Solution-Processed Functionalized MoS2 Nanosheets Composite for Photodetection Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
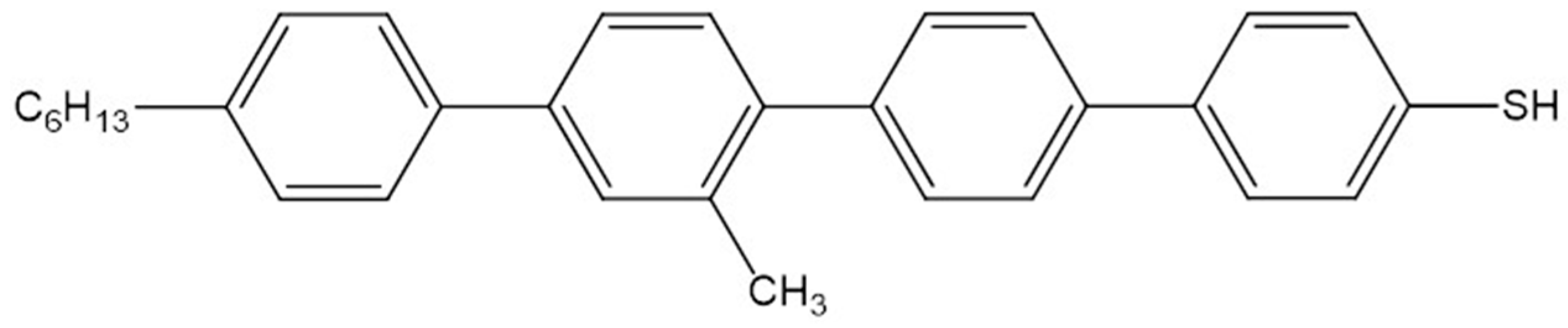
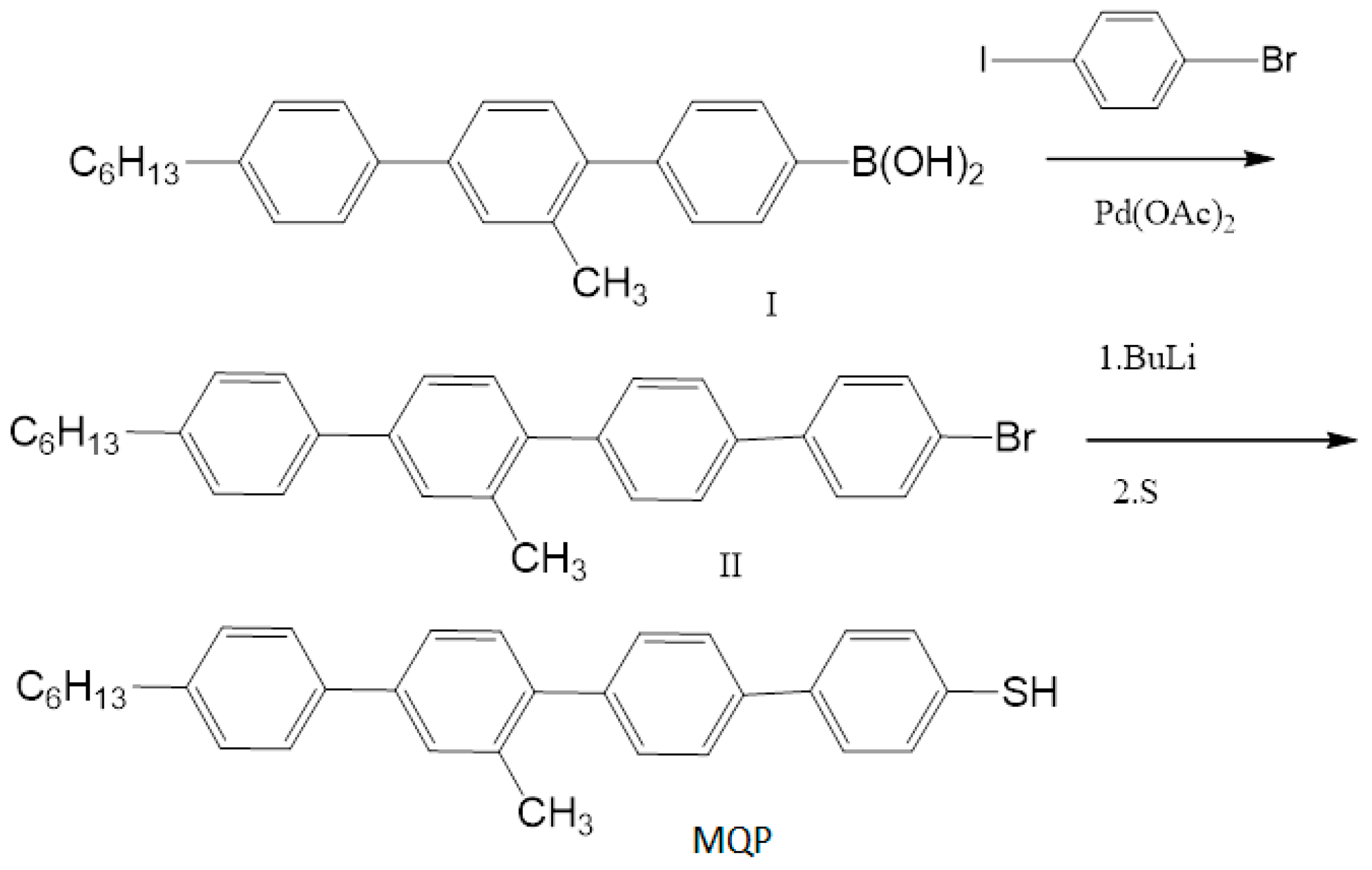
2.2. MoS2 Nanosheets Functionalization
2.3. Thin Film Preparation
2.4. Device Fabrication
2.5. Characterization
3. Results and Discussion
3.1. Microscopy
3.2. Raman Spectra
3.3. Absorption and Luminescence
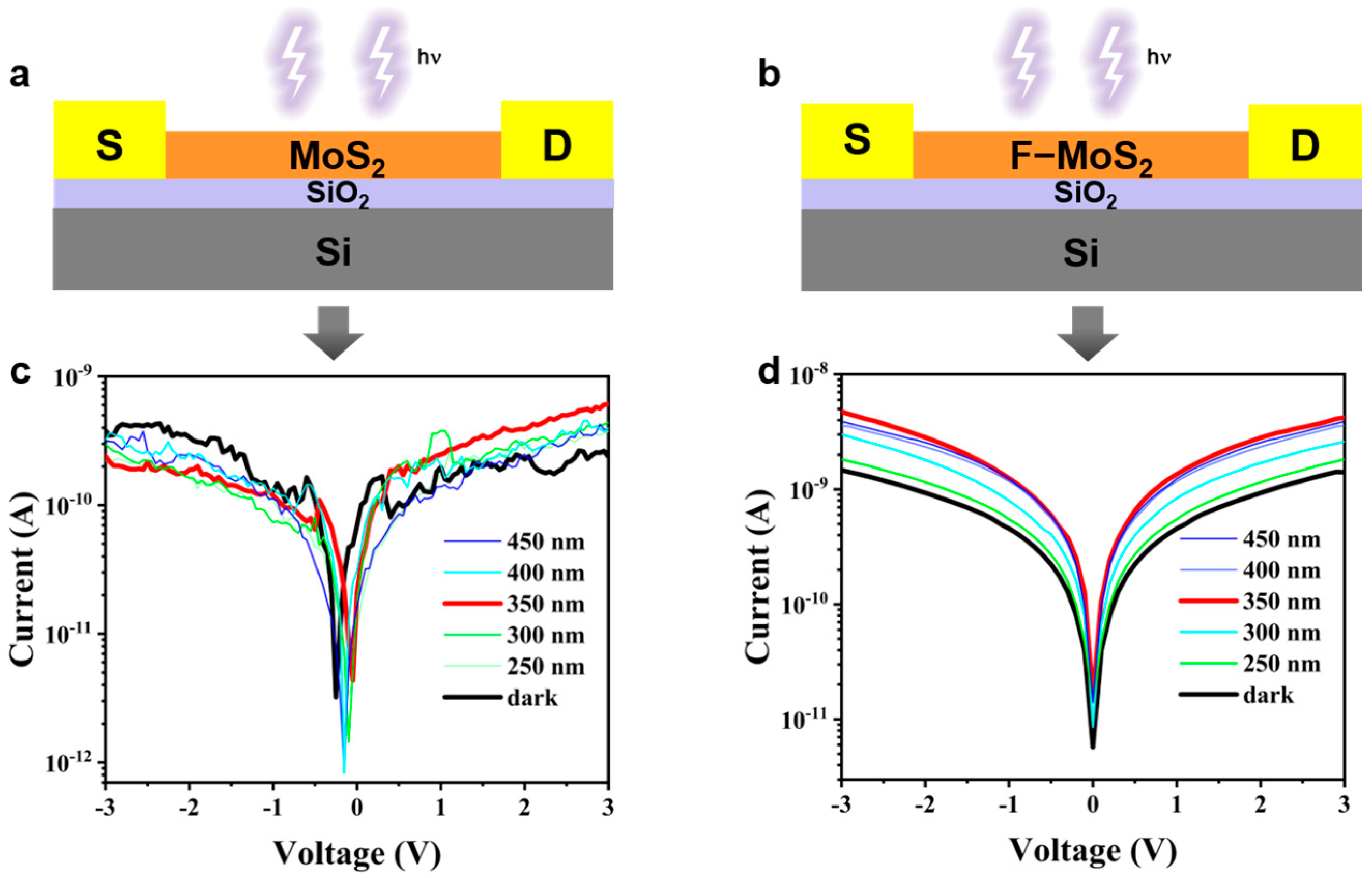
3.4. Current-Voltage Characteristics of Photodetectors
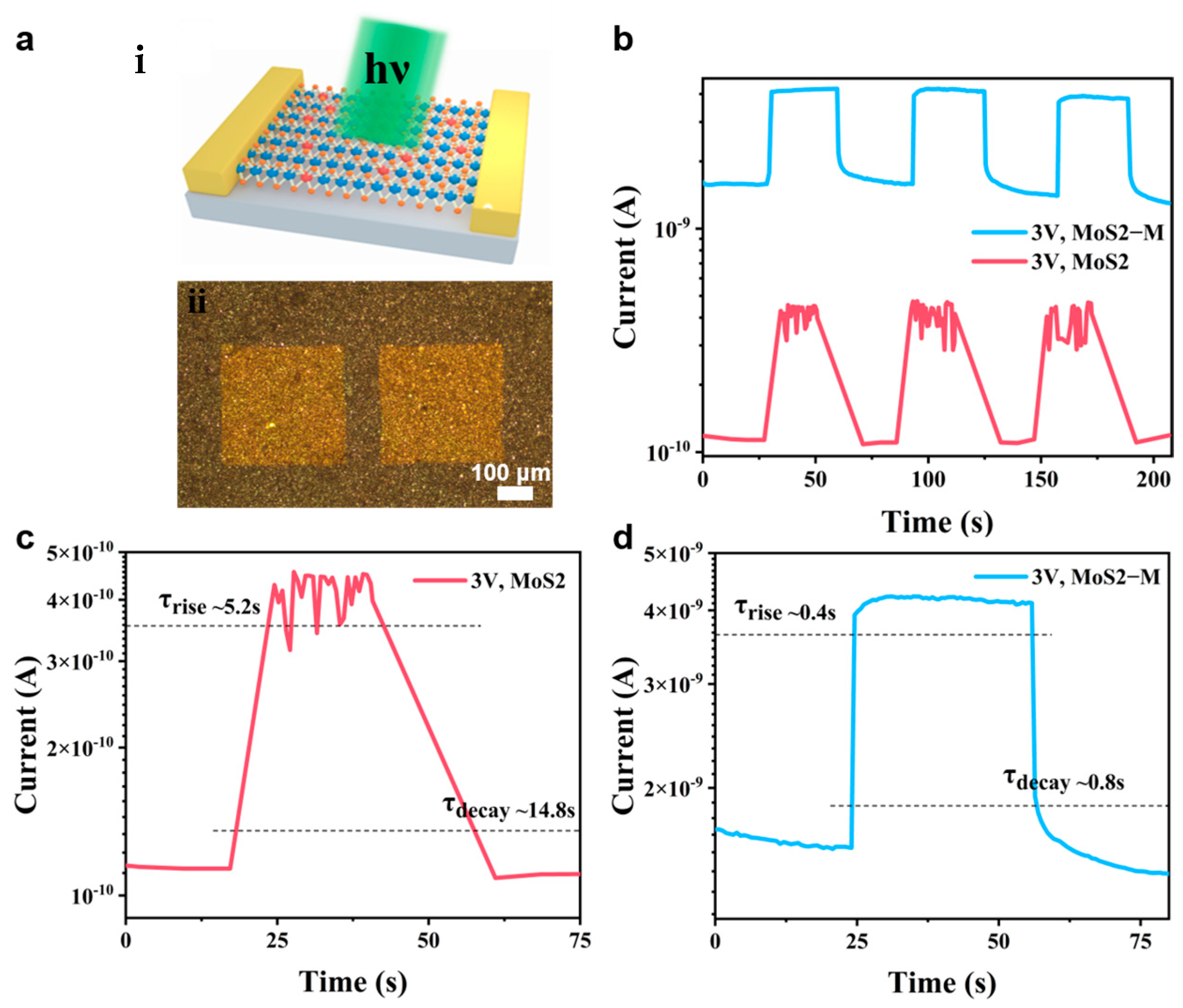
3.5. Photoresponse Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.Q.; Yan, T.T.; Fang, X.S. Low-dimensional wide-bandgap semiconductors for UV photodetectors. Nat. Nanotech. Nat. Rev. Mater. 2023, 8, 587–603. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, C.; Zou, X.; Liao, L. Recent advances in optoelectronic devices based on 2D materials and their heterostructures. Adv. Opt. Mater. 2019, 7, 1800441. [Google Scholar] [CrossRef]
- Malik, M.; Iqbal, M.A.; Choi, J.R.; Pham, P.V. 2D materials for efficient photodetection: Overview, mechanisms, performance and UV-IR range applications. Front. Chem. 2022, 10, 905404. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A review on MoS2 properties, synthesis, sensing applications, and challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Nalwa, H.S. A review of molybdenum disulfide (MoS2) based photodetectors: From ultra-broadband, selfpowered to flexible devices. RSC Adv. 2020, 10, 30529–30602. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Hu, M.; Kang, F.; Lv, R. Flexible photodetector based on large-area few-layer MoS2. Progress. Nat. Sci. Mater. Int. 2018, 28, 563–568. [Google Scholar] [CrossRef]
