A High-Sensitivity Fiber Biosensor Based on PVDF-Excited Surface Plasmon Resonance in the Terahertz Band
Abstract
:1. Introduction
2. Structural Design and Analysis
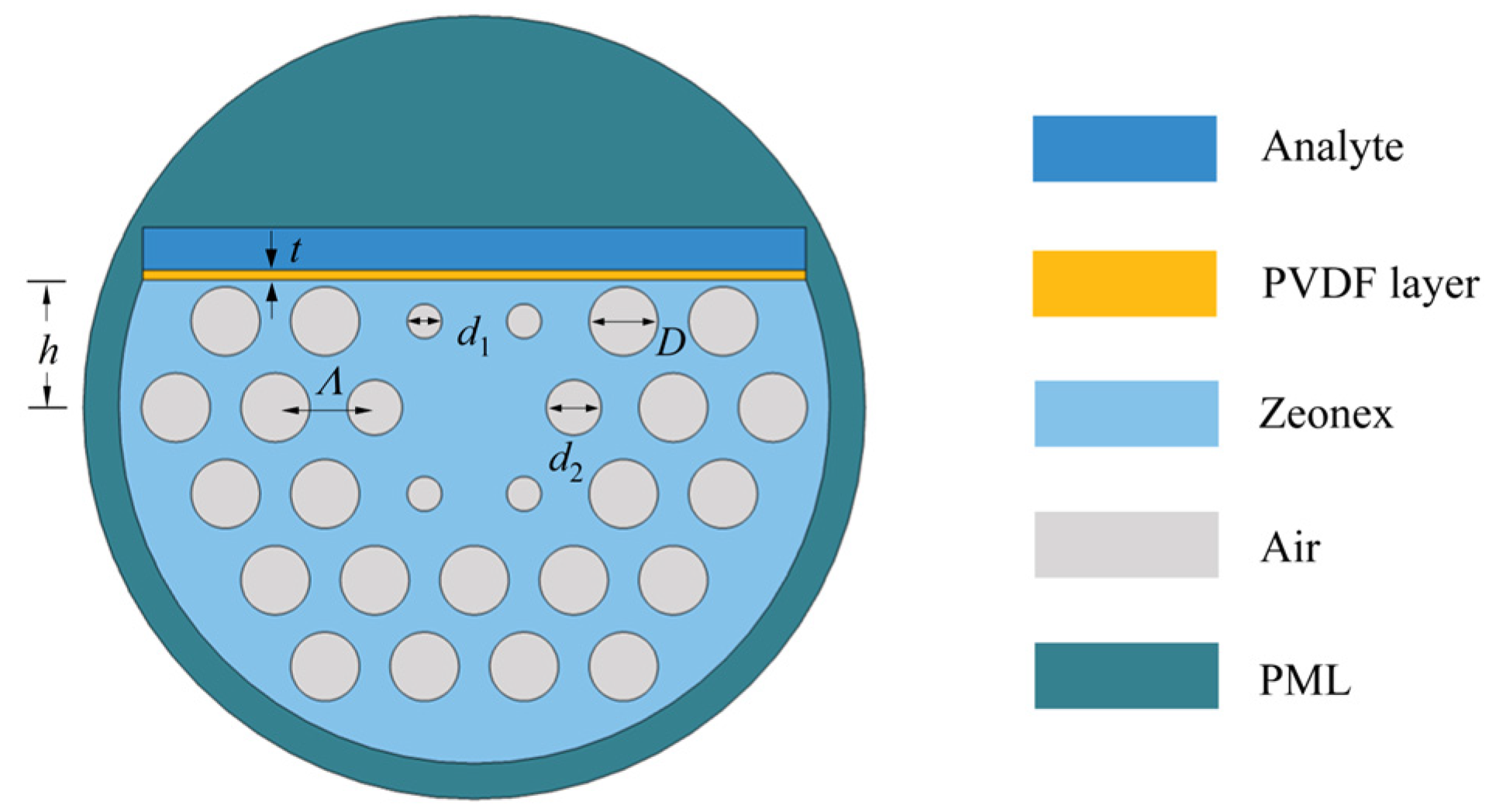
2.1. Structural Design
2.2. Performance Analysis
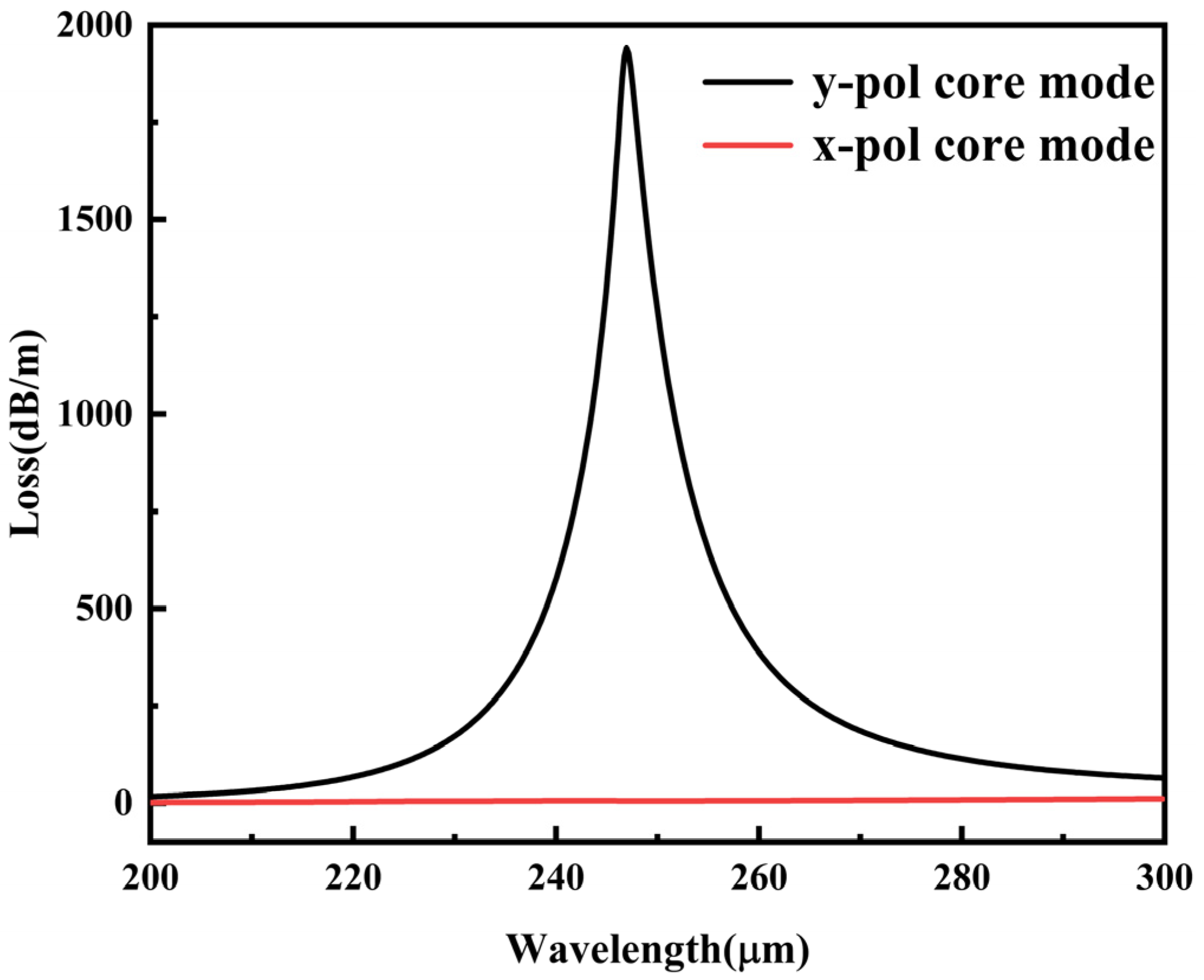
3. Simulation Results and Discussion
3.1. Structural Optimization of the Proposed Sensing Structure
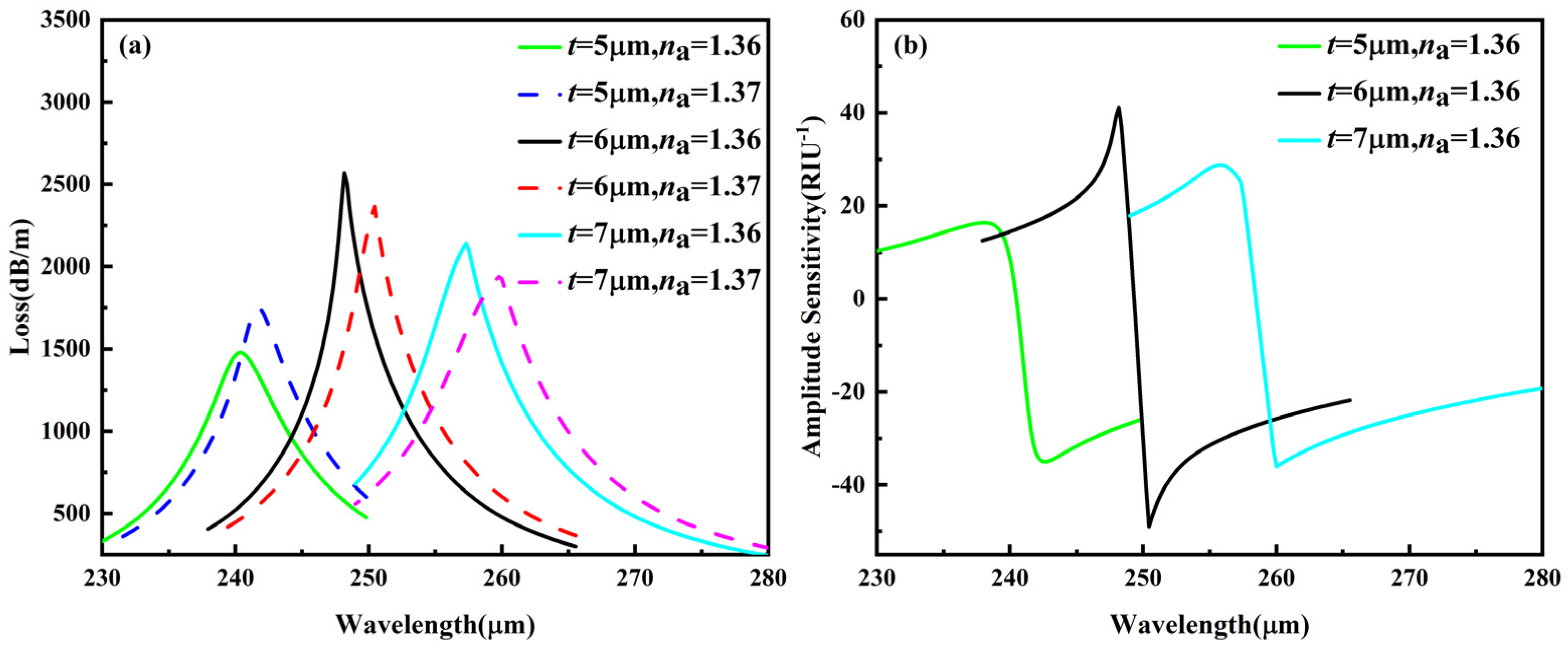
3.1.1. Analysis of PVDF Thickness t
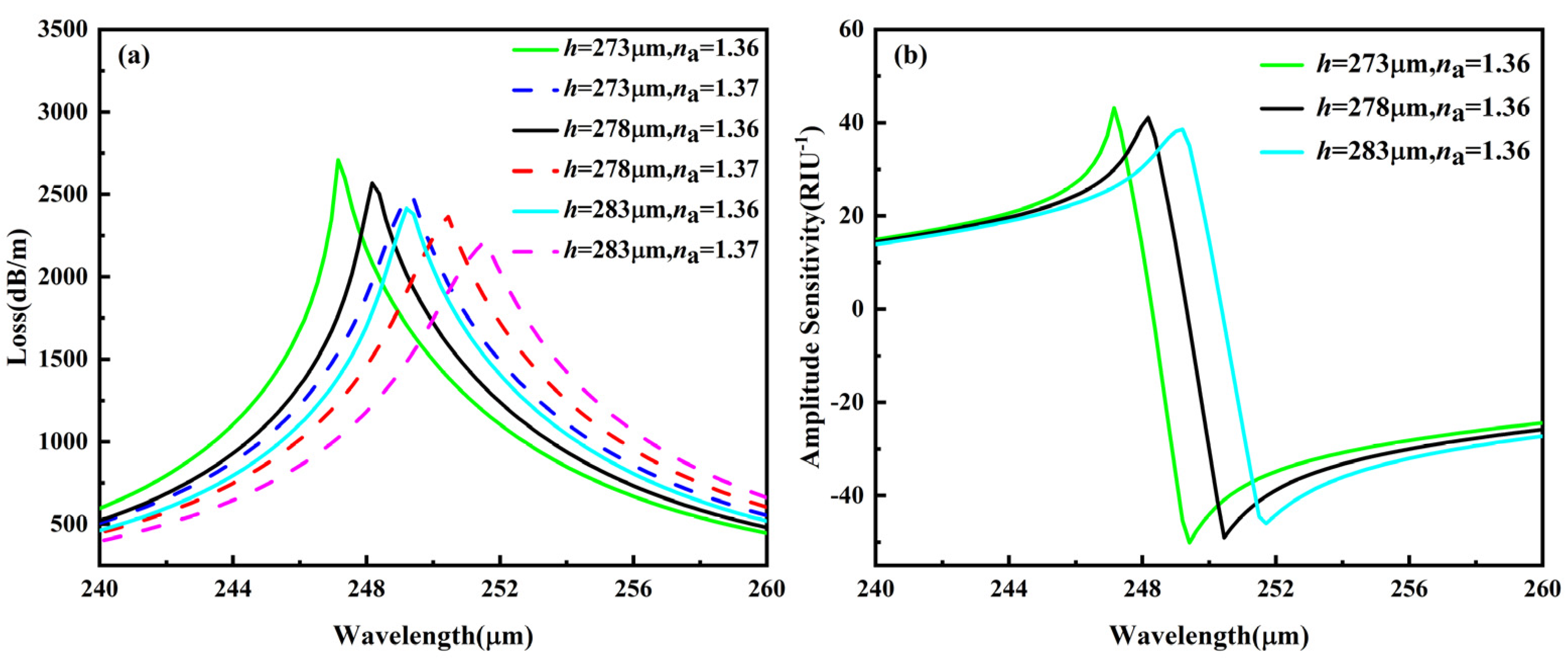
3.1.2. Analysis of Polishing Depth h
3.1.3. Analysis of Air Hole Diameter D
3.1.4. Analysis of Air Hole Diameter d1 and d2
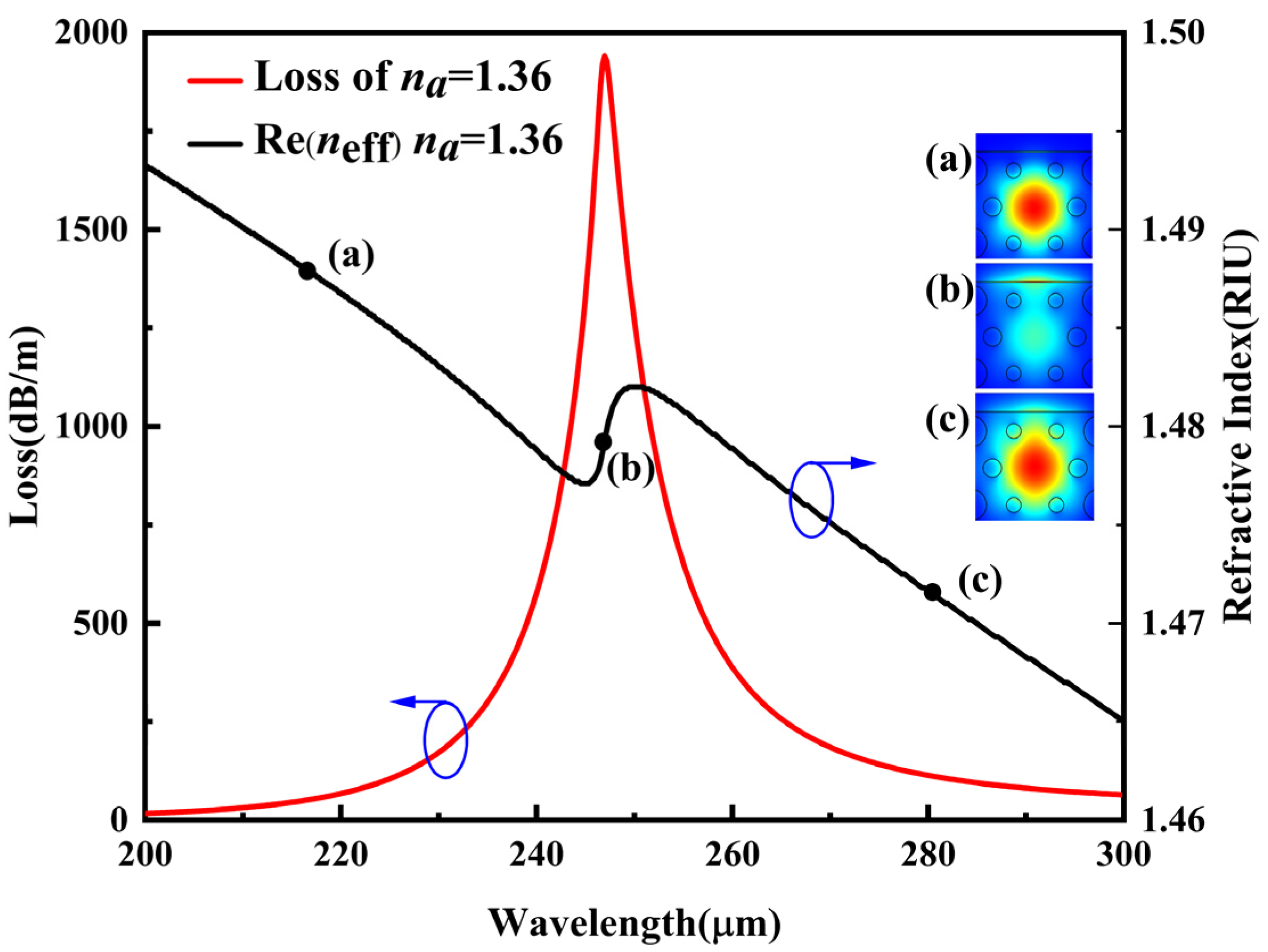
3.2. Sensor Performance at Optimized Parameters
3.3. Analysis of Different Biomaterials
3.4. Response of Linearity
3.5. Fabrication Tolerance
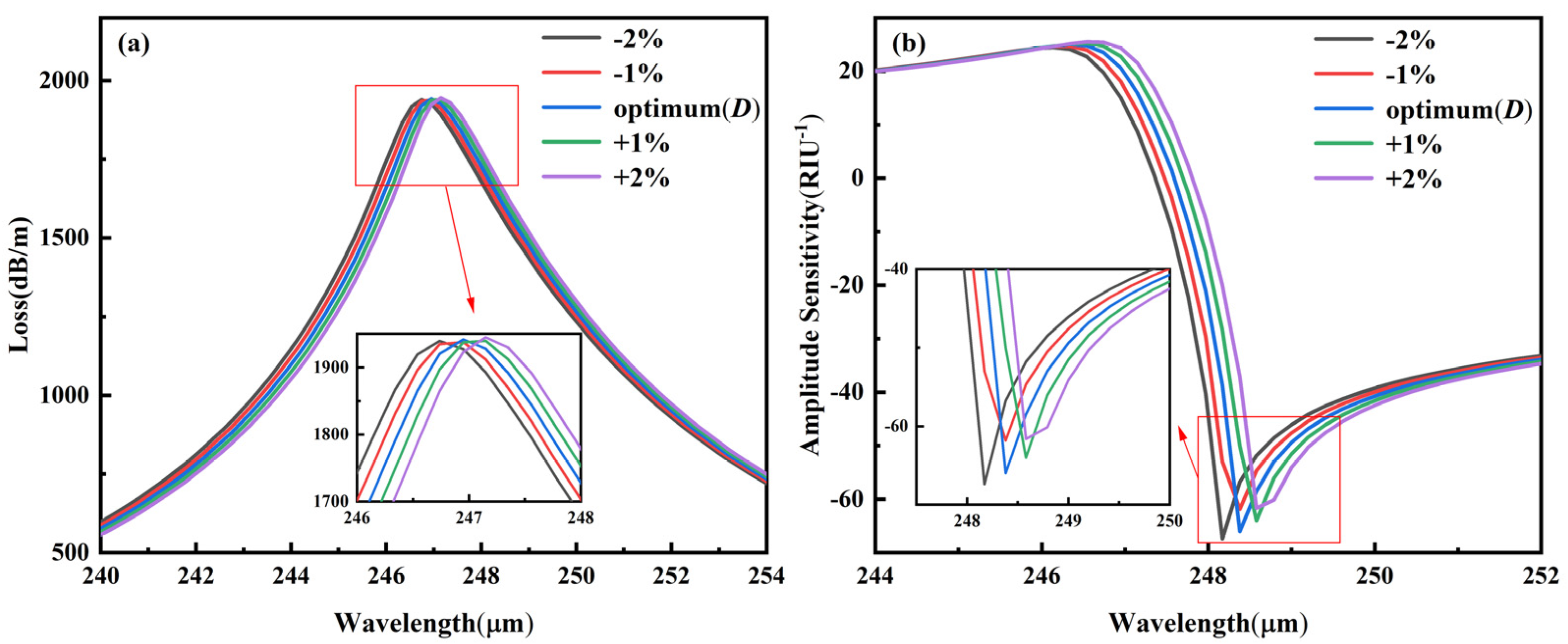
3.5.1. Fabrication Tolerance for ±1–2% Variation in D
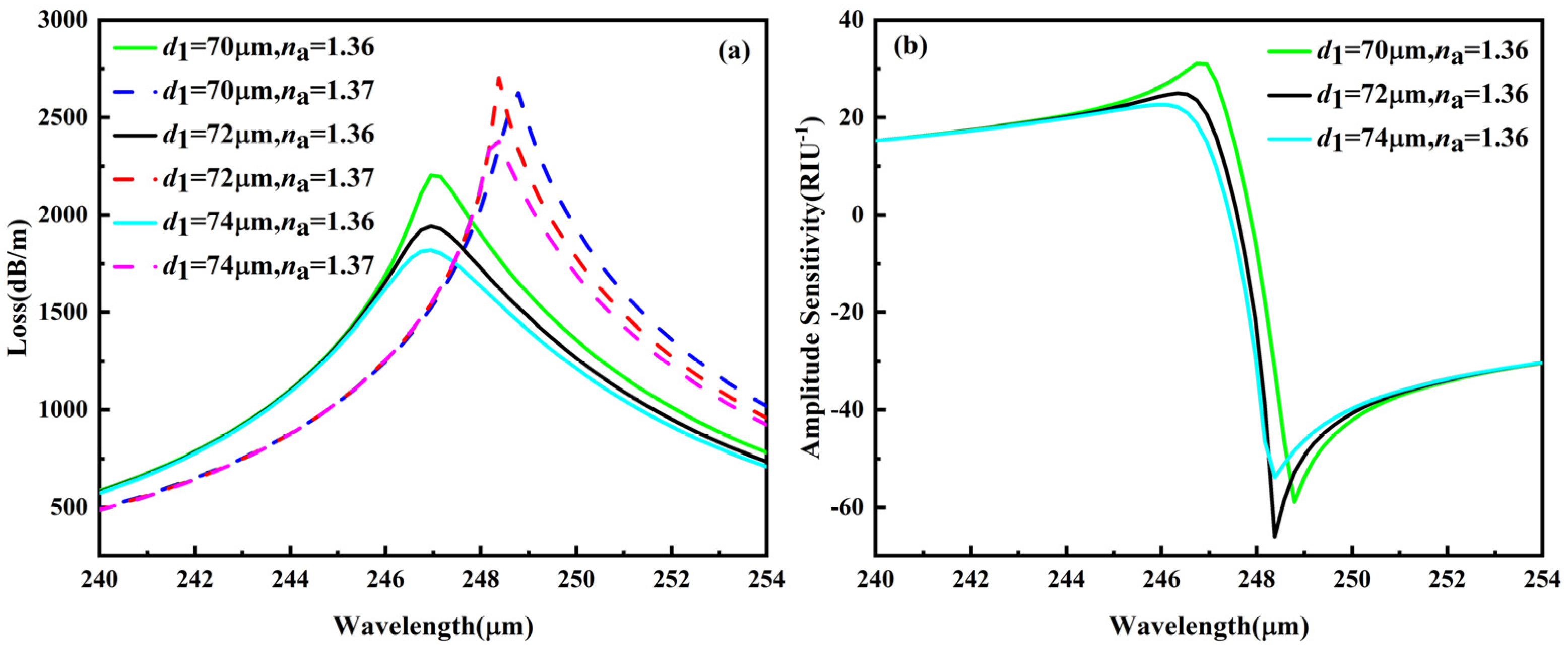
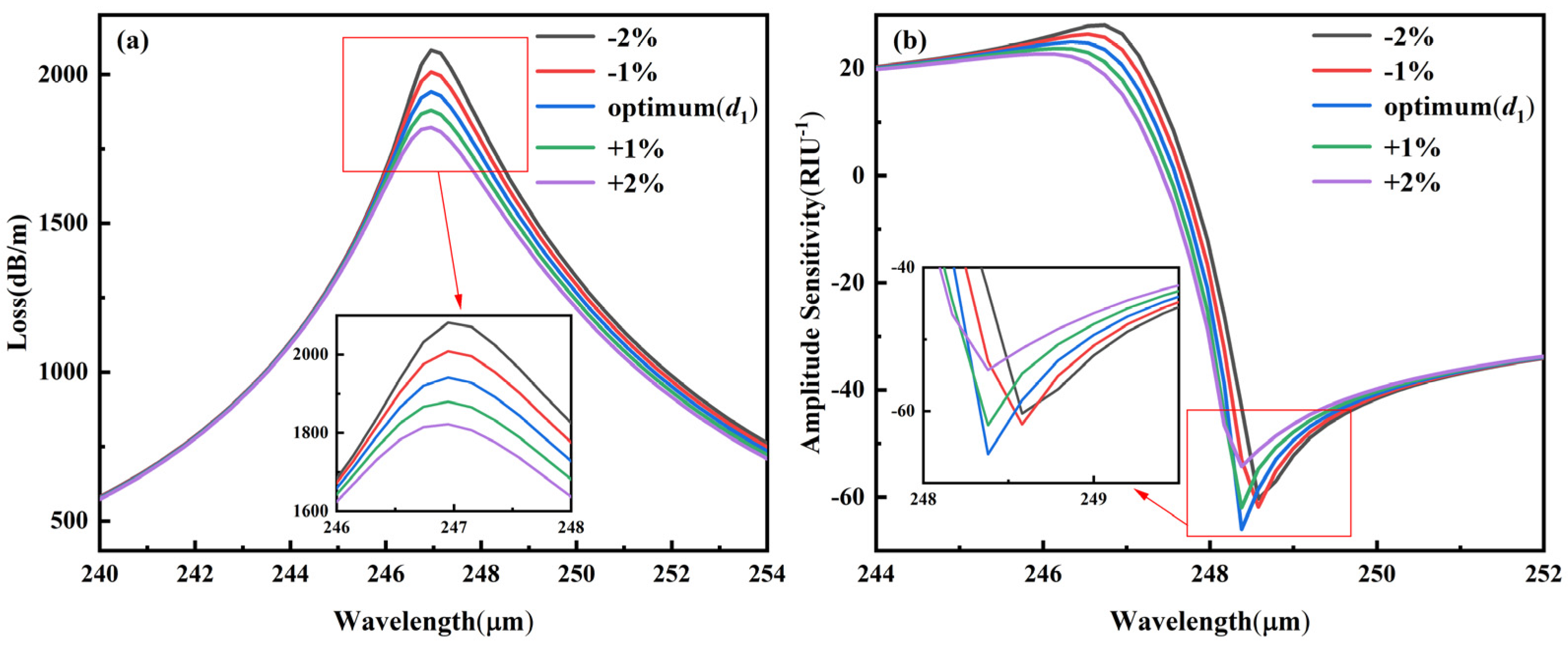
3.5.2. Fabrication Tolerance for ±1–2% Variation in d1
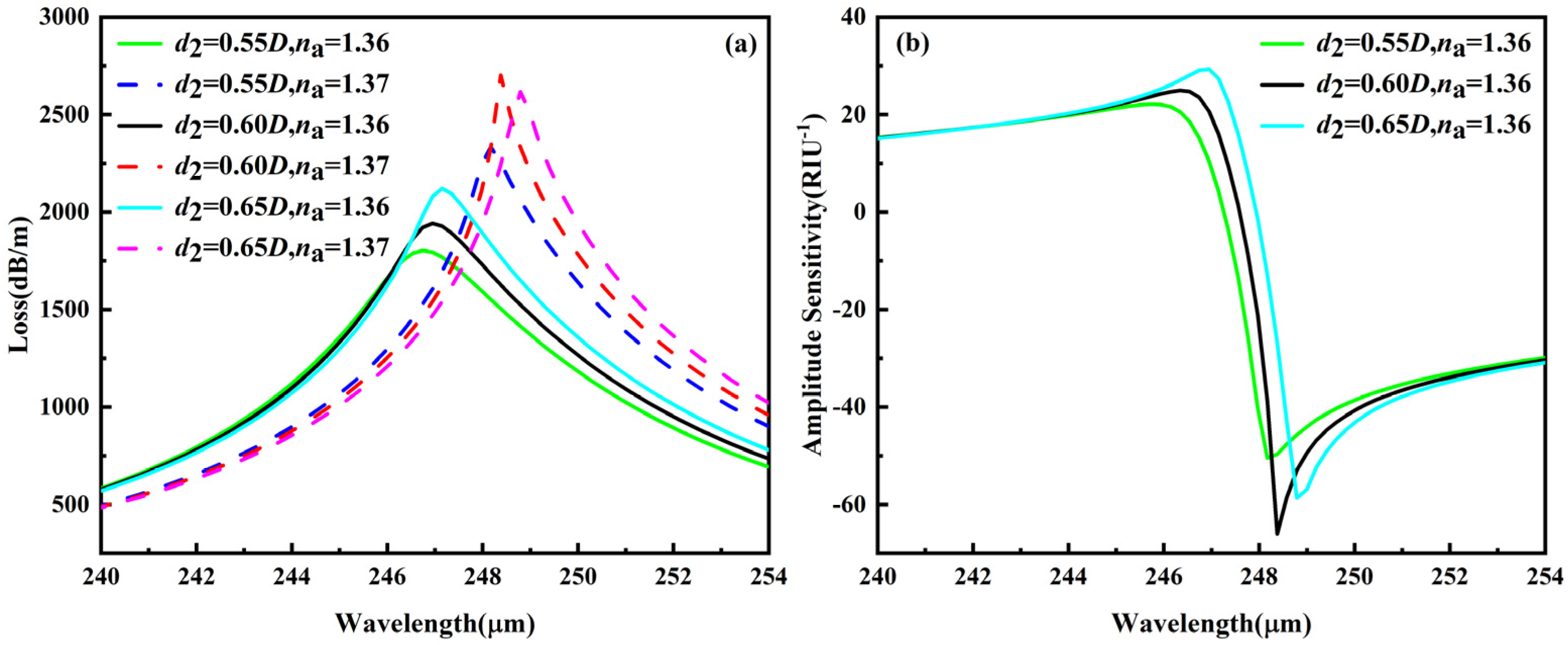
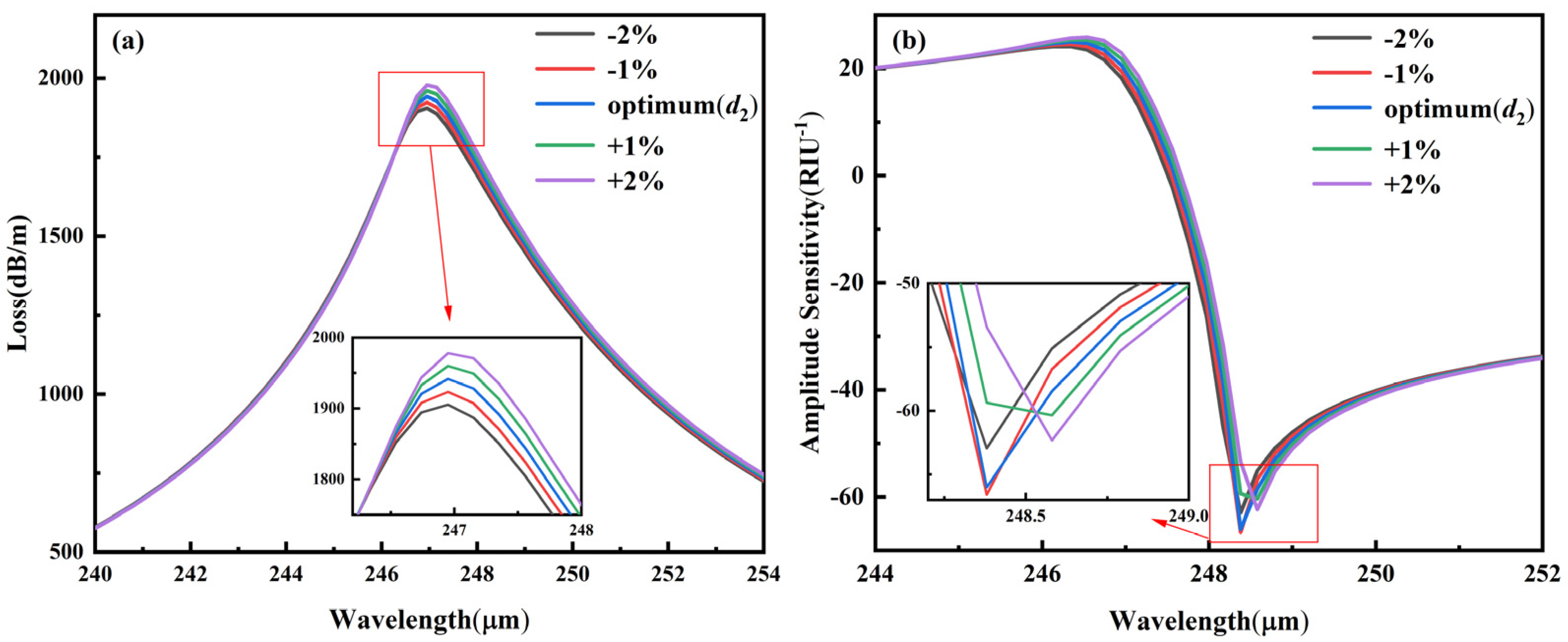
3.5.3. Fabrication Tolerance for ±1–2% Variation in d2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Nakkeeran, K.; Abobaker, A.M.; Aphale, S.S.; Babu, P.R.; Senthilnathan, K. Design and Analysis of Surface-Plasmon-Resonance-Based Photonic Quasi-Crystal Fiber Biosensor for High-Refractive-Index Liquid Analytes. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 6900309. [Google Scholar] [CrossRef]
- Jabin, M.A.; Ahmed, K.; Rana, M.J.; Paul, B.K.; Luo, Y.; Vigneswaran, D. Titanium-Coated Dual-Core D-Shaped SPR-Based PCF for Hemoglobin Sensing. Plasmonics 2019, 14, 1601–1610. [Google Scholar] [CrossRef]
- Wong, W.C.; Chan, C.C.; Boo, J.L.; Teo, Z.Y.; Tou, Z.Q.; Yang, H.B.; Li, C.M.; Leong, K.C. Photonic Crystal Fiber Surface Plasmon Resonance Biosensor Based on Protein G Immobilization. IEEE J. Sel. Top. Quantum Electron. 2013, 19, 4602107. [Google Scholar] [CrossRef]
