Release of Selected Metals (Al, Cd, Cu, Mn, Ni, Fe, Zn) from River Bottom Sediments: An Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
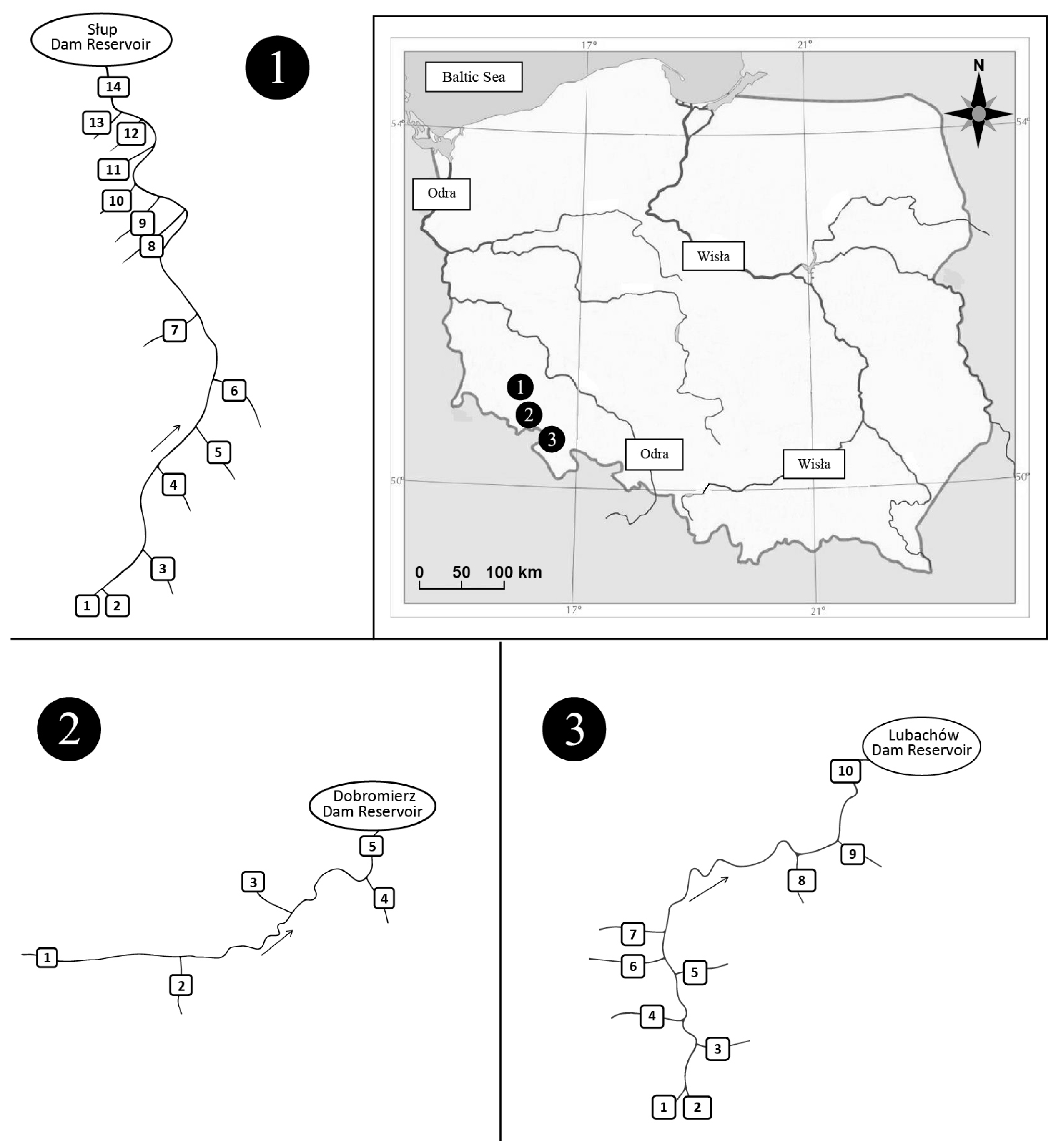
2.1. Study Area
2.2. Material
2.3. Analytical Methods
2.4. Laboratory Experiment
2.5. Statistical Analysis
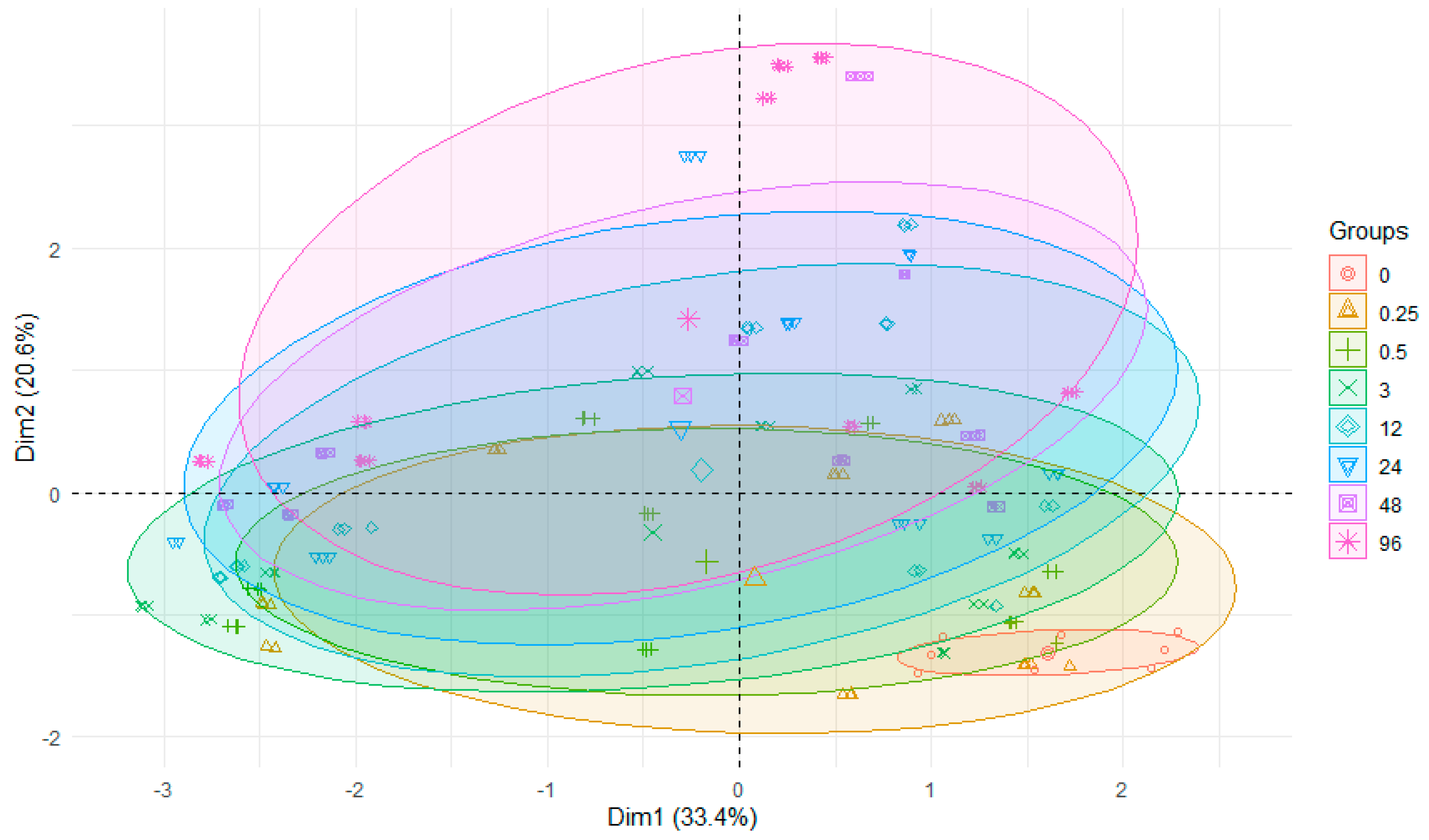
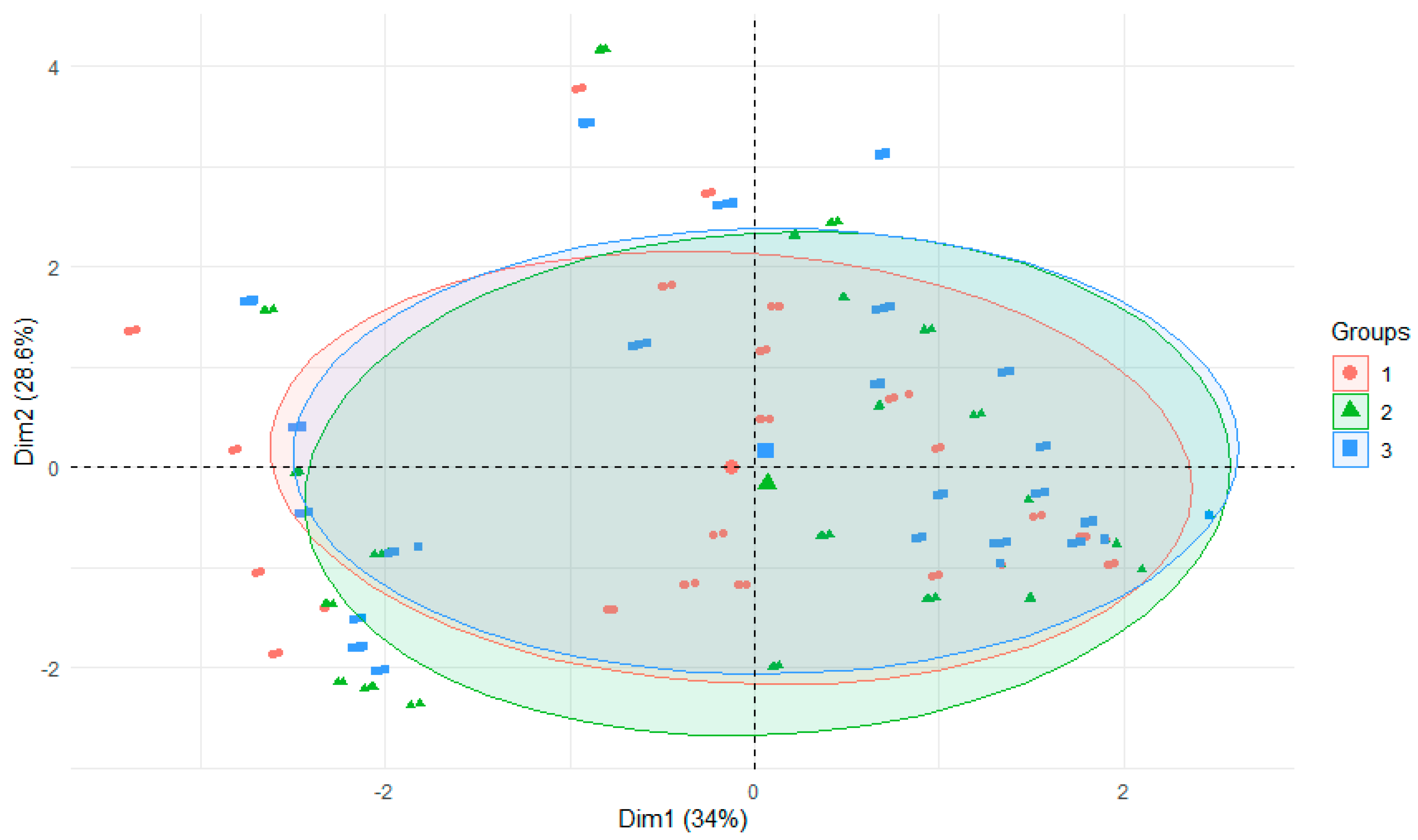
3. Results and Discussion
3.1. Characteristics of Bottom Sediments Used for the Experiment
3.2. Experiment on the Release of Metals from Bottom Sediments into Water
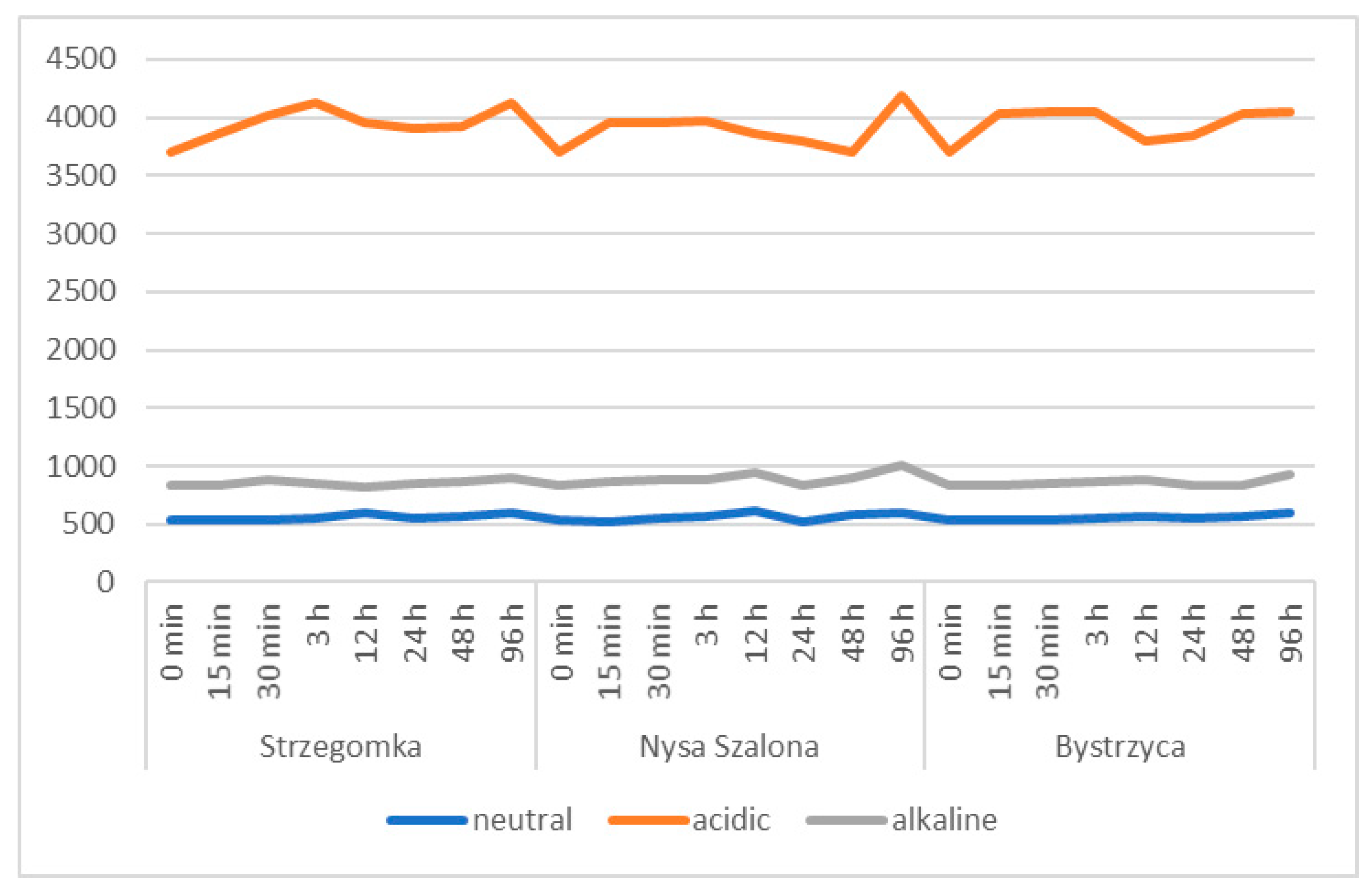
3.2.1. The pH and Electrolytic Conductivity
3.2.2. Metals
3.2.3. Aluminum
3.2.4. Copper
3.2.5. Manganese
3.2.6. Iron
3.2.7. Nickel
3.2.8. Cadmium
3.2.9. Zinc
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; PWN: Warsaw, Poland, 1999. [Google Scholar]
- Market, B. Presence and significance of naturally occurring chemical elements of the periodic system in the plant organism and consequences for future investigations on inorganic environmental chemistry in ecosystems. Vegetatio 1992, 103, 1–30. [Google Scholar] [CrossRef]
