Process Modification of Pharmaceutical Tablet Manufacturing Operations: An Eco-Efficiency Approach
Abstract
:1. Introduction
2. Materials and Methods
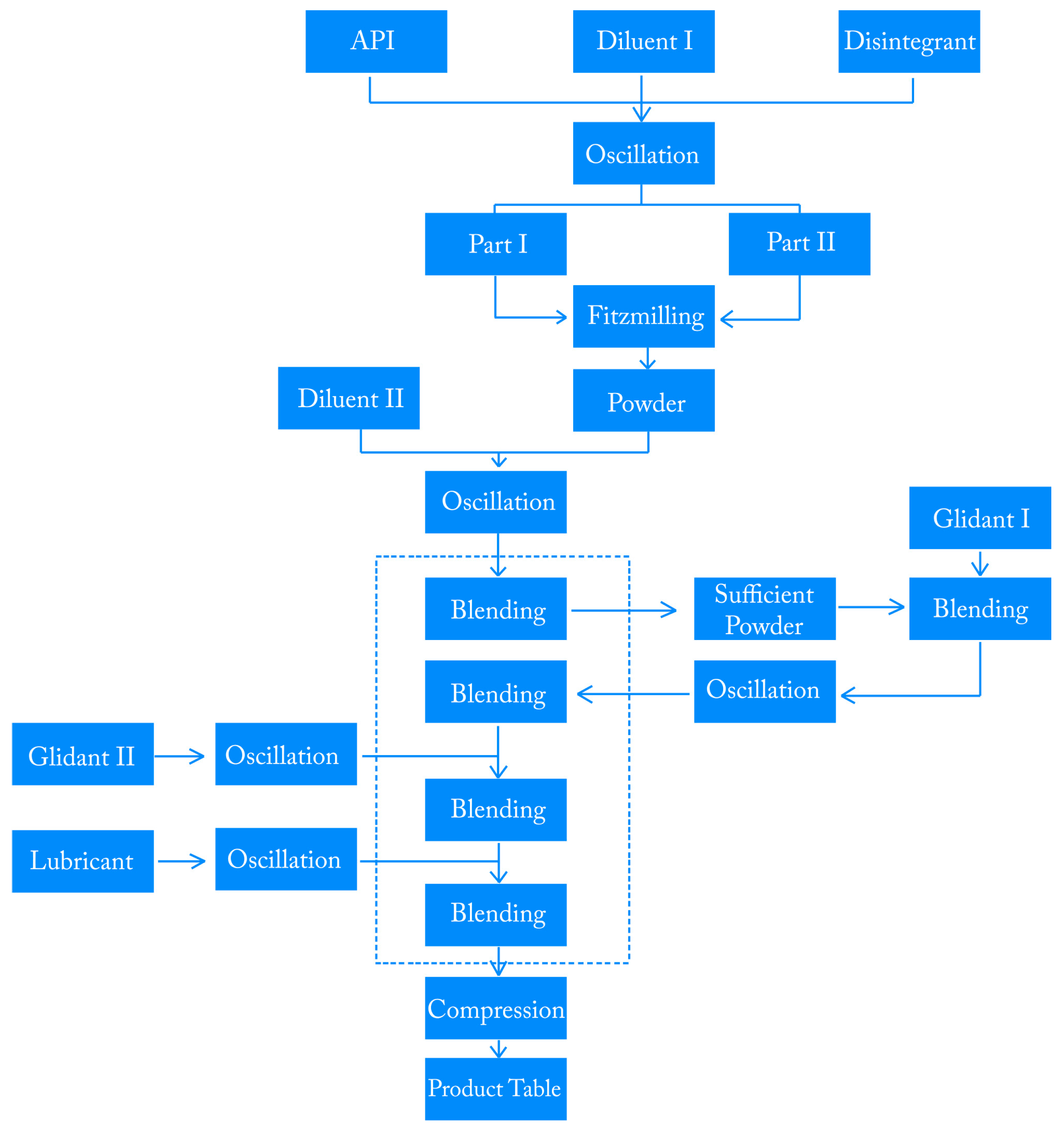
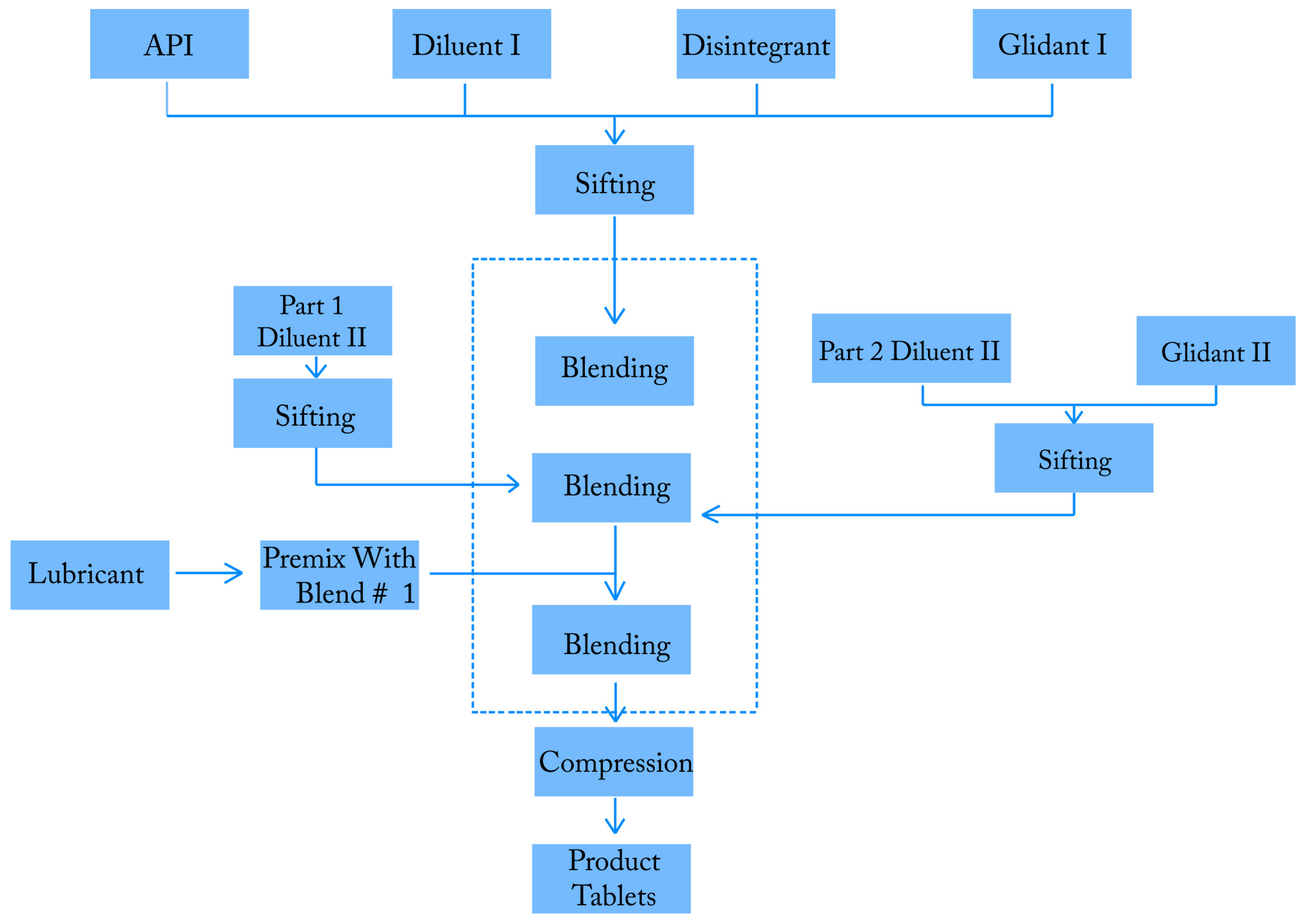
2.1. Overview of the Manufacturing Process
2.2. Consumption
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Järvinen, M.A.; Paaso, J.; Paavola, M.; Leiviskä, K.; Juuti, M.; Muzzio, F.; Järvinen, K. Continuous direct tablet compression: Effects of impeller rotation rate, total feed rate and drug content on the tablet properties and drug release. Drug Dev. Ind. Pharm. 2012, 39, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.; Basu, P.K. Improving pharmaceutical product development and manufacturing: Impact on cost of drug development and cost of goods sold of pharmaceuticals. J. Pharm. Innov. 2008, 3, 175–187. [Google Scholar] [CrossRef]
- Shah, N. Pharmaceutical supply chains: Key issues and strategies for optimisation. Comput. Chem. Eng. 2004, 28, 929–941. [Google Scholar] [CrossRef]
- McKenzie, P.; Kiang, S.; Tom, J.; Rubin, A.E.; Futran, M. Can pharmaceutical process development become high tech? AIChE J. 2006, 52, 3990–3994. [Google Scholar] [CrossRef]
- Amanda, J.; Rogers, A.H.; Ierapetritou, G.M. Modeling of Particulate Processes for the Continuous Manufacture of Solid-Based Pharmaceutical Dosage Forms. Processes 2013, 1, 67–127. [Google Scholar] [CrossRef]
- Basu, P.; Joglekar, G.; Rai, S.; Suresh, P.; Vernon, J. Analysis of manufacturing costs in pharmaceutical companies. J. Pharm. Innov. 2008, 3, 30–40. [Google Scholar] [CrossRef]
- Plumb, K. Continuous processing in the pharmaceutical industry—Changing the mindset. Chem. Eng. Res. Des. 2005, 83, 730–738. [Google Scholar] [CrossRef]