- Arif, M.F.H.; Ahmed, K.; Asaduzzaman, S.; Azad, M.A.K. Design and optimization of photonic crystal fiber for liquid sensing applications. Photonic Sens. 2016, 6, 279–288. [Google Scholar] [CrossRef]
- Ayyanar, N.; Raja, G.T.; Sharma, M.; Kumar, D.S. Photonic Crystal Fiber-Based Refractive Index Sensor for Early Detection of Cancer. IEEE Sens. J. 2018, 18, 7093–7099. [Google Scholar] [CrossRef]
- Noh, T.K.; Ryu, U.-C.; Lee, Y.W. Compact and wide range polarimetric strain sensor based on polarization-maintaining photonic crystal fiber. Sens. Actuators A Phys. 2014, 213, 89–93. [Google Scholar] [CrossRef]
- Xing, R.; Dong, C.; Wang, Z.; Wu, Y.; Yang, Y.; Jian, S. Simultaneous strain and temperature sensor based on polarization maintaining fiber and multimode fiber. Opt. Laser Technol. 2018, 102, 17–21. [Google Scholar] [CrossRef]
- Yan, W.; Han, Q.; Chen, Y.; Song, H.; Tang, X.; Liu, T. Fiber-loop ring-down interrogated refractive index sensor based on an SNS fiber structure. Sens. Actuators B: Chem. 2018, 255, 2018–2022. [Google Scholar] [CrossRef]
- Yesudasu, V.; Pradhan, H.S.; Pandya, R.J. Recent progress in surface plasmon resonance based sensors: A comprehensive review. Heliyon 2021, 7, e06321. [Google Scholar] [CrossRef]
- Zha, F.; Li, J.; Sun, P.; Ma, H. Highly sensitive selectively coated D-shape photonic crystal fibers for surface plasmon resonance sensing. Phys. Lett. A 2019, 383, 1825–1830. [Google Scholar] [CrossRef]
- Hassani, A.; Skorobogatiy, M. Design of the Microstructured Optical Fiber-based Surface Plasmon Resonance sensors with enhanced microfluidics. Opt. Express 2006, 14, 11616–11621. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Sharma, A.K.; Marques, C. On The Application of SiO2/SiC Grating on Ag for High-Performance Fiber Optic Plasmonic Sensing of Cortisol Concentration. Materials 2020, 13, 1623. