Variations in Lens Thickness Affecting the Anterior Chamber Length and Their Potential Measurement Using a Biometer
Abstract
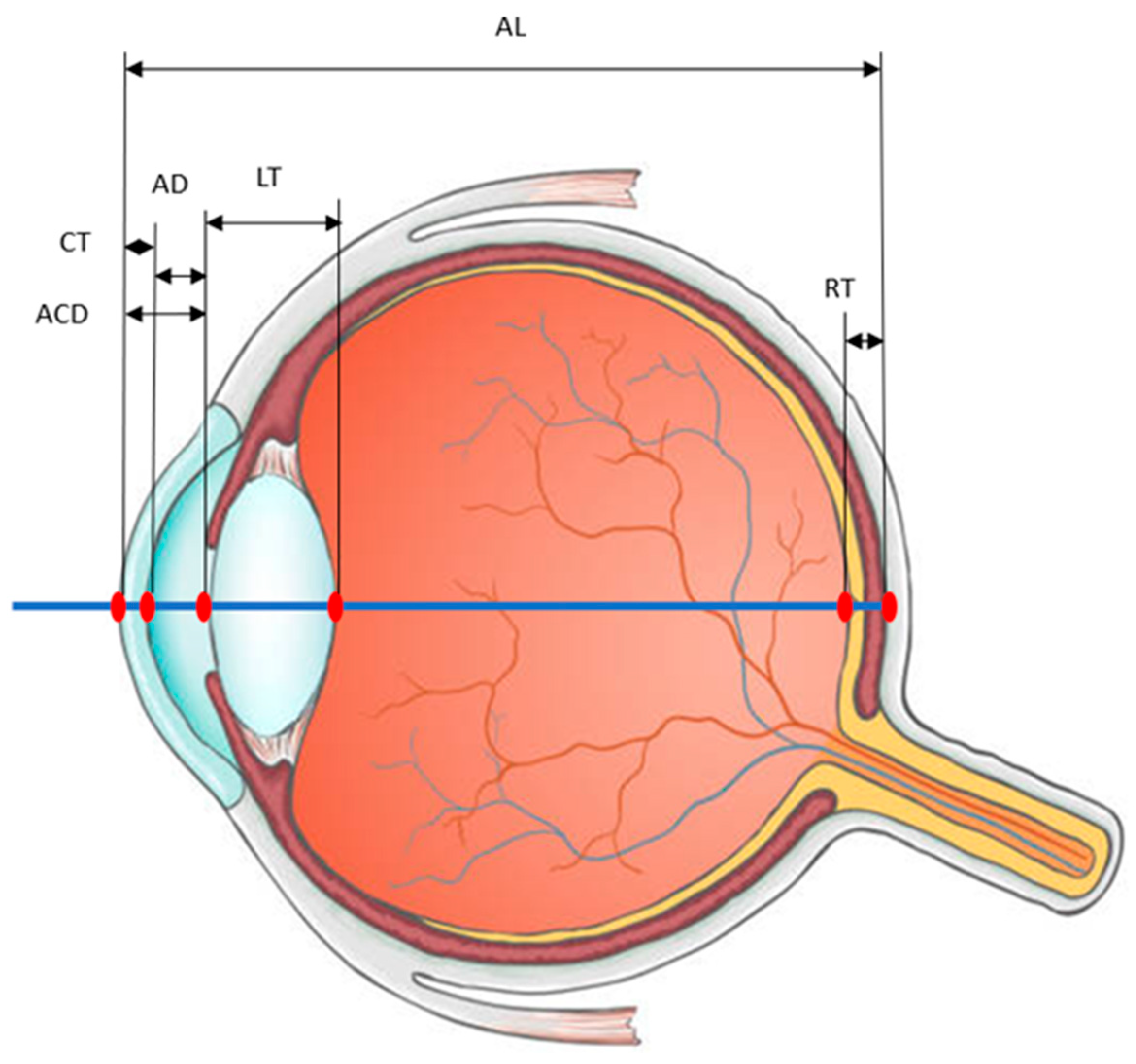
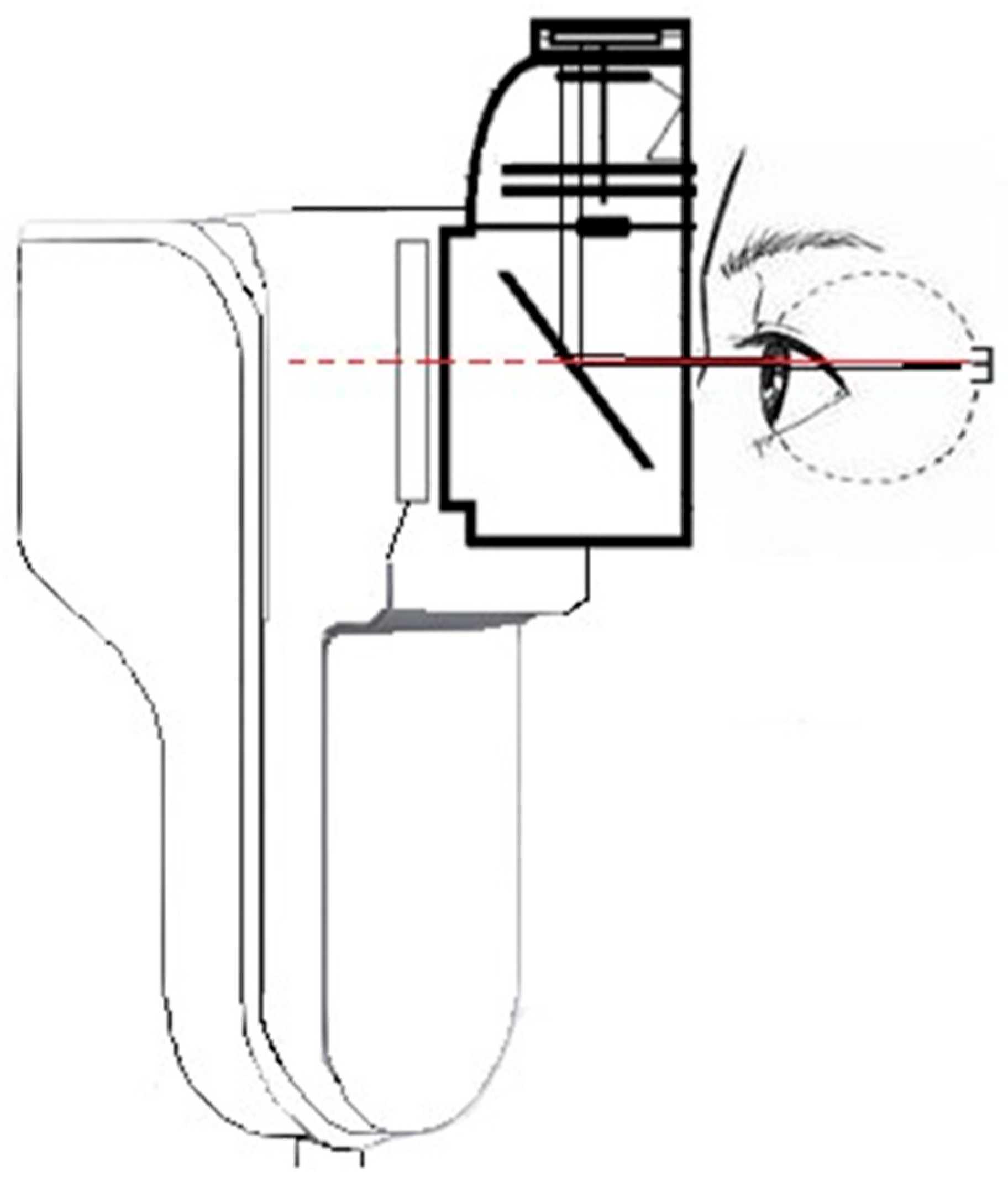
:1. Introduction
2. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dervisogullari, M.S.; Totan, Y.; Guragac, B. Comparison of anterior chamber depth measurements of Nidek AL-Scan and Galilei Dual Scheimpflug Analyzer. Contact Lens Anterior Eye 2015, 38, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Chamarty, S.; Verkicharla, P.K. Accuracy and Precision of New Optical Biometer Designed for Myopia Management in Measurement of Ocular Biometry. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2023. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.; Sneha, C.; Subramanian, S.; Raman, G.V. Comparison between ocular biometry parameters in patients with unilateral congenital glaucoma. Indian J. Ophthalmol. 2023, 71, 2962–2966. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Qazi, M.A.; Pepose, J.S. Biometry and intraocular lens power calculation. Curr. Opin. Ophthalmol. 2008, 19, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Savur, S.; Kaup, S.; Dinesh, A.; Shivalli, S.; Kondal, D. Can ultrasonic biometric indices with optimal cut-offs be a potential screening tool for primary angle closure disease? A case-control study. Eye 2023, 37, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.L.; Gong, T.; Nongpiur, M.E.; How, A.C.; Lee, H.K.; Cheng, L.; Perera, S.A.; Aung, T. Myopia in asian subjects with primary angle closure: Implications for glaucoma trends in East Asia. Ophthalmology 2014, 121, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Santodomingo-Rubido, J.; Mallen, E.A.; Gilmartin, B.; Wolffsohn, J.S. A new non-contact optical device for ocular biometry. Br. J. Ophthalmol. 2002, 86, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Pakuliene, G.; Zimarinas, K.; Nedzelskiene, I.; Siesky, B.; Kuzmiene, L.; Harris, A.; Januleviciene, I. Anterior segment optical coherence tomography imaging and ocular biometry in cataract patients with open angle glaucoma comorbidity. BMC Ophthalmol. 2021, 21, 127. [Google Scholar] [CrossRef]
- Holzer, M.P.; Mamusa, M.; Auffarth, G.U. Accuracy of a new partial coherence interferometry analyser for biometric measurements. Br. J. Ophthalmol. 2009, 93, 807–810. [Google Scholar] [CrossRef]