- Matschullat, R.; Ottenstein, R.; Reimann, C. Geochemical background—Can we calculate it? Environ. Geol. 2000, 39, 9. [Google Scholar] [CrossRef]
- Siregar, A.S.; Sulistyo, I.; Prayogo, N.A. Heavy metal contamination in water, sediments and Planiliza subviridis tissue in the Donan River, Indonesia. J. Water Land Dev. 2020, 45, 157–164. [Google Scholar]
- Mandeng, E.P.B.; Bidjeck, L.M.B.; Bessa, A.M.E.; Ntomb, Y.D.; Wadjou, J.W.; Doumo, E.P.E.; Dieudonne, L.B. Contamination and risk assessment of heavy metals, and uranium of sediments in two watershed in Abiete-Toko gold district, Southern Cameroon. Heliyon 2019, 5, e02591. [Google Scholar] [CrossRef] [Green Version]
- Luoma, S.N.; Rainbow, P.S.; DiLeo, J. Metal Contamination in Aquatic Environments. Science and Lateral Management; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Gaillardet, J.; Viers, J.; Dupré, B. Trace Elements in River Waters. In Treatise Geochem, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, pp. 195–235. [Google Scholar]
- Kotowski, M.; Wieteska, E.; Pawłowski, L.; Kozak, Z. Characteristics of the Occurrence of Various Forms of Aluminum in Selected Elements of the Environment in Poland; PIOŚ, OW OIKOS: Warsaw, Poland, 1994. [Google Scholar]