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Pandey, A.K.; Kaur, B. Fluoride Fiber-Based Plasmonic Biosensor with Two-Dimensional Material Heterostructures: Enhancement of Overall Figure-of-Merit via Optimization of Radiation Damping in Near Infrared Region. Materials 2019, 12, 1542. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-J.; Gu, B.; An, Q.-F.; Yang, C.; Guan, Y.L.; Yong, K.-T. Recent development of fiber-optic chemical sensors and biosensors: Mechanisms, materials, micro/nano-fabrications and applications. Coord. Chem. Rev. 2018, 376, 348–392. [Google Scholar] [CrossRef]
- Homola, J.; Koudela, I.; Yee, S.S. Surface plasmon resonance sensors based on diffraction gratings and prism couplers: Sensitivity comparison. Sens. Actuators B Chem. 1999, 54, 16–24. [Google Scholar] [CrossRef]
- Gupta, B.D.; Verma, R.K. Surface Plasmon Resonance-Based Fiber Optic Sensors: Principle, Probe Designs, and Some Applications. J. Sens. 2009, 2009, 979761. [Google Scholar] [CrossRef]
- Rifat, A.A.; Ahmed, R.; Yetisen, A.K.; Butt, H.; Sabouri, A.; Mahdiraji, G.A.; Yun, S.H.; Adikan, F.R.M. Photonic crystal fiber based plasmonic sensors. Sens. Actuators B Chem. 2017, 243, 311–325. [Google Scholar] [CrossRef]
- Vengelis, J.; Jarutis, V.; Sirutkaitis, V. Measurement of the phase refractive index of a photonic crystal fiber mode. Opt. Lett. 2018, 43, 2571–2574. [Google Scholar] [CrossRef]
- Sypabekova, M.; Korganbayev, S.; González-Vila, Á.; Caucheteur, C.; Shaimerdenova, M.; Ayupova, T.; Bekmurzayeva, A.; Vangelista, L.; Tosi, D. Functionalized etched tilted fiber Bragg grating aptasensor for label-free protein detection. Biosens. Bioelectron. 2019, 146, 111765. [Google Scholar] [CrossRef]
- Kiroriwal, M.; Singal, P.; Sharma, M.; Singal, A. Hemoglobin sensor based on external gold-coated photonic crystal fiber. Opt. Laser Technol. 2022, 149, 107817. [Google Scholar] [CrossRef]
