On-Line Fabric Disk Sorptive Extraction via a Flow Preconcentration Platform Coupled with Atomic Absorption Spectrometry for the Determination of Essential and Toxic Elements in Biological Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents and Samples
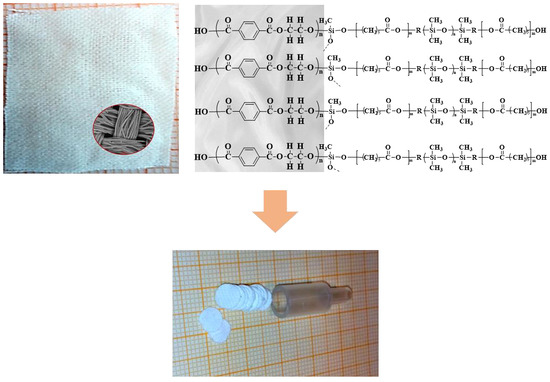
2.3. Preparation of Sol-Gel Poly(caprolactone-dimethylsiloxane-caprolactone) Coating on Polyester Substrate
2.4. FI-FDSE-FAAS Analytical Procedure
3. Results and Discussion
3.1. Surface Morphology and Properties of Sol-Gel Sorbent FDSE Medium
3.2. Study of Preconcentration Conditions
3.2.1. Retention of the Analytes onto the Microcolumn
3.2.2. pH Studies
3.2.3. Effect of Loading Flow Rate
3.2.4. Selection of Eluent and Elution Flow Rate
3.2.5. Effect of Preconcentration Time
3.3. Test for Interferences
3.4. Analytical Performance Characteristics
4. Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. Trends Anal. Chem. 2015, 71, 2–8. [Google Scholar] [CrossRef]
- Miró, M.; Hansen, E.H. On-line sample processing involving microextraction techniques as a front-end to atomic spectrometric detection for trace metal assays: A review. Anal. Chim. Acta 2013, 782, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Locatelli, M.; Ulusoy, H. Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation. Separations 2017, 4, 36. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Masqué, N.; Marcé, R.M.; Borrull, F. New polymeric and other types of sorbents for solid-phase extraction of polar organic micropollutants from environmental water. Trends Anal. Chem. 1998, 17, 384–394. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Marć, M.; Szczepańska, N.; Namieśnik, J. New Polymeric Materials for Solid Phase Extraction. Crit. Rev. Anal. Chem. 2017, 47, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Gilart, N.; Borrull, F.; Fontanals, N.; Marcé, R.M. Selective materials for solid-phase extraction in environmental analysis. Trends Environ. Anal. Chem. 2014, 1, 8–18. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Giakisikli, G.; Mitani, C. Flow injection dual-syringe sorbent extraction platform for metal determination in environmental matrices utilizing a new strong cation exchange sorbent micro-cartridge and flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2012, 92, 1276–1288. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Giakisikli, G.; Zachariadis, G. The HyperSep SCX micro-cartridge for on-line flow injection inductively coupled plasma atomic emission spectrometric determination of trace elements in biological and environmental samples. Anal. Methods 2011, 3, 2108–2114. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Xidia, S.; Giakisikli, G. Study of bond Elut Plexa PCX cation exchange resin in flow injection column preconcentration system for metal determination by flame atomic absorption spectrometry. Talanta 2012, 97, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Sixto, A.; Fiedoruk-Pogrebniak, M.; Rosende, M.; Cocovi-Solberg, D.; Knochen, M.; Miró, M. A mesofluidic platform integrating restricted access-like sorptive microextraction as a front end to ICP-AES for the determination of trace level concentrations of lead and cadmium as contaminants in honey. J. Anal. At. Spectrom. 2016, 31, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Anthemidis, A.N.; Maloumidou, T. Flow injection online solid phase extraction system using Oasis-HLBTM micro-cartridge for chromium(vi) and copper determination by flame atomic absorption spectrometry. Anal. Methods 2011, 3, 1392–1398. [Google Scholar] [CrossRef]