- Sposito, G. The Environmental Chemistry of Aluminum; ImprintCRC Press: London, UK, 1996. [Google Scholar]
- Barabasz, W.; Albińska, D.; Jaśkowska, M.; Lipiec, J. Ecotoxicology of aluminium. Pol. J. Environ. Stud. 2002, 11, 199–203. [Google Scholar]
- Kotowski, M.; Saczuk, M. Aluminium in water and soil environment. Ekoinżynieria 1997, 2, 22–29. [Google Scholar]
- Miller, T.E.; Iqbal, N.; Reader, S.M.; Mahmood, A.; Cant, K.A.; King, I.P. A cytogenetic approach to the improvement of aluminium tolerance in wheat. New Phytol. 1997, 137, 93–98. [Google Scholar] [CrossRef]
- Borkowska, B. Aluminium toxicity (Al). Wiad. Bot. 1998, 32, 157–166. [Google Scholar]
- Ciszewski, D.; Malik, I.; Wardas, M. Geomorphological influences on heavy metal migration in fluvial deposits: The Mała Panew River valley (Southern Poland). Przegl. Geol. 2004, 52, 163–174. [Google Scholar]
- Appelo, C.; Postma, D. Modeling the Hydrogeochemical Processes and Source of Ions in the Groundwater of Aquifers within Kasra-Nukhaib Region (West Iraq). In Geochemistry, Groundwater and Pollution, 2nd ed.; Balkmema: Rotterdam, The Netherlands, 2005. [Google Scholar]