- Liu, X.; Hu, S.; Lin, Z.; Li, X.; Song, L.; Yu, W.; Wang, Q.; He, W. High-performance MoS2 photodetectors using a patterned gallium nitride substrate. ACS Appl. Mater. Interfaces 2021, 13, 15820–15826. [Google Scholar] [CrossRef] [PubMed]
- Mutz, N.; Park, S.; Schultz, T.; Sadofev, S.; Dalgleish, S.; Reissig, L.; Koch, N.; List-Kratochvil, E.J.W.; Blumstengel, S. Excited-state charge transfer enabling MoS2/phthalocyanine photodetectors with extended spectral sensitivity. J. Phys. Chem. C 2020, 124, 2837–2843. [Google Scholar] [CrossRef]
- Kiriya, D.; Tosun, M.; Zhao, P.; Kang, J.S.; Javey, A. Air-stable surface charge transfer doping of MoS2 by benzyl viologen. J. Am. Chem. Soc. 2014, 136, 7853–7856. [Google Scholar] [CrossRef] [PubMed]
- Nur, R.; Tsuchiya, T.; Toprasertpong, K.; Terabe, K.; Takagi, S.; Takenaka, M. High responsivity in MoS2 phototransistors based on charge trapping HfO2 dielectrics. Communs. Mat. 2020, 1, 103. [Google Scholar] [CrossRef]
- Wu, S.; Wu, G.; Wang, X.; Chen, Y.; Lin, T.; Shen, H.; Hu, W.; Meng, X.; Wang, J.; Chu, J. A gate-free MoS2 phototransistor assisted by ferroelectrics. J. Semicond. 2019, 40, 092002. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Liang, Z.-Y.; Lin, Y.-C.; Wang, C.-C.; Lin, K.-I.; Suenaga, K.; Chiu, P.-W. Photogating WS2 photodetectors using embedded WSe2 charge puddles. ACS Nano. 2020, 14, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhuge, F.; Hou, J.; Lv, L.; Luo, P.; Zhou, N.; Gan, L.; Zhai, Z. Van der Waals coupled organic molecules with monolayer MoS2 for fast response photodetectors with gate-tunable responsivity. ACS Nano. 2018, 12, 4062–4073. [Google Scholar] [CrossRef] [PubMed]