- Kaswin, G.; Rousseau, A.; Mgarrech, M.; Barreau, E.; Labetoulle, M. Biommetry and intraocular lens power calculation results with a new optical biometry device: Comparison with the gold standard. J. Cataract Refract. Surg. 2014, 40, 593–600. [Google Scholar] [CrossRef]
- Huang, J.H.; Savini, G.; Wu, F.; Yu, X.X.; Yang, J.; Yu, A.; Yu, Y.; Wang, Q.M. Repeatability and reproducibility of ocular biometry using a new noncontact optical low-coherence interferometer. J. Cataract Refract. Surg. 2015, 41, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Upasna; Singh, V.K.; Rana, J.; Kumar, S.; Singh, A.; Singh, K. An evaluation of intraoperative and postoperative outcomes of phacoemulsification surgery in eyes with shallow anterior chamber. Indian J. Ophthalmol. 2021, 69, 1346–1347. [Google Scholar] [CrossRef] [PubMed]
- El-Haddad, N.; Abd Elwahab, A.; Shalaby, S.; Farag, M.M.A.; Alkassaby, M.; Ahmed, S.; Shawky, S. Comparison between open-angle glaucoma and angle-closure glaucoma regarding the short-term optic disc vessel density changes after trabeculectomy. Lasers Med. Sci. 2023, 38, 246. [Google Scholar] [CrossRef] [PubMed]
- Noya-Padin, V.; Garcia-Queiruga, J.; Iacubitchii, M.; Giraldez, M.J.; Yebra-Pimentel, E.; Pena-Verdeal, H. Lenstar LS900 vs EchoScan US-800: Comparison between optical and ultrasound biometry with and without contact lenses and its relationship with other biometric parameters. Expert Rev. Med. Devices 2023, 20, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, H.; Dhurjon, L.; Francis, C.; Bhambhwani, V. Comparative Analysis of Axial Length Measurements by Optical Biometers Based on Partial Coherence Interferometry versus Optical Low-Coherence Interferometry: An Office Audit. Cureus 2022, 14, e21883. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Lin, Y.; Zhao, Z.; Wang, H.; Zhang, G. Evaluation and comparison of ocular biometric parameters obtained with Tomey OA-2000 in silicone oil-filled aphakic eyes. BMC Ophthalmol. 2023, 23, 218. [Google Scholar] [CrossRef] [PubMed]
- Shammas, H.J.; Hoffer, K.J. Repeatability and Reproducibility of Biometry and Keratometry Measurements Using a Noncontact Optical Low-Coherence Reflectometer and Keratometer. Am. J. Ophthalmol. 2012, 153, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Berrow, E.J.; Naroo, S.A.; Wolffsohn, J.S.; Uthoff, D.; Holland, D.; Shah, S. Validity and repeatability of the Aladdin ocular biometer. Br. J. Ophthalmol. 2014, 98, 256–258. [Google Scholar] [CrossRef]