- Ponnamperuma, F.N. The Chemistry of Submerged Soils. Adv. Agron. 1972, 24, 29–98. [Google Scholar]
- Korzeniewski, K. Hydrochemistry; WSP: Słupsk, Poland, 1986. [Google Scholar]
- Miranda, L.S.; Wijesiri, B.; Ayoko, G.A.; Egpdawatta, P.; Goonetilleke, A. Water-sediment interactions and mobility of heavy metals in aquatic environments. Water Res. 2021, 202, 117386. [Google Scholar] [CrossRef]
- Lu, J.; Cai, H.; Zhang, X.; Fu, Y. Release flux of heavy metals from river sediments at different flow rates. Water Supply 2022, 22, 542–554. [Google Scholar] [CrossRef]
- Wen, X.; E Allen, H. Mobilization of heavy metals from Le An River sediment. Sci. Total Environ. 1999, 227, 101–108. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, D.; Xu, Z.; Yuan, S.; Li, Y.; Wang, L. Effect of overlying water pH, dissolved oxygen and temperature on heavy metal release from river sediments under laboratory conditions. Arch. Environ. Prot. 2017, 43, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, H.; Zhang, Z.; Liu, C.; Sun, C.; Zhang, W.; Marhaba, T. pH Effect on Heavy Metal Release from a Polluted Sediment. J. Chem. 2018, 2018, 7597640. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, W. Experimental study on release of heavy metals in sediment under hydrodynamic conditions. IOP Conf. Ser. Earth Environ. Sci. 2018, 208, 012040. [Google Scholar] [CrossRef]
- Senze, M.; Kowalska-Góralska, M.; Wondołowska-Grabowska, A. Evaluation of bottom sediment contamination with trace metals on the example of a lowland dam reservoir in Slup, Lower Silesia. Ochrona Środ. 2017, 39, 51–56. [Google Scholar]
- Senze, M.; Kowalska-Góralska, M.; Czyż, K.; Wondołowska-Grabowska, A.; Łuczyńska, J. Aluminum in Bottom Sediments of the Lower Silesian Rivers Supplying Dam Reservoirs vs. Selected Chemical Parameters. Int. J. Environ. Res. Public Health 2021, 18, 13170. [Google Scholar] [CrossRef]
- PIG 2022. State Research Institute—National Research Institute in Warsaw. Characteristics of the Geological Structure of Lower Silesia. Available online: https://www.pgi.gov.pl/wroclaw/oddzial-dolnoslaski/opracowania/geologia-dolnego-slaska/charakterystyka-budowy-geologicznej-dolnego-slaska.html (accessed on 15 May 2023).