- Buchholz, S. Future manufacturing approaches in the chemical and pharmaceutical industry. Chem. Eng. Process. Process Intensif. 2010, 49, 993–995. [Google Scholar] [CrossRef]
- Singh, R.; Ierapetritou, M.; Ramachandran, R. An engineering study on the enhanced control and operation of continuous manufacturing of pharmaceutical tablets via roller compaction. Int. J. Pharm. 2012, 438, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Schmidheiny, S. Changing Course; MIT Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Ciccozzi, E.; Checkenya, R.; Rodriguez, A.V. Recent experiences and challenges in promoting cleaner production investments in developing countries. J. Clean. Prod. 2003, 11, 629–638. [Google Scholar] [CrossRef]
- Kjaerheim, G. Cleaner production and sustainability. J. Clean. Prod. 2005, 13, 329–339. [Google Scholar] [CrossRef]
- Sangwon, S.; Kun Mo, L.; Sangsun, H. Eco-efficiency for pollution prevention in small to medium-sized enterprises: A case from South Korea. J. Ind. Ecol. 2005, 9, 223–240. [Google Scholar]
- Rana, A.; Khokra, S.L.; Chandel, A.; Nanda, G.P.; Sahu, R.K. Overview On Roll Compaction/Dry Granulation Process. Pharmacologyonline 2011, 3, 286–298. [Google Scholar]
- Boltic, Z.; Ruzic, N.; Jovanovic, M.; Savic, M.; Jovanovic, J.; Petrovic, S. Cleaner production aspects of tablet coating process in pharmaceutical industry: Problem of VOCs emission. J. Clean. Prod. 2013, 44, 123–132. [Google Scholar] [CrossRef]
- Basu, S.; Mukhopadhyay, S.K.; Gangopadhyay, A.; Dastidar, S.G. Materials Saving and Waste Minimization in Indian Pharmaceutical Industries. Res. J. Pharm. Sci. 2013, 2, 20–25. [Google Scholar]
- Young, D.; Scharp, R.; Cabezas, H. The waste reduction (WAR) algorithm: Environmental impacts, energy consumption, and engineering economics. Waste Manag. 2000, 20, 605–615. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Neto, B.; Martins, M.L.; Salced, R.L.R.; Costa, C.A.V. LCA Tool for Sustainability Evaluations in the Pharmaceutical Industry. Chem. Eng. Trans. 2012, 26, 261–266. [Google Scholar]