- Raoufi, M.; Rühl, S.; Chandrabose, S.; Shukla, A.; Sun, B.; Kratochvil, E.-L.; Blumstengel, S.; Neher, D. Fast Photoresponse from hybrid monolayer MoS2/organic photodetector. Phys. Status Solidi A 2023, 2300107. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Kumar, A.; Durante, O.; Sessa, A.; Faella, E.; Viscardi, L.; Intonti, K.; Giubileo, F.; Martucciello, N.; Romano, P.; et al. Temperature-dependent photoconductivity in two-dimensional MoS2 transistors. Mater. Today Nano. 2023, 24, 100382. [Google Scholar] [CrossRef]
- Andleeb, S.; Wang, X.; Dong, H.; Valligatla, S.; Saggau, C.N.; Ma, L.; Schmidt, O.G.; Zhu, F. Fast-response micro-phototransistor based on MoS2/organic molecule heterojunction. Nanomaterials 2023, 13, 1491. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; He, M.; Wu, Q.; Wu, C.C.; Li, X.B.; Liu, B.L.; Tang, M.-C.; Yao, J.; Wei, G. Ultrafast charge transfer 2D MoS2/organic heterojunction for sensitive photodetector. Adv. Sci. 2023, 10, 2207743. [Google Scholar] [CrossRef]
- Cunningham, G.; Khan, U.; Backes, C.; Hanlon, D.; McCloskey, D.; Donegan, J.; Coleman, J.N. Photoconductivity of solution-processed MoS2 films. J. Mater. Chem. C 2013, 1, 6899–6904. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Z.Q.; Fang, X.S. Solution-prepared AgBi2I7 Thin Films and Their Photodetecting Properties. J. Inorg. Mater. 2023, 38, 1055–1061. [Google Scholar] [CrossRef]
- Daukiya, L.; Seibel, J.; De Feyter, S. Chemical modification of 2D materials using molecules and assemblies of molecules. Adv. Phys. X 2019, 4, 1625723. [Google Scholar] [CrossRef]
- Molina-Mendoza, A.J.; Vaquero-Garzon, L.; Leret, S.; de Juan-Fernández, L.; Pérez, E.M.; Castellanos-Gomez, A. Engineering the optoelectronic properties of MoS2 photodetectors through reversible noncovalent functionalization. Chem. Communs. 2016, 52, 14365–14368. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Okabe, S.; Takaguchi, Y. Photoinduced electron transfer in a MoS2/anthracene mixed-dimensional heterojunction in aqueous media. Bull. Chem. Soc. Jpn. 2020, 93, 745–750. [Google Scholar] [CrossRef]