- GIOŚ. Chief Inspectorate of Environmental Protection. In Report on the State of the Environment; Chief Inspectorate of Environmental Protection: Warsaw, Poland, 2018. (In Polish) [Google Scholar]
- PN-EN ISO 5667-15:2009E; Water Quality—Sampling—Guidelines for the Fixation and Handling of Sewage Sludge and Bottom Sediment Samples. The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 2009.
- PN-EN ISO 5667-19:2005E; Water Quality—Sampling—Part 19: Guidelines for Mari ne Sediment Sampling. The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 2005.
- PN-90/C-04540.01; Water and Wastewater—pH, Acidity and Alkalinity Tests—Determination of pH of Water and Wastewater with Specific Electrolytic Conductivity of 10 Microsiemens/cm and above by Electrometric Method. The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 1990.
- PN-EN ISO 10390:2022-0; Soil, Treated Bio-Waste and Sewage Sludge—Determination of pH. The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 2022.
- PN-75/C-04616.01; Water and Wastewater. Special Tests of Sludge. Determination of Water, Dry Matter, Organic Matter and Mineral Content in Sewage Sludge. The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 1975.
- PN-ISO 11048:2002; Soil Quality—Determination of Water-Soluble and Acid-Soluble Sulfate(VI). The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 2002.
- PN-EN ISO 12020:2002; Total Aluminium by Electrothermal Atomic Absorption Spectrometry. The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 2002.
- PN-ISO 8288:2002; Water Quality. Determination of Cobalt, Nickel, Copper, Zinc, Cadmium and Lead—Flame Atomic Absorption Spectrometry Methods. The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 2002.
- PB-10/I-1998; Test Procedure. VARIAN’s Analytical Methods. The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warsaw, Poland, 1998.
- Senze, M.; Kowalska-Góralska, M.; Dobicki, W. Migration of aluminium from bottom sediments into water in laboratory conditions. Teka Kom. Ochr. Kształtowania Sr. Przyr.-OL PAN 2009, 6, 285–291. [Google Scholar]
- Baran, A.; Tarnawski, M.; Jasiewicz, C.Z. Assessment of the content and solubility of heavy metals in bottom sediments of the Chańcza reservoir. Ecol. Chem. Eng. A 2011, 18, 941–950. [Google Scholar]
- Gäbler, H.E. Mobility of heavy metals as a function of pH of samples from an overbank sediment profile contaminated by mining activities. J. Geochem. Explor. 1997, 58, 185–194. [Google Scholar] [CrossRef]
- Riba, I.; Del Valls, T.Á.; Forja, J.M.; Gòmez-Parra, A. The influence of pH and salinity on the toxicity of heavy metals in sediment to the estuarine clam Ruditapes philippinarum. Environ. Toxicol. Chem. 2004, 23, 1100–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.F.; Chen, Y.; Qian, X.; Guo, L.; Zhu, H.Y. A study of the effect of soil pH on chemical species of cadmium by simulated experiments. Earth Sci. Front. 2005, 12, 252–260. [Google Scholar]
- Linnik, P.M.; Zubenko, I.B. Role of bottom sediments in the secondary pollution of aquatic environments by heavy-metal compounds. Lake Reserv. Manag. 2000, 5, 11–21. [Google Scholar] [CrossRef]
- Lynn, D.C.; Bonatti, E. Mobility of manganese in diagenesis of deep-sea sediments. Mar. Geol. 1965, 3, 457–474. [Google Scholar] [CrossRef]
- Förstner, U.; Ahlf, W.; Calmano, W.; Kersten, M.; Salomons, W. Mobility of Heavy Metals in Dredged Harbor Sediments. In Sly, P.G. Sediments and Water Interactions; Springer: New York, NY, USA, 1986; pp. 371–380. [Google Scholar]
- Osae, R.; Nukpezah, D.; Darko, D.A.; Mensah, A. Heavy metal mobility, bioavailability, and potential toxicity in sediments of the Korle lagoon in Ghana. Int. J. Environ. Stud. 2022. [Google Scholar] [CrossRef]
- Marques, L.; Reis, D.; Nascimento, L.; Oliveira, E.; Santiago, A.; Roeser, H. Mobility of metals in river sediments from a watershed in the Iron Quadrangle, Brazil. Geochim. Bras. 2019, 33, 273–285. [Google Scholar] [CrossRef]
- Ahdy, H.; Rifaat, A.; Draz, S. The Speciation and Potential Mobility of Pb, Cd, Cu and Zn in Lake Qarun Bottom Sediments, Fayioum, Egypt. J. King Abdulaziz Univ. Mar. Sci. 2011, 22, 111–133. [Google Scholar] [CrossRef]
- Regulation of the Minister of Maritime Affairs and Inland Navigation of March 1, 2019 on the List of Priority Substances Journal of Laws of March 20, 2019, Item 528); Government Legislation Center: Warsaw, Poland, 2019.