- Zuhayer, A.; Shafkat, A. Design and analysis of a gold-coated dual-core photonic crystal fiber bio-sensor using surface plasmon resonance. Sens. Bio-Sens. Res. 2021, 33, 100432. [Google Scholar] [CrossRef]
- Stawska, H.I.; Popenda, M.A.; Bereś-Pawlik, E. Combining Hollow Core Photonic Crystal Fibers with Multimode, Solid Core Fiber Couplers through Arc Fusion Splicing for the Miniaturization of Nonlinear Spectroscopy Sensing Devices. Fibers 2018, 6, 77. [Google Scholar] [CrossRef]
- Sardar, M.R.; Faisal, M. Gas sensor based on octagonal hollow core photonic crystal Fiber. In Proceedings of the 2017 IEEE International Conference on Imaging, Vision & Pattern Recognition (icIVPR), Dhaka, Bangladesh, 13–14 February 2017; pp. 1–4. [Google Scholar]
- Morshed, M.; Imran Hassan, M.; Roy, T.K.; Uddin, M.S.; Abdur Razzak, S.M. Microstructure core photonic crystal fiber for gas sensing applications. Appl. Opt. 2015, 54, 8637–8643. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dong, X.; Chan, C.C.; Ni, K.; Zhang, S.; Shum, P.P. Humidity Sensor With a PVA-Coated Photonic Crystal Fiber Interferometer. IEEE Sens. J. 2013, 13, 2214–2216. [Google Scholar] [CrossRef]
- Larrión, B.; Hernáez, M.; Arregui, F.J.; Goicoechea, J.; Bravo, J.; Matías, I.R. Photonic Crystal Fiber Temperature Sensor Based on Quantum Dot Nanocoatings. J. Sens. 2009, 2009, 932471. [Google Scholar] [CrossRef]
- Chao, C.-T.C.; Chen, S.-H.; Huang, H.J.; Kooh, M.R.; Lim, C.M.; Thotagamuge, R.; Mahadi, A.H.; Chau, Y.-F.C. Improving Temperature-Sensing Performance of Photonic Crystal Fiber via External Metal-Coated Trapezoidal-Shaped Surface. Crystals 2023, 13, 813. [Google Scholar] [CrossRef]
- Choi, E.; Choi, Y.; Park, J. Specific and label-free immunoglobulin G antibody detection using nano porous hydrogel photonic crystals. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 2203–2206. [Google Scholar]
- Ramola, A.; Marwaha, A.; Singh, S. Design and investigation of a dedicated PCF SPR biosensor for CANCER exposure employing external sensing. Appl. Phys. A 2021, 127, 643. [Google Scholar] [CrossRef]