- Fang, H.; Hu, W. Photogating in low dimensional photodetectors. Adv. Sci. 2017, 4, 1700323. [Google Scholar] [CrossRef]
- Shin, J.; Yoo, H. Photogating effect-driven photodetectors and their emerging applications. Nanomaterials 2023, 13, 882. [Google Scholar] [CrossRef]
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; O’Neill, A.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G.; et al. Large-scale exfoliation of inorganic layered compounds in aqueous surfactant solutions. Adv. Mater. 2011, 23, 3944–3948. [Google Scholar] [CrossRef]
- Palenzuela, C.L.M.; Luxa, J.; Sofer, Z.; Pumera, M. MoSe2 dispersed in stabilizing surfactant media: Effect of the surfactant type and concentration on electron transfer and catalytic properties. ACS Appl. Mater. Interfaces 2018, 10, 17820–17826. [Google Scholar] [CrossRef] [PubMed]
- Shyyko, L.O.; Kotsyubynsky, V.O.; Budzulyak, I.M. The importance of surfactant and its type on MoS2 nanoparticles formation. J. Nanosci. Nanotech. 2016, 16, 7792–7796. [Google Scholar] [CrossRef]
- Sasnouski, G.; Lapanik, V.; Bezborodov, V.; Dabrowski, R.; Dziaduczek, J. Synthesis of fluoro substituted quaterphenyl liquid crystals. Phase Transit. 2014, 87, 783–789. [Google Scholar] [CrossRef]
- Chen, X.; McGlynn, C.; McDonald, A.R. Two-dimensional MoS2 catalyzed oxidation of organic thiols. Chem. Mater. 2018, 30, 6978–6982. [Google Scholar] [CrossRef]
- Makarova, M.; Okawa, Y.; Aono, M. Selective adsorption of thiol molecules at sulfur vacancies on MoS2(0001), followed by vacancy repair via S−C dissociation. J. Phys. Chem. C 2012, 116, 22411–22416. [Google Scholar] [CrossRef]