- Strang, N.C.; Schmid, K.L.; Carney, L.G. Hyperopia is predominantly axial in nature. Curr. Eye Res. 1998, 17, 380–383. [Google Scholar] [CrossRef]
- Wallman, J. Nature and nurture of myopia. Nature 1994, 371, 201–202. [Google Scholar] [CrossRef]
- Harrington, S.; O’Dwyer, V. The association between time spent on screens and reading with myopia, premyopia and ocular biometric and anthropometric measures in 6-to 7-year-old schoolchildren in Ireland. Ophthalmic Physiol. Opt. 2023, 43, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Atchison, D.A.; Smith, G. Possible errors in determining axial length changes during accommodation with the IOLMaster. Optom. Vis. Sci. 2004, 81, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, N.; Pradhan, C.; Joshi, P.; Subedi, P.; Shrestha, P. Comparison of Ocular Biometry between Primary Open Angle Glaucoma Patients and Normal subjects. Nepal. J. Ophthalmol. 2021, 13, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Oku, Y.; Oku, H.; Park, M.; Hayashi, K.; Takahashi, H.; Shouji, T.; Chihara, E. Long axial length as risk factor for normal tension glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Hrebcová, J.; Skorkovská, S.; Vasků, A. Comparison of contact and immersion techniques of ultrasound biometry in terms of target postoperative refraction. Czech Slovak Ophthalmol. 2009, 65, 143–146. [Google Scholar]
- Norrby, S. Sources of error in intraocular lens power calculation. J. Cataract Refract. Surg. 2008, 34, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.; Hoffmann, P. C constant: New concept for ray tracing-assisted intraocular lens power calculation. J. Cataract Refract. Surg. 2014, 40, 764–773. [Google Scholar] [CrossRef]
- Liang, Y.B.; Friedman, D.S.; Zhou, Q.; Yang, X.H.; Sun, L.P.; Guo, L.X.; Chang, D.S.; Lian, L.Y.; Wang, N.L.; Handan Eye Study, G. Prevalence and Characteristics of Primary Angle-Closure Diseases in a Rural Adult Chinese Population: The Handan Eye Study. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8672–8679. [Google Scholar] [CrossRef]
- Wang, D.D.; Huang, W.Y.; Li, Y.T.; Zheng, Y.F.; Foster, P.J.; Congdon, N.; He, M.G. Intraocular Pressure, Central Corneal Thickness, and Glaucoma in Chinese Adults: The Liwan Eye Study. Am. J. Ophthalmol. 2011, 152, 454–462. [Google Scholar] [CrossRef]
- Ogbeide, O.U.; Omoti, A.E. Ultrasonographic ocular diameters in Nigerians. West Afr. J. Med. 2009, 28, 97–101. [Google Scholar]