- Chao, C.-T.C.; Kooh, M.R.; Chau, Y.-F.C.; Thotagamuge, R. Susceptible Plasmonic Photonic Crystal Fiber Sensor with Elliptical Air Holes and External-Flat Gold-Coated Surface. Photonics 2022, 9, 916. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, X.; Stepanov, A.L.; Ren, F.; Wu, W.; Cai, G.; Zhang, S.; Dai, Z.; Mei, F.; Jiang, C. Efficiency enhancements in Ag nanoparticles-SiO2-TiO2 sandwiched structure via plasmonic effect-enhanced light capturing. Nanoscale Res. Lett. 2013, 8, 73. [Google Scholar] [CrossRef]
- Rifat, A.A.; Mahdiraji, G.A.; Sua, Y.M.; Ahmed, R.; Shee, Y.G.; Adikan, F.R.M. Highly sensitive multi-core flat fiber surface plasmon resonance refractive index sensor. Opt. Express 2016, 24, 2485–2495. [Google Scholar] [CrossRef]
- Shafkat, A. Analysis of a gold coated plasmonic sensor based on a duplex core photonic crystal fiber. Sens. Bio-Sens. Res. 2020, 28, 100324. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wang, F.; Su, W.; Yang, L.; Lv, J.; Fu, G.; Li, X.; Liu, Q.; Sun, T.; et al. Surface plasmon resonance (SPR) infrared sensor based on D-shape photonic crystal fibers with ITO coatings. Opt. Commun. 2020, 464, 125496. [Google Scholar] [CrossRef]
- West, P.R.; Ishii, S.; Naik, G.V.; Emani, N.K.; Shalaev, V.M.; Boltasseva, A. Searching for better plasmonic materials. Laser Photonics Rev. 2010, 4, 795–808. [Google Scholar] [CrossRef]
- Zhu, J.; Ruan, B.; You, Q.; Guo, J.; Dai, X.; Xiang, Y. Terahertz imaging sensor based on the strong coupling of surface plasmon polaritons between PVDF and graphene. Sens. Actuators B Chem. 2018, 264, 398–403. [Google Scholar] [CrossRef]
- Liu, S.; Ma, R.; Li, Y.; Zhao, L.; Xia, Y.; Dong, X.; Pang, Y. D-shaped surface plasmon resonance biosensor based on MoS2 in terahertz band. Opt. Fiber Technol. 2021, 66, 102631. [Google Scholar] [CrossRef]