- Canton-Vitoria, R.; Sayed-Ahmad-Baraza, Y.; Pelaez-Fernandez, M.; Arenal, R.; Bittencourt, C.; Ewels, C.P.; Tagmatarchis, N. Functionalization of MoS2 with 1,2-dithiolanes: Toward donoracceptor nanohybrids for energy conversion. Npj 2D Mater. Appl. 2017, 1, 13. [Google Scholar] [CrossRef]
- Chen, X.; Denninger, P.; Stimpel-Lindner, T.; Spiecker, E.; Duesberg, G.S.; Backes, C.; Knirsch, K.C.; Hirsch, A. Defect engineering of two-dimensional molybdenum disulfide. Chem. Eur. J. 2020, 26, 6535–6544. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Bacaksiz, C.; Unsal, E.; Akbali, B.; Tomak, A.; Zareie, H.M.; Sahin, H. Theoretical and experimental investigation of conjugation of 1,6- hexanedithiol on MoS2. Mater. Res. Express. 2018, 5, 036415. [Google Scholar] [CrossRef]
- Wu, S.Q.; Wang, X.D.; Jiang, W.; Tu, L.; Chen, Y.; Liu, J.; Lin, T.; Shen, H.; Ge, J.; Hu, W.; et al. Interface engineering of ferroelectric-gated MoS2 phototransistor. Sci. China Inf. Sci. 2021, 64, 140407. [Google Scholar] [CrossRef]
- O’Neill, A.; Khan, U.; Coleman, J.N. Preparation of high concentration dispersions of exfoliated MoS2 with increased flake size. Chem. Mater. 2012, 24, 2414–2421. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Z.; Sun, M.; Lin, F.; Cheng, T.; Shi, J.; Xie, C.; Shi, Y.; Jiang, S.; Huan, Y.; et al. Thickness tunable wedding-cake-like MoS2 flakes for high-performance optoelectronics. ACS Nano 2019, 26, 3649–3658. [Google Scholar] [CrossRef]
- Ha, H.D.; Han, D.J.; Choi, J.S.; Park, M.; Seo, T.S. Dual role of blue luminescent MoS2 quantum dots in fluorescence resonance energy transfer phenomenon. Small 2014, 10, 3858–3862. [Google Scholar] [CrossRef]
- Park, S.J.; Pak, S.W.; Qiu, D.; Kang, J.H.; Song, D.Y.; Kim, E.K. Structural and optical characterization of MoS2 quantum dots defined by thermal annealing. J. Lum. 2017, 183, 62–67. [Google Scholar] [CrossRef]