- Miao, Z.; Bian, N.; Dong, L.; Sun, W. Exploring execution of ecological engineering and cleaner production in pharmaceutical industry. Energy Procedia 2011, 5, 679–683. [Google Scholar]
- Li, Z.D.; Zhang, S.-S.; Zhang, Y.; Zhang, Y.; Wei, L. Evaluation of cleaner production audit in pharmaceutical production industry: Case study of the pharmaceutical plant in Dalian, P.R. China. Clean Technol. Environ. Policy 2011, 13, 195–206. [Google Scholar]
- Searcy, C.; Morali, O.; Karapetrovic, S.; Wichuk, K.; McCartney, D.; McLeod, S.; Fraser, D. Challenges in implementing a functional ISO 14001 environmental management system. Int. J. Qual. Reliab. Manag. 2011, 29, 779–796. [Google Scholar] [CrossRef]
- Saidan, M. Sustainable Energy Mix and Policy Framework for Jordan; Friedrich Ebert Stiftung: Amman, Jordan, 2012. [Google Scholar]
- Al-Hamamre, Z.; Saidan, M.; Hararah, M.; Rawajfeh, K.; Alkhasawneh, H.; Al-Shannag, M. Wastes and biomass materials as sustainable-renewable energy resources for Jordan. Renew. Sustain. Energy Rev. 2017, 67, 295–314. [Google Scholar] [CrossRef]
- Saidan, M.; Tarawneh, A. Estimation of Potential E-waste Generation in Jordan. Ekoloji Derg. 2015, 24, 60–64. [Google Scholar] [CrossRef]
- Hararah, M.A.; Saidan, M.N.; Alhamamre, Z.; Abu-Jrai, A.M.; Alsawair, J.; Damra, R.A. The PCDD/PCDF emission inventory in Jordan: Aqaba city. J. Chem. Technol. Metall. 2016, 51, 112–120. [Google Scholar]
- Saidan, M.N.; Al-Weshah, R.A.; Obada, I. Potential Rainwater Harvesting: Adaptation Measure for Urban Areas in Jordan. Am. Water Works Assoc. 2015, 107, 594–602. [Google Scholar] [CrossRef]