- Portugal, L.A.; Laglera, L.M.; Anthemidis, A.N.; Ferreira, S.L.; Miró, M. Pressure-driven mesofluidic platform integrating automated on-chip renewable micro-solid-phase extraction for ultrasensitive determination of waterborne inorganic mercury. Talanta 2013, 110, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Anthemidis, A.N.; Giakisikli, G.; Xidia, S.; Miró, M. On-line sorptive preconcentration platform incorporating a readily exchangeable Oasis HLB extraction micro-cartridge for trace cadmium and lead determination by flow injection-flame atomic absorption spectrometry. Microchem. J. 2011, 98, 66–71. [Google Scholar] [CrossRef]
- Giakisikli, G.; Zachariadis, P.; Kila, I.; Teshima, N.; Anthemidis, A. Flow Injection Solid Phase Extraction for Trace Metal Determination Using a Chelating Resin and Flame Atomic Absorption Spectrometry Detection. Anal. Lett. 2015, 49, 929–942. [Google Scholar] [CrossRef]
- Giakisikli, G.; Ayala Quezada, A.; Tanaka, J.; Anthemidis, A.N.; Murakami, H.; Teshima, N.; Sakai, T. Automatic On-line Solid-phase Extraction—Electrothermal Atomic Absorption Spectrometry Exploiting Sequential Injection Analysis for Trace Vanadium, Cadmium and Lead Determination in Human Urine Samples. Anal. Sci. 2015, 31, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Conway, T.M.; Rosenberg, A.D.; Adkins, J.F.; John, S.G. A new method for precise determination of iron, zinc and cadmium stable isotope ratios in seawater by double-spike mass spectrometry. Anal. Chim. Acta 2013, 793, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kazantzi, V.; Giakisikli, G.; Anthemidis, A. Reversed phase StrataTM-X resin as sorbent for automatic on-line solid phase extraction atomic absorption spectrometric determination of trace metals: Comparison of polymeric-based sorbent materials. Int. J. Environ. Anal. Chem. 2017, 97, 508–519. [Google Scholar] [CrossRef]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Ayazi, Z. Application of nanocomposite-based sorbents in microextraction techniques: A review. Analyst 2017, 142, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractor (FPSE). U.S. Patent 14,216,121, 17 March 2014. [Google Scholar]
- Kazantzi, V.; Anthemidis, A. Fabric Sol-gel Phase Sorptive Extraction Technique: A Review. Separations 2017, 4, 20. [Google Scholar] [CrossRef]
- Heena; Kaur, R.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Determination of cobalt(II), nickel(II) and palladium(II) Ions via fabric phase sorptive extraction in combination with high-performance liquid chromatography-UV detection. Sep. Sci. Technol. 2017, 52, 81–90. [Google Scholar] [CrossRef]
- Anthemidis, A.; Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G. An automated flow injection system for metal determination by flame atomic absorption spectrometry involving on-line fabric disk sorptive extraction technique. Talanta 2016, 156–157, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Elmongy, H.; Madrakian, T.; Abdel-Rehim, M. Nanomaterials as sorbents for sample preparation in bioanalysis: A review. Anal. Chim. Acta 2017, 958, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fontanals, N.; Marcé, R.M.; Borrull, F. New materials in sorptive extraction techniques for polar compounds. J. Chromatogr. A 2007, 1152, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1998; pp. 345–347. [Google Scholar]