- Islam, M.R.; Khan, M.M.I.; Al Rafid, R.; Mehjabin, F.; Rashid, M.S.; Chowdhury, J.A.; Zerin, N.; Islam, M. Trigonal cluster-based ultra-sensitive surface plasmon resonance sensor for multipurpose sensing. Sens. Bio-Sens. Res. 2022, 35, 100477. [Google Scholar] [CrossRef]
- Atakaramians, S.; Afshar, V.S.; Fischer, B.M.; Abbott, D.; Monro, T.M. Low loss, low dispersion and highly birefringent terahertz porous fibers. Opt. Commun. 2009, 282, 36–38. [Google Scholar] [CrossRef]
- Saiful, I.; Mohammad Rakibul, I.; Mohammad, F.; Abu, S.M.S.A.; Hasan, R.; Jakeya, S.; Sohel, R. Extremely low-loss, dispersion flattened porous-core photonic crystal fiber for terahertz regime. Opt. Eng. 2016, 55, 076117. [Google Scholar] [CrossRef]
- Hidaka, T.; Minamide, H.; Ito, H.; Nishizawa, J.; Tamura, K.; Ichikawa, S. Ferroelectric PVDF cladding terahertz waveguide. J. Light. Technol. 2005, 23, 2469–2473. [Google Scholar] [CrossRef]
- Liu, G.; Tian, F.; Lu, Y.; Han, Z.; Yao, C.; Wang, B.; Zhang, Y.; Li, L.; Zhang, J. Zeonex-based high sensitivity dual-channel SPR optical fiber sensor for gaseous analytes in terahertz regime. Optik 2022, 255, 168656. [Google Scholar] [CrossRef]
- Shakya, A.K.; Singh, S. Design of dual polarized tetra core PCF based plasmonic RI sensor for visible-IR spectrum. Opt. Commun. 2021, 478, 126372. [Google Scholar] [CrossRef]
- Liu, C.; Su, W.; Liu, Q.; Lu, X.; Wang, F.; Sun, T.; Chu, P.K. Symmetrical dual D-shape photonic crystal fibers for surface plasmon resonance sensing. Opt. Express 2018, 26, 9039–9049. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Jeon, D.I.; Ahn, S.-G.; Yoon, J.-H.; Kim, S.; Lee, B.H. Full-field optical coherence microscopy for identifying live cancer cells by quantitative measurement of refractive index distribution. Opt. Express 2010, 18, 23285–23295. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lee, C.-W.; Lin, E.-H.; Wei, P.-K. Single live cell refractometer using nanoparticle coated fiber tip. Appl. Phys. Lett. 2008, 93, 173110. [Google Scholar] [CrossRef]
