Med. 2011, 39, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Izban, M.G.; Nowicki, B.J.; Nowicki, S. 1,25-dihydroxy Vitamin D3 promotes a sustained LPS-induced NF-κB-dependent expression of CD55 in human monocytic THP-1 cells. PLoS ONE 2012, 7, e49318. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a 25 OH Vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the 25-OH Vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxy Vitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Lemonovich, T.L.; Salata, R.A. An update on the association of Vitamin D deficiency with common infectious diseases. Can. J. Physiol. Pharmacol. 2015, 93, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, C.; Natale, F.; Pascone, R. C-reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin. Chim. Acta 2011, 412, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Posen, R.; Delemos, R.A. C-reactive protein levels in the extremely premature infant: Case studies and literature review. J. Perinatol. 1998, 18, 138–141. [Google Scholar] [PubMed]
- Shah, M.D.; Shah, S.R. Nutrient deficiencies in the premature infant. Pediatric Clin. North. Am. 2009, 56, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.A.; McKenna, M.J.; Oyefeso, O.; Uduma, O.; Murray, B.F.; Brady, J.J.; Kilbane, M.T.; Murphy, J.F.; Twomey, A.; Donnell, C.P.; et al. Vitamin D nutritional status in preterm infants and response to supplementation. Br. J. Nutr. 2013, 110, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Weisman, Y. Maternal, fetal and neonatal 25-OH Vitamin D and calcium metabolism during pregnancy and lactation. Endocr. Dev. 2003, 6, 34–49. [Google Scholar] [PubMed]
- Aydemir, G.; Cekmez, F.; Kalkan, G.; Fidanci, M.K.; Kaya, G.; Karaoglu, A.; Meral, C.; Arzıman, İ.; Karademir, F.; Ayar, G.; et al. High Serum 25-Hydroxyvitamin D Levels Are Associated with Pediatric Sepsis. Tohoku J. Exp. Med. 2014, 234, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Ratzinger, F.; Haslacher, H.; Stadlberger, M.; Schmidt, R.L.J.; Obermüller, M.; Schmetterer, K.G.; Perkmann, T.; Makristathis, A.; Marculescu, R.; Burgmann, H. 25(OH)D and 1,25(OH)D vitamin D fails to predict sepsis and mortality in a prospective cohort study. Sci. Rep. 2017, 7, 40646. [Google Scholar] [CrossRef]
- El-Mazary, A.M.; Abdel-Maaboud, M.; Mohamed, M.; Nasef, K.A. 25-OH Vitamin D supplementation and the risk of infections in fullterm infants. Correlations with the maternal serum 25 OH Vitamin D. Arch. Dis. Child. 2012, 97, A257. [Google Scholar] [CrossRef]
- Binkley, N.; Novotny, R.; Krueger, D.; Kawahara, T.; Daida, Y.G.; Lensmeyer, G.; Hollis, B.W.; Drezner, M.K. Low Vitamin D status despite abundant sun exposure. J. Clin. Endocrinol. Metab. 2007, 92, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- de Haan, K.; Groeneveld, A.J.; de Geus, H.R.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care. 2014, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Berry, D.J.; Vimaleswaran, K.S.; Whittaker, J.C.; Hingorani, A.D.; Hypponen, E. Evaluation of Genetic Markers as Instruments for Mendelian Randomization Studies on Vitamin D. PloS ONE 2012, 7, e37465. [Google Scholar] [CrossRef] [PubMed]
- Barragan, M.; Good, M.; Kolls, J.K. Regulation of Dendritic Cell Function by Vitamin D. Nutrients 2015, 7, 8127–8151. [Google Scholar] [CrossRef] [PubMed]

| Item | Full-term (n = 40) | Preterm (n = 40) | |||||
|---|---|---|---|---|---|---|---|
| Patients (n = 25) | Control (n = 15) | p-value | Patients (n = 25) | Control (n = 15) | p-value | ||
| Gestational age (weeks) | Mean ± SD | 37.5 ± 0.98 | 37.4 ± 0.58 | 0.08 | 34.1 ± 1.26 | 35.2 ± 2.14 | 0.15 |
| Birth weight (kg) | Mean ± SD | 3.2 ± 0.43 | 3.19 ± 0.35 | 0.08 | 2.78 ± 0.3 | 2.14 ± 0.26 | 0.06 |
| Gender | Male Female | 14 (56%) 11 (44%) | 12 (80%) 3 (20%) | 0.19 | 15 (60%) 10 (40%) | 8 (53%) 7 (47%) | 0.63 |
| Mode of delivery | NVD C.S | 17 (68%) 8 (32%) | 12 (80%) 3 (20%) | 0.54 | 7 (28%) 18 (72%) | 3 (20%) 12 (80%) | 0.66 |
| Maternal risk factors | Yes No | 13 (52%) 12 (48%) | 3 (20%) 12 (80%) | 0.04 | 14 (56%) 11 (44%) | 13 (86.6%) 2 (13.4%) | 0.04 |
| Item | Patients (n = 50) | Controls (n = 30) | p-Value |
|---|---|---|---|
| Hb (gm/dL) | 12.9 ± 3.4 | 15.4 ± 2.2 | 0.04 |
| WBCs (×10³/µL) | 20,038 ± 18,237.4 | 10,304 ± 3201.6 | 0.003 |
| Platelets (×10³/µL) | 89 ± 8.4 | 255 ± 5.9 | 0.002 |
| Neutrophils(×10³/µL) | 63.2 ± 12.9 | 37.5 ± 7.7 | 0.001 |
| Staff (×10³/µL) | 11.8 ± 7.4 | 3.0 ± 1.2 | 0.004 |
| Segmented(×10³/µL) | 51.9 ± 11.9 | 34.4 ± 7.5 | 0.01 |
| ANC | 11,184.5 ± 75.7 | 3847.2 ± 13.7 | 0.001 |
| I/T ratio | 0.2 ± 0.1 | 0.1 ± 0.03 | 0.01 |
| CRP (mg/L) | 37.6 ± 9.02 | 6.0 ± 1.05 | 0.001 |
| IL-6 (pg/mL) | 139.9 ± 70.19 | 5.8 ± 3.8 | 0.001 |
| Neonatal. 25-OH Vit.D (nmol/L) | 6.4 ± 1.8 | 24.6 ± 2.2 | 0.001 |
| Maternal. 25-OH Vit.D (nmol/L) | 42.5 ± 20.7 | 50.4 ± 21.4 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamal, T.S.; Madiha, A.-A.S.; Hanan, M.K.; Abdel-Azeem, M.E.-M.; Marian, G.S. Neonatal and Maternal 25-OH Vitamin D Serum Levels in Neonates with Early-Onset Sepsis. Children 2017, 4, 37. https://doi.org/10.3390/children4050037
Gamal TS, Madiha A-AS, Hanan MK, Abdel-Azeem ME-M, Marian GS. Neonatal and Maternal 25-OH Vitamin D Serum Levels in Neonates with Early-Onset Sepsis. Children. 2017; 4(5):37. https://doi.org/10.3390/children4050037
Chicago/Turabian StyleGamal, Taha Soliman, Abd-Allah Sayed Madiha, Mostafa Kamel Hanan, Mohamed El-Mazary Abdel-Azeem, and Gamil S. Marian. 2017. "Neonatal and Maternal 25-OH Vitamin D Serum Levels in Neonates with Early-Onset Sepsis" Children 4, no. 5: 37. https://doi.org/10.3390/children4050037