- Regulation of the Minister of Maritime Economy and Inland Navigation of August 29, 2019 on the Requirements to Be Met by Surface Waters Used for Supplying the Public with Water Intended for Human Consumption (Journal of Laws of September 13, 2019, Item 1747); Government Legislation Center: Warsaw, Poland, 2019.
- Regulation of the Minister of Health of December 7, 2017 on the Quality of Water Intended for Human Consumption (Journal of Laws of December 11, 2017, Item 2294); Government Legislation Center: Warsaw, Poland, 2017.


| Metal/Unit | Air | Waters | Soils | Plants |
|---|---|---|---|---|
| ng∙m−3 | mg/L | mg∙kg−1 | mg∙kg−1 | |
| Cu | 0.03–4900 | 0.001–0.02 | 3–25 | 5–30 |
| Cd | 0.003–0.6 | 0.00001–0.00002 | 0.0002–0.6 | 0.05–0.2 |
| Ni | 0.10–1 | 0.0001–0.0075 | 5–22 | 0.1–5 |
| Pb | 0.50–10 | 0.0002–0.0003 | 25–40 | 0.10–5 |
| Zn | 0.002–0.05 | 0.01–0.22 | 10–220 | 10.00–70.00 |
| Fe | 0.5–6000 | 0.49–1.4 | 8000–18,000 | 50–200 |
| Mn | 0.02–900 | 0.02–0.06 | 100–1300 | 70–500 |
| Parameter | Unit | Nysa Szalona (14 Sites) | Bystrzyca (10 Sites) | Strzegomka (5 Sites) |
|---|---|---|---|---|
| Min–Max ± SD | ||||
| Reaction | pH | 7.19–7.23 7.21 ± 0.02 | 7.01–7.03 7.02 ± 0.01 | 7.50–7.54 7.52 ± 0.02 |
| Mineral compounds | % | 94.48–94.66 94.55 ± 0.08 | 92.14–92.26 92.19 ± 0.05 | 96.68–97.68 97.33 ± 0.46 |
| Organic compounds | 5.62–5.64 5.63 ± 0.01 | 7.82–7.86 7.84 ± 0.02 | 2.31–2.36 2.34 ± 0.02 | |
| Sulphates | mg·kg−1 | 21.48–22.15 21.74 ± 0.29 | 12.74–12.85 12.79 ± 0.05 | 13.63–13.68 13.65 ± 0.02 |
| Al | 12,241–12,243 12,242 ± 0.51 | 17,345–17,347 17,346 ± 0.54 | 167–168 168 ± 0.63 | |
| Cu | 86.15–86.25 86.21 ± 0.04 | 60.23–60.25 60.24 ± 0.01 | 9.22–9.28 9.25 ± 0.02 | |
| Ni | 142.2–142.9 142.5 ± 0.32 | 55.14–55.17 55.16 ± 0.01 | 22.13–22.18 22.16 ± 0.02 | |
| Cd | 1.25–1.28 1.26 ± 0.01 | 1.07–1.09 1.08 ± 0.01 | 0.62–0.69 0.65 ± 0.03 | |
| Zn | 405.2–406.6 405.9 ± 0.55 | 328.2–328.7 328.43 ± 0.22 | 65.01–65.09 65.06 ± 0.04 | |
| Fe | 19,447–19,448 19,448 ± 0.77 | 9290–9290 9290 ± 0.01 | 10,256–10,257 10,256 ± 0.49 | |
| Mn | 170.9–172.2 171.7 ± 0.53 | 101.3–101.5 101.3 ± 0.11 | 114.3–114.5 114.3 ± 0.09 | |
| Variable | pH | Conductivity | Al | Cu | Mn | Fe | Ni | Cd | Zn | Sediment | Time | River |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.0000 | −0.4420 | −0.4897 | 0.2323 | −0.5696 | 0.1362 | 0.1361 | 0.3766 | −0.7659 | 0.4549 | 0.0966 | −0.0187 |