- Feng, S.; Lv, J.; Pei, F.; Lv, X.; Wu, Y.; Hao, Q.; Zhang, Y.; Tong, Z.; Lei, W. Fluorescent MoS2 QDs based on IFE for turn-off determination of FOX-7 in real water samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 231, 118131. [Google Scholar] [CrossRef]
- Manamel, L.T.; Mukherjee, A.; Das, B.C. Two-dimensional nanohybrid of MoS2 and Rose Bengal: Facile solution growth and band structure probing. Appl. Surf. Sci. 2020, 530, 147063. [Google Scholar] [CrossRef]
- Kukhta, A.V.; Paddubskaya, A.G.; Kuzhir, P.P.; Maksimenko, S.A.; Vorobyova, S.A.; Bistarelli, S.; Cataldo, A.; Belucchi, S. Copper nanoparticles decorated graphene nanoplatelets and their polymer composites. Synth. Met. 2016, 222, 192–197. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukhta, A.V.; Hong, E.; Valynets, N.I.; Maksimenko, S.A.; Parkhomenka, U.; Belko, N.; Lugovsky, A.; Pavich, T.A.; Kukhta, I.N.; Li, Z.; et al. Solution-Processed Functionalized MoS2 Nanosheets Composite for Photodetection Application. Photonics 2023, 10, 1295. https://doi.org/10.3390/photonics10121295
Kukhta AV, Hong E, Valynets NI, Maksimenko SA, Parkhomenka U, Belko N, Lugovsky A, Pavich TA, Kukhta IN, Li Z, et al. Solution-Processed Functionalized MoS2 Nanosheets Composite for Photodetection Application. Photonics. 2023; 10(12):1295. https://doi.org/10.3390/photonics10121295
Chicago/Turabian StyleKukhta, Alexander V., Enliu Hong, Nadzeya I. Valynets, Sergei A. Maksimenko, Uladzislau Parkhomenka, Nikita Belko, Anatoly Lugovsky, Tatiana A. Pavich, Iryna N. Kukhta, Ziqing Li, and et al. 2023. "Solution-Processed Functionalized MoS2 Nanosheets Composite for Photodetection Application" Photonics 10, no. 12: 1295. https://doi.org/10.3390/photonics10121295