- Saidan, M.N.; Abu Drais, A.; Al-Manaseer, E. Solid waste composition analysis and recycling evaluation: Zaatari Syrian Refugees Camp, Jordan. Waste Manag. 2017, 61, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, A.; Saidan, M. Households Awareness, Behaviors, and Willingness to Participate in E-waste Management in Jordan. Int. J. Ecosyst. 2013, 3, 124–131. [Google Scholar]
- Saidan, M.; Khasawneh, H.J.; Tayyem, M.; Hawari, M. Getting Energy from Poultry Waste in Jordan: Cleaner Production Approach. J. Chem. Technol. Metall. 2017, 52, 595–601. [Google Scholar]
- Saidan, M.N.; Ansour, L.M.; Saidan, H. Management of Plastic Bags Waste: An assessment of scenarios in Jordan. J. Chem. Technol. Metall. 2017, 52, 148–154. [Google Scholar]
- United States Environmental Protection Agency (EPA). Guide to Industrial Assessment for Pollution Prevention and Energy Efficiency; EPA/625/R-99/003; EPA: Washington, DC, USA, 2001. [Google Scholar]
- National Development and Reform Commission (NDRC). Interim Measures of Cleaner Production Auditing; Ministry of Environmental Protection of the People’s Republic of China (MEPC): Beijing, China, 2004.
- Guo, X.; Zhang, X.; Fang, P. Cleaner Production Audit Guide; China Environmental Science Press: Beijing, China, 2007. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). IPCC Guidelines for National Greenhouse Gas Inventories. In National Greenhouse Gas Inventory Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; The Initial Graphics Exchange Specification (IGES): Hayama, Japan, 2006. [Google Scholar]
- Mehrotra, A.; Chaudhuri, B.; Faqih, A.; Tomassone, M.S.; Muzzio, F.J. A modeling approach for understanding effects of powder flow properties on tablet weight variability. Powder Technol. 2009, 188, 295–300. [Google Scholar] [CrossRef]