| conductivity | −0.4420 | 1.0000 | 0.2190 | 0.1996 | 0.4559 | −0.0308 | 0.5239 | 0.2638 | 0.3238 | 0.4715 | 0.1755 | −0.0002 |
| Al | −0.4898 | 0.2190 | 1.0000 | 0.1739 | 0.7146 | 0.4154 | 0.0428 | −0.1293 | 0.5431 | −0.3804 | 0.2008 | 0.1642 |
| Cu | 0.2323 | 0.1996 | 0.1739 | 1.0000 | 0.0826 | 0.4809 | 0.6469 | 0.7256 | −0.2698 | 0.3788 | 0.3886 | 0.0346 |
| Mn | −0.5696 | 0.4559 | 0.7146 | 0.0826 | 1.0000 | 0.0136 | 0.1789 | −0.0031 | 0.5499 | −0.3300 | 0.5081 | 0.0913 |
| Fe | 0.1362 | −0.0308 | 0.4154 | 0.4809 | 0.0136 | 1.0000 | 0.3942 | 0.3571 | 0.0470 | 0.1119 | −0.1125 | −0.0443 |
| Ni | 0.1361 | 0.5239 | 0.0428 | 0.6469 | 0.1789 | 0.3942 | 1.0000 | 0.8436 | −0.1483 | 0.6403 | 0.3075 | −0.0743 |
| Cd | 0.3766 | 0.2638 | −0.1293 | 0.7256 | −0.0031 | 0.3571 | 0.8436 | 1.0000 | −0.3768 | 0.5786 | 0.3513 | −0.0966 |
| Zn | −0.7659 | 0.3238 | 0.5431 | −0.2698 | 0.5499 | 0.0470 | −0.1483 | −0.3768 | 1.0000 | −0.3927 | −0.2992 | 0.0209 |
| sediment | 0.4549 | 0.4715 | −0.3804 | 0.3788 | −0.3300 | 0.1119 | 0.6403 | 0.5786 | −0.3927 | 1.0000 | 0.0000 | 0.0000 |
| time | 0.0966 | 0.1755 | 0.2008 | 0.3886 | 0.5081 | −0.1125 | 0.3075 | 0.3513 | −0.2992 | 0.0000 | 1.0000 | 0.0000 |
| river | −0.0187 | −0.0002 | 0.1642 | 0.0346 | 0.0913 | −0.0443 | −0.0743 | −0.0966 | 0.0209 | 0.0000 | 0.0000 | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senze, M.; Kowalska-Góralska, M.; Czyż, K.; Wondołowska-Grabowska, A. Release of Selected Metals (Al, Cd, Cu, Mn, Ni, Fe, Zn) from River Bottom Sediments: An Experimental Study. Limnol. Rev. 2023, 23, 50-69. https://doi.org/10.3390/limnolrev23020004
Senze M, Kowalska-Góralska M, Czyż K, Wondołowska-Grabowska A. Release of Selected Metals (Al, Cd, Cu, Mn, Ni, Fe, Zn) from River Bottom Sediments: An Experimental Study. Limnological Review. 2023; 23(2):50-69. https://doi.org/10.3390/limnolrev23020004
Chicago/Turabian StyleSenze, Magdalena, Monika Kowalska-Góralska, Katarzyna Czyż, and Anna Wondołowska-Grabowska. 2023. "Release of Selected Metals (Al, Cd, Cu, Mn, Ni, Fe, Zn) from River Bottom Sediments: An Experimental Study" Limnological Review 23, no. 2: 50-69. https://doi.org/10.3390/limnolrev23020004