- Curl, C.L.; Bellair, C.J.; Harris, T.; Allman, B.E.; Harris, P.J.; Stewart, A.G.; Roberts, A.; Nugent, K.A.; Delbridge, L.M.D. Refractive index measurement in viable cells using quantitative phase-amplitude microscopy and confocal microscopy. Cytom. Part A 2005, 65A, 88–92. [Google Scholar] [CrossRef]
- Rakibul Islam, M.; Khan, M.M.I.; Mehjabin, F.; Alam Chowdhury, J.; Islam, M. Design of a fabrication friendly & highly sensitive surface plasmon resonance-based photonic crystal fiber biosensor. Results Phys. 2020, 19, 103501. [Google Scholar] [CrossRef]
- Ramani, U.; Kumar, H.; Singh, B.K.; Pandey, P.C. Design of surface plasmon resonance based both side polished photonic crystal fiber for highly efficient refractive index sensor. Optik 2021, 248, 168062. [Google Scholar] [CrossRef]
- Anthony, J.; Leonhardt, R.; Argyros, A.; Large, M.C.J. Characterization of a microstructured Zeonex terahertz fiber. J. Opt. Soc. Am. B 2011, 28, 1013–1018. [Google Scholar] [CrossRef]
- Woyessa, G.; Fasano, A.; Markos, C.; Stefani, A.; Rasmussen, H.K.; Bang, O. Zeonex microstructured polymer optical fiber: Fabrication friendly fibers for high temperature and humidity insensitive Bragg grating sensing. Opt. Mater. Express 2017, 7, 286–295. [Google Scholar] [CrossRef]
- Cordeiro, C.M.B.; Ng, A.K.L.; Ebendorff-Heidepriem, H. Ultra-simplified Single-Step Fabrication of Microstructured Optical Fiber. Sci. Rep. 2020, 10, 9678. [Google Scholar] [CrossRef]
- Mahfuz, M.A.; Hossain, M.A.; Haque, E.; Hai, N.H.; Namihira, Y.; Ahmed, F. Dual-Core Photonic Crystal Fiber-Based Plasmonic RI Sensor in the Visible to Near-IR Operating Band. IEEE Sens. J. 2020, 20, 7692–7700. [Google Scholar] [CrossRef]

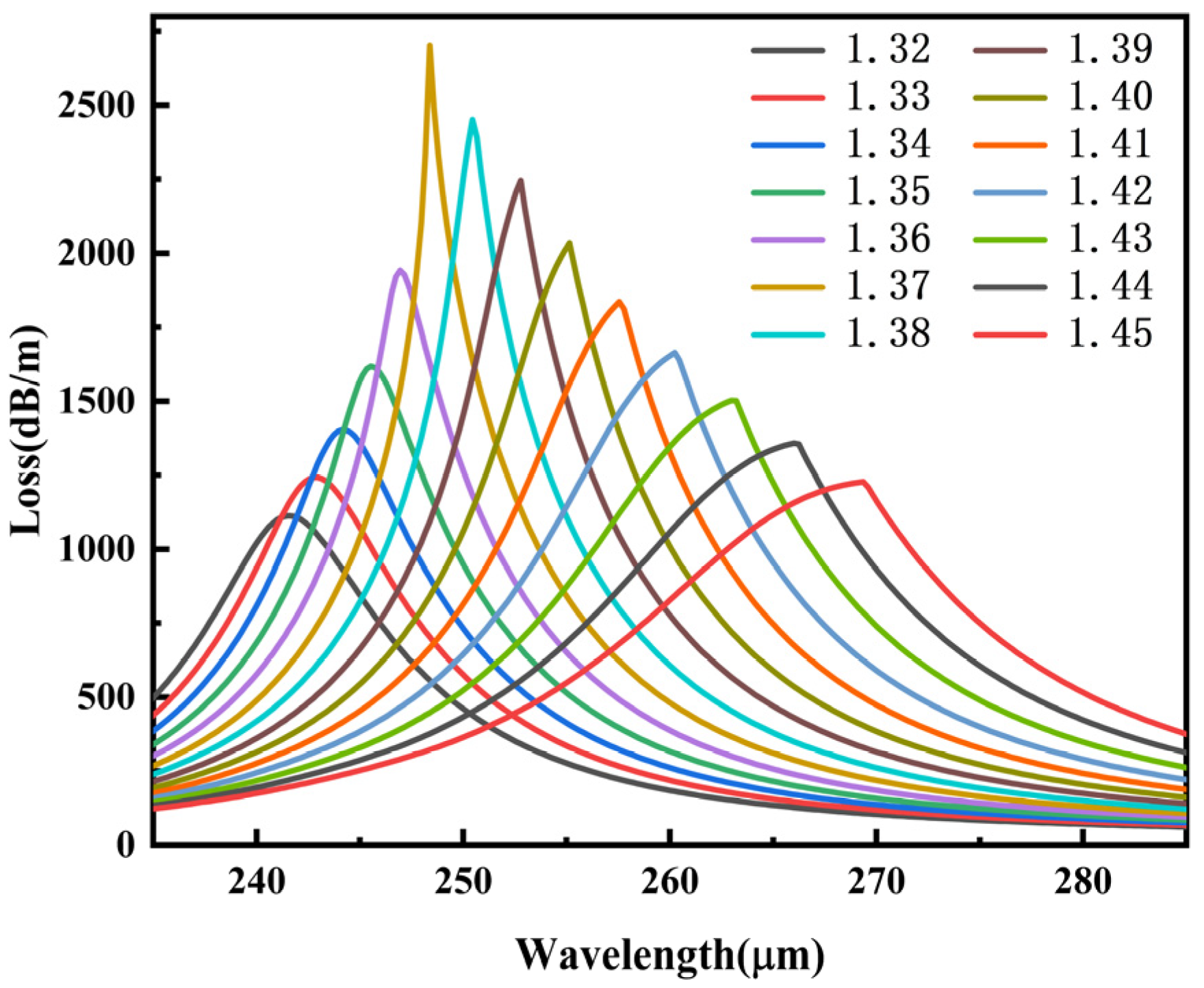


| na | AS (RIU−1) | WS (μm/RIU) | SR (RIU) | FWHM (μm) | FOM (RIU−1) |
|---|---|---|---|---|---|
| 1.32 | −27.40 | 137.00 | 7.30 × 10−7 | 12.64 | 10.84 |
| 1.33 | −29.41 | 119.00 | 8.40 × 10−7 | 12.02 | 9.90 |