- Kuentz, M.; Lunenberger, H. A new model for the hardness of a compacted particle systems, applied to tablets of pharmaceutical polymers. Powder Technol. 2000, 111, 145–153. [Google Scholar] [CrossRef]

| Process Unit | Power (kW) | Operating Time (h) | Energy Consumption (kWh) |
|---|---|---|---|
| Oscillation | 2 | 130 | 5200 |
| Fitzmilling | 3 | ||
| Sifter | 0 | ||
| Blending | 35 | ||
| Tableting | 10 | 40 | 400 |
| Total | 50 | 170 | 5600 |
| Process Unit | The Power (kW) | The Operating Time (h) | The Energy Consumption (kWh) |
|---|---|---|---|
| Oscilaation | 0 | 37 | 1369 |
| Fitzmilling | 0 | ||
| Sifter | 2 | ||
| Blending | 35 | ||
| Tableting | 10 | 13 | 130 |
| Total | 47 | 50 | 1499 |
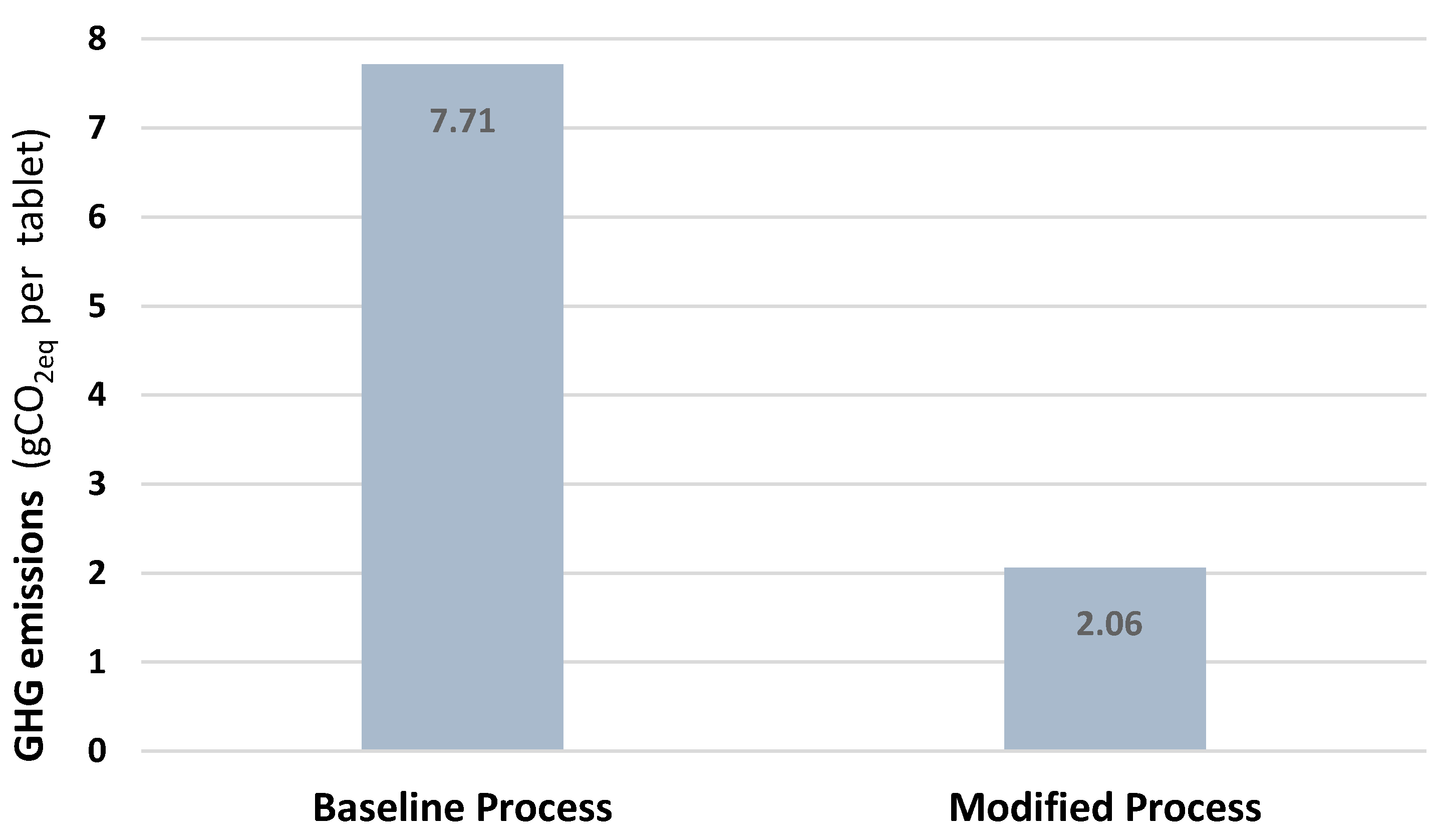
| Process | The Operating Time (h)/Batch | The Energy Consumption (kWh)/Batch | The Amount of Emissions * (kg CO2eq)/Batch | Energy Cost (JD)/Batch |
|---|---|---|---|---|
| Old Process | 170 | 5600 | 3855 | 476 |
| New Process | 50 | 1499 | 1032 | 127 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hindiyeh, M.; Altalafha, T.; Al-Naerat, M.; Saidan, H.; Al-Salaymeh, A.; Sbeinati, L.; Saidan, M.N. Process Modification of Pharmaceutical Tablet Manufacturing Operations: An Eco-Efficiency Approach. Processes 2018, 6, 15. https://doi.org/10.3390/pr6020015
Hindiyeh M, Altalafha T, Al-Naerat M, Saidan H, Al-Salaymeh A, Sbeinati L, Saidan MN. Process Modification of Pharmaceutical Tablet Manufacturing Operations: An Eco-Efficiency Approach. Processes. 2018; 6(2):15. https://doi.org/10.3390/pr6020015
Chicago/Turabian StyleHindiyeh, Muna, Tala Altalafha, Manar Al-Naerat, Hakam Saidan, Ahmed Al-Salaymeh, Lu’ay Sbeinati, and Motasem N. Saidan. 2018. "Process Modification of Pharmaceutical Tablet Manufacturing Operations: An Eco-Efficiency Approach" Processes 6, no. 2: 15. https://doi.org/10.3390/pr6020015