- Sivrikaya, S.; Imamoglu, M.; Yıldız, S.Z.; Kara, D. Novel Functionalized Silica Gel for On-line Preconcentration of Cadmium(II), Copper(II), and Cobalt(II) with Determination by Flame Atomic Absorption Spectrometry. Anal. Lett. 2016, 49, 943–957. [Google Scholar] [CrossRef]
- Chamjangali, M.A.; Bagherian, G.; Mokhlesian, A.; Bahramian, B. Synthesis and application of chloromethylated polystyrene modified with 1-phenyl-1,2-propanedione-2-oxime thiosemicarbazone (PPDOT) as a new sorbent for the on-line preconcentration and determination of copper in water, soil, and food samples by FAAS. J. Hazard. Mater. 2011, 192, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Saçmaci, Ş.; Şahan, S.; Şahin, U.; Kartal, Ş.; Ülgen, A. On-line solid-phase separation/preconcentration for the determination of copper in urine by flame atomic absorption spectrometry. Mater. Sci. Eng. C 2014, 44, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.M.; Song, H.; Chen, M.L. Dithizone immobilized silica gel on-line preconcentration of trace copper with detection by flame atomic absorption spectrometry. Talanta 2011, 85, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Çetin, T.; Ülgen, A.; Tokalıoğlu, Ş. On-line Solid Phase Extraction of Copper in Water Samples with Flow Injection Flame Atomic Absorption Spectrometry. Clean Soil Air Water 2011, 39, 244–249. [Google Scholar] [CrossRef]
- Tobiasz, A.; Walas, S.; Trzewik, B.; Grzybek, P.; Zaitz, M.M.; Gawin, M.; Mrowiec, H. Cu(II)-imprinted styrene-divinylbenzene beads as a new sorbent for flow injection-flame atomic absorption determination of copper. Microchem. J. 2009, 93, 87–92. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, H.; Zhao, S.; Yan, H.; Han, D. On-line solid phase extraction coupled to flame atomic absorption spectrometry for the determination of trace copper and zinc in environmental and biological samples. Int. J. Environ. Anal. Chem. 2008, 88, 689–699. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Zachariadis, G.A.; Stratis, J.A. On-line preconcentration and determination of nickel and zinc in natural water samples by flow injection—Flame atomic absorption spectrometry using ptfe-turnings for column packing. Int. J. Environ. Anal. Chem. 2010, 90, 127–136. [Google Scholar] [CrossRef]
- Lemos, V.A.; Novaes, C.G.; Lima Ada, S.; Vieira, D.R. Flow injection preconcentration system using a new functionalized resin for determination of cadmium and nickel in tobacco samples. J. Hazard. Mater. 2008, 155, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wutke, N.B.; Diniz, K.M.; Corazza, M.Z.; de Oliveira, F.M.; Ribeiro, E.S.; da Fonseca, B.T.; Segatelli, M.G.; Teixeira Tarley, C.R. Preconcentration of nickel(II) by a mini-flow system with a novel ternary oxide solid phase and flame atomic absorption spectrometry. Anal. Lett. 2016, 49, 723–736. [Google Scholar] [CrossRef]
- Escudero, L.A.; Blanchet, A.J.; Sombra, L.L.; Salonia, J.A.; Gasquez, J.A. Determination of the total and extractable fraction of Ni in lake sediments and natural waters of San Luis (Argentina) by FAAS using a simple solid phase extraction system. Microchem. J. 2014, 116, 92–97. [Google Scholar] [CrossRef]
- Yilmaz, S.; Tokalioĝlu, Ş.; Şahan, S.; Ülgen, A.; Şahan, A.; Soykan, C. On-line preconcentration/determination of zinc from water, Biological and food samples using synthesized chelating resin and flame atomic absorption spectrometry. J. Trace Elem. Med. Biol. 2013, 27, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.N.; Mosquetta, R.; Carasek, E.; Coelho, N.M.M. Determination of Zn(II) in alcohol fuel by flame atomic absorption spectrometry after on-line preconcentration using a solid phase extraction system. J. Anal. Chem. 2012, 67, 448–454. [Google Scholar] [CrossRef]