- Bolz, M.; Prinz, A.; Drexler, W.; Findl, O. Linear relationship of refractive and biometric lenticular changes during accommodation in emmetropic and myopic eyes. Br. J. Ophthalmol. 2007, 91, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ostrin, L.; Kasthurirangan, S.; Win-Hall, D.; Glasser, A. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom. Vis. Sci. 2006, 83, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Dubbelman, M.; Van der Heijde, G.L.; Weeber, H.A. Change in shape of the aging human crystalline lens with accommodation. Vis. Res. 2005, 45, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Koretz, J.F.; Handelman, G.H. Model of the accommodative mechanism in the human eye. Vis. Res. 1982, 22, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Koretz, J.F.; Handelman, G.H. A model for accommodation in the young human eye: The effects of lens elastic anisotropy on the mechanism. Vis. Res. 1983, 23, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Rosales, P.; Wendt, M.; Marcos, S.; Glasser, A. Changes in crystalline lens radii of curvature and lens tilt and decentration during dynamic accommodation in rhesus monkeys. J. Vis. 2008, 8, 18.1–18.12. [Google Scholar] [CrossRef] [PubMed]
- Koretz, J.F.; Handelman, G.H.; Brown, N.P. Analysis of human crystalline lens curvature as a function of accommodative state and age. Vis. Res. 1984, 24, 1141–1151. [Google Scholar] [CrossRef]
- Garner, L.F. Mechanisms of accommodation and refractive error. Ophthalmic Physiol. Opt. 1983, 3, 287–293. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Povedano-Montero, F.J.; Bernardez-Villaboa, R.; Martínez-Florentín, G.; López-Muñoz, F.; Cedrún-Sánchez, J.E. Variations in Lens Thickness Affecting the Anterior Chamber Length and Their Potential Measurement Using a Biometer. Photonics 2023, 10, 1351. https://doi.org/10.3390/photonics10121351
Povedano-Montero FJ, Bernardez-Villaboa R, Martínez-Florentín G, López-Muñoz F, Cedrún-Sánchez JE. Variations in Lens Thickness Affecting the Anterior Chamber Length and Their Potential Measurement Using a Biometer. Photonics. 2023; 10(12):1351. https://doi.org/10.3390/photonics10121351
Chicago/Turabian StylePovedano-Montero, F. Javier, Ricardo Bernardez-Villaboa, Gema Martínez-Florentín, Francisco López-Muñoz, and Juan E. Cedrún-Sánchez. 2023. "Variations in Lens Thickness Affecting the Anterior Chamber Length and Their Potential Measurement Using a Biometer" Photonics 10, no. 12: 1351. https://doi.org/10.3390/photonics10121351