| 1.34 | −32.40 | 140.00 | 7.14 × 10−7 | 11.23 | 12.47 |
| 1.35 | −38.38 | 142.00 | 7.04 × 10−7 | 10.15 | 13.99 |
| 1.36 | −66.01 | 143.00 | 6.99 × 10−7 | 8.54 | 16.74 |
| 1.37 | −51.31 | 207.00 | 4.83 × 10−7 | 5.25 | 39.42 |
| 1.38 | −44.15 | 233.00 | 4.29 × 10−7 | 6.94 | 33.57 |
| 1.39 | −38.15 | 236.00 | 4.24 × 10−7 | 8.48 | 27.83 |
| 1.40 | −34.14 | 241.00 | 4.15 × 10−7 | 10.22 | 23.58 |
| 1.41 | −31.13 | 269.00 | 3.72 × 10−7 | 12.13 | 22.17 |
| 1.42 | −28.73 | 274.00 | 3.65 × 10−7 | 14.10 | 19.43 |
| 1.43 | −26.71 | 303.00 | 3.30 × 10−7 | 16.29 | 28.60 |
| 1.44 | −24.98 | 335.00 | 2.98 × 10−7 | 18.65 | 17.96 |
| 1.45 | 21.27 |
| Reference | RI Range | Operating Wavelength (μm) | AS (RIU−1) | WS (μm/RIU) | SR (RIU) |
|---|---|---|---|---|---|
| [42] | 1.00–1.03 | 300–460 | −44.84 | 110.00 | 2.60 × 10−4 |
| [37] | 1.33–1.40 | 150–300 | - | 715.59 | 1.40 × 10−7 |
| [20] | 1.33–1.40 | 0.55–0.90 | 2080.00 | 10.00 | 4.80 × 10−6 |
| [21] | 1.35–1.40 | 0.55–1.10 | 1443.00 | 8.00 | 1.25 × 10−5 |
| [49] | 1.30–1.40 | 0.60–1.65 | 947.00 | 22.80 | 4.38 × 10−6 |
| This work | 1.32–1.45 | 235–285 | −66.01 | 335.00 | 8.40 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yao, Y.; Guang, Z.; Xue, J.; Wang, Q.; Gong, J.; Ali, Z.; Yang, Z. A High-Sensitivity Fiber Biosensor Based on PVDF-Excited Surface Plasmon Resonance in the Terahertz Band. Photonics 2023, 10, 1159. https://doi.org/10.3390/photonics10101159
Zhang Y, Yao Y, Guang Z, Xue J, Wang Q, Gong J, Ali Z, Yang Z. A High-Sensitivity Fiber Biosensor Based on PVDF-Excited Surface Plasmon Resonance in the Terahertz Band. Photonics. 2023; 10(10):1159. https://doi.org/10.3390/photonics10101159
Chicago/Turabian StyleZhang, Yani, Yiming Yao, Zhe Guang, Jia Xue, Qiuyang Wang, Jiaqin Gong, Zohaib Ali, and Zhongtian Yang. 2023. "A High-Sensitivity Fiber Biosensor Based on PVDF-Excited Surface Plasmon Resonance in the Terahertz Band" Photonics 10, no. 10: 1159. https://doi.org/10.3390/photonics10101159