- Peixoto, R.R.A.; Macarovscha, G.T.; Cadore, S. On-line Preconcentration and Determination of Zinc Using Zincon and Flame Atomic Absorption Spectrometry. Food Anal. Methods 2012, 5, 814–820. [Google Scholar] [CrossRef]




| Injection Valve | Peristaltic Pumps | ||||||
|---|---|---|---|---|---|---|---|
| Step | Position | P1 | P2 | Delivered Medium | Flow Rate (mL min−1) | Time (s) | Operation |
| 1 | Loading | OFF | ON | Sample/APDC | 11.5/1.0 | 90/30 * | Preconcentration |
| 2 | Elution | ON | OFF | MIBK | 4.8 | 20 | Elution, measurement |
| Cu(II) | Ni(II) | Zn(II) | Pb(II) | Cd(II) | |
|---|---|---|---|---|---|
| SC (mL) | 19.0 | 19.0 | 6.0 | 19.0 | 19.0 |
| LT (s) | 90 | 90 | 30 | 90 | 90 |
| Sampling frequency (h−1) | 33 | 33 | 72 | 33 | 33 |
| EF | 250 | 130 | 49 | 185 | 36 |
| cL, (μg L−1) | 0.15 | 0.41 | 0.12 | 1.62 | 0.49 |
| RSD, (%, n = 10) | 2.2 (5.0 μg L−1) | 2.9 (10.0 μg L−1) | 3.5 (2.0 μg L−1) | 2.5 (50.0 μg L−1) | 3.2 (10.0 μg L−1) |
| Linear range (μg L−1) | 0.50–30.0 | 1.4–60.0 | 0.39–15.0 | 5.4–250.0 | 1.6–60.0 |
| Regression equation | A = 0.0149 [Cu(II)] + 0.0025 | A = 0.006 [Ni(II)] + 0.0022 | A = 0.0223 [Zn(II)] + 0.0034 | A = 0.0013 [Pb(II)] + 0.0045 | A = 0.0062 [Cd(II)] + 0.0016 |
| Correlation coefficient (r) | 0.9991 | 0.9993 | 0.9989 | 0.9977 | 0.9985 |
| CRM | Analyte | Certified value | Found * | Relative error (%) | texp |
|---|---|---|---|---|---|
| Seronorm™ | (μg L−1) | (μg L−1) | |||
| Cu ** | 31 | 30.5 ± 0.8 | 1.6 | 1.083 | |
| Zn | 334 ± 67 | 318 ± 12 | 4.8 | 2.309 | |
| (mg kg−1) | (mg kg−1) | ||||
| BCR 278-R | Cu | 9.45 ± 0.13 | 9.05 ± 0.3 | 4.2 | 2.309 |
| Zn | 83.1 ± 1.7 | 84.2 ± 3.5 | −1.3 | −0.544 | |
| Pb | 2.00 ± 0.04 | 1.95 ± 0.06 | 2.5 | 1.443 | |
| Cd | 0.35 ± 0.01 | 0.34 ± 0.01 | 2.9 | 1.732 |
| Analyte | Added a | Found a,b | Recovery (%) | |
|---|---|---|---|---|
| Urine | Cu(II) | - | 9.5 ± 0.3 | - |
| 5.0 | 14.4 ± 0.5 | 98.0 | ||
| Ni(II) | - | N.D. | - | |
| 5.0 | 5.1 ± 0.2 | 102.0 | ||
| Zn(II) | - | 220.2 ± 0.35 | - | |
| 10.0 | 229.7 ± 0.8 | 95.0 | ||
| Pb(II) | - | N.D. | - | |
| 10.0 | 9.6 ± 0.3 | 96.0 | ||
| Cd(II) | - | N.D. | - | |
| 5.0 | 4.8 ± 0.2 | 96.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G.; Anthemidis, A. On-Line Fabric Disk Sorptive Extraction via a Flow Preconcentration Platform Coupled with Atomic Absorption Spectrometry for the Determination of Essential and Toxic Elements in Biological Samples. Separations 2018, 5, 34. https://doi.org/10.3390/separations5030034
Kazantzi V, Samanidou V, Kabir A, Furton KG, Anthemidis A. On-Line Fabric Disk Sorptive Extraction via a Flow Preconcentration Platform Coupled with Atomic Absorption Spectrometry for the Determination of Essential and Toxic Elements in Biological Samples. Separations. 2018; 5(3):34. https://doi.org/10.3390/separations5030034
Chicago/Turabian StyleKazantzi, Viktoria, Victoria Samanidou, Abuzar Kabir, Kenneth G. Furton, and Aristidis Anthemidis. 2018. "On-Line Fabric Disk Sorptive Extraction via a Flow Preconcentration Platform Coupled with Atomic Absorption Spectrometry for the Determination of Essential and Toxic Elements in Biological Samples" Separations 5, no. 3: 34. https://doi.org/10.3390/separations5030034