Highly Efficient Solution-Processed Bluish-Green Thermally Activated Delayed Fluorescence Compounds Using Di(pyridin-3-yl)methanone as Acceptor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Thermal Stability and Film-Forming Properties of ACCz-DPyM and POxCz-DPyM
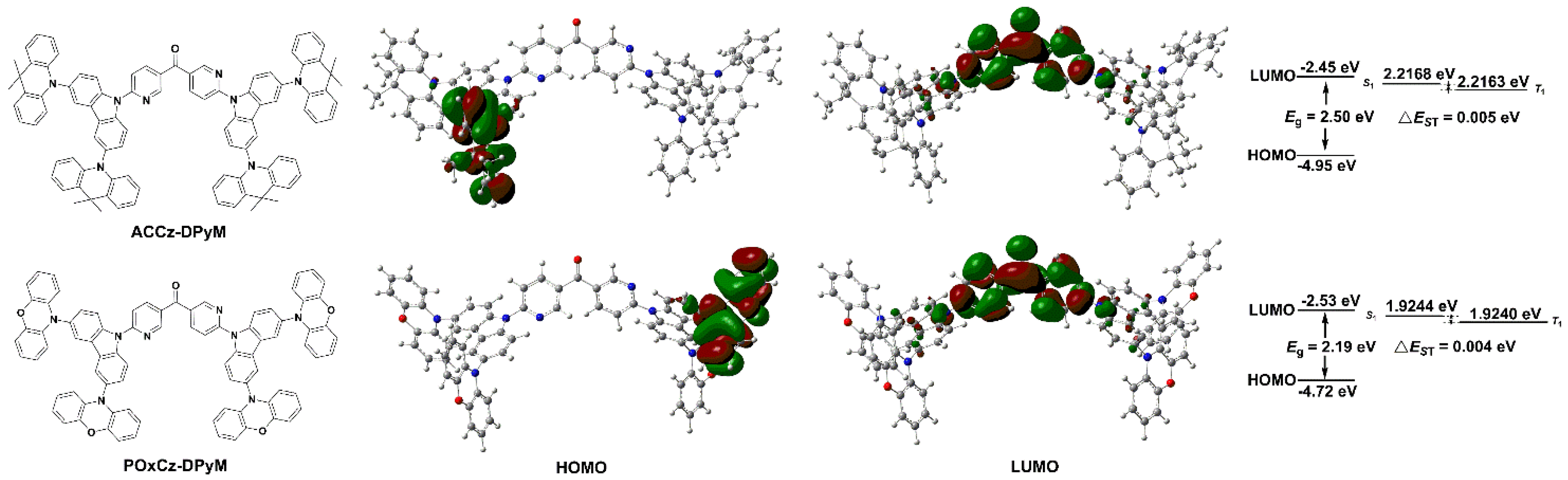
2.3. Electrochemical Properties and Theoretical Calculation
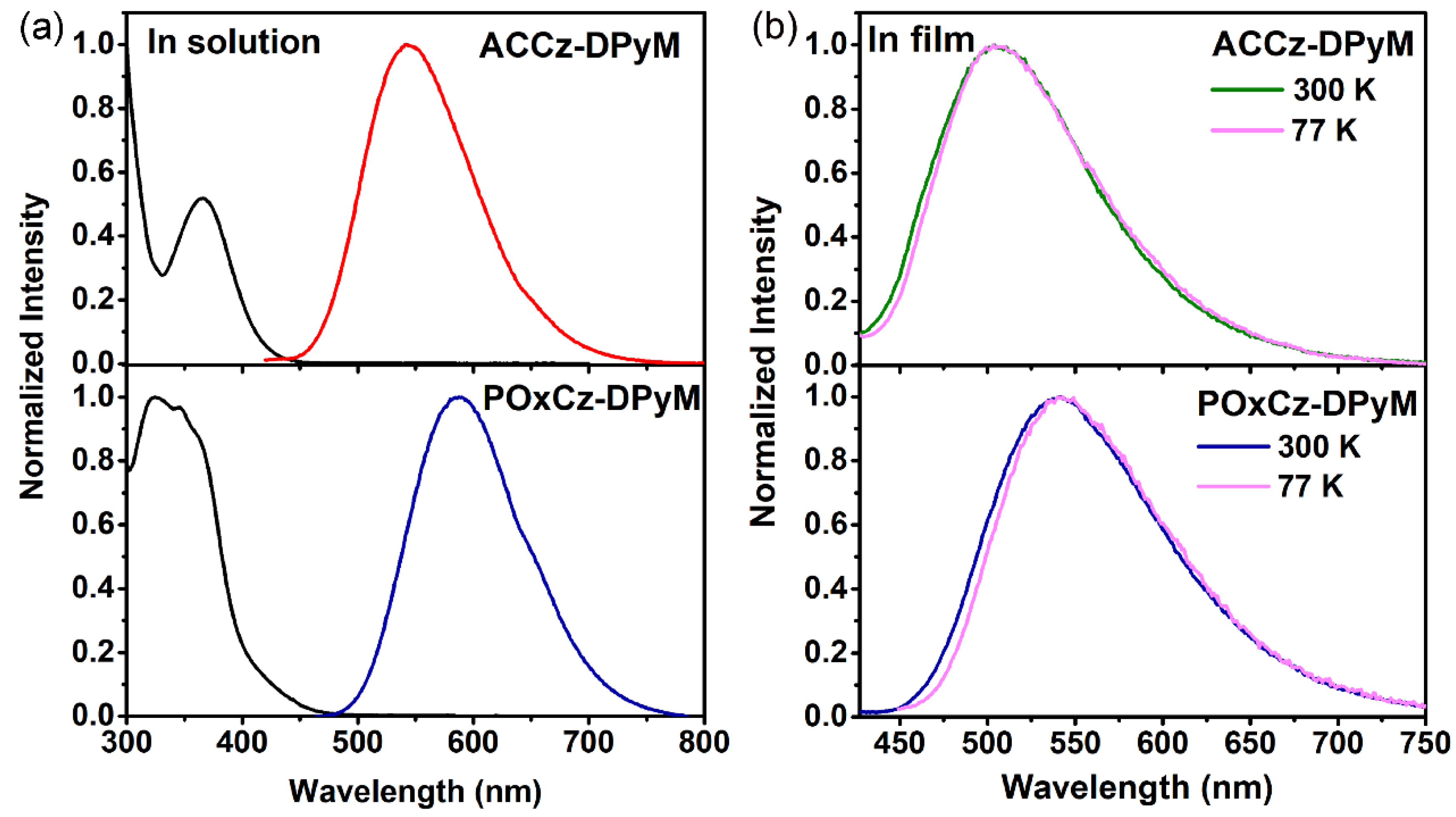
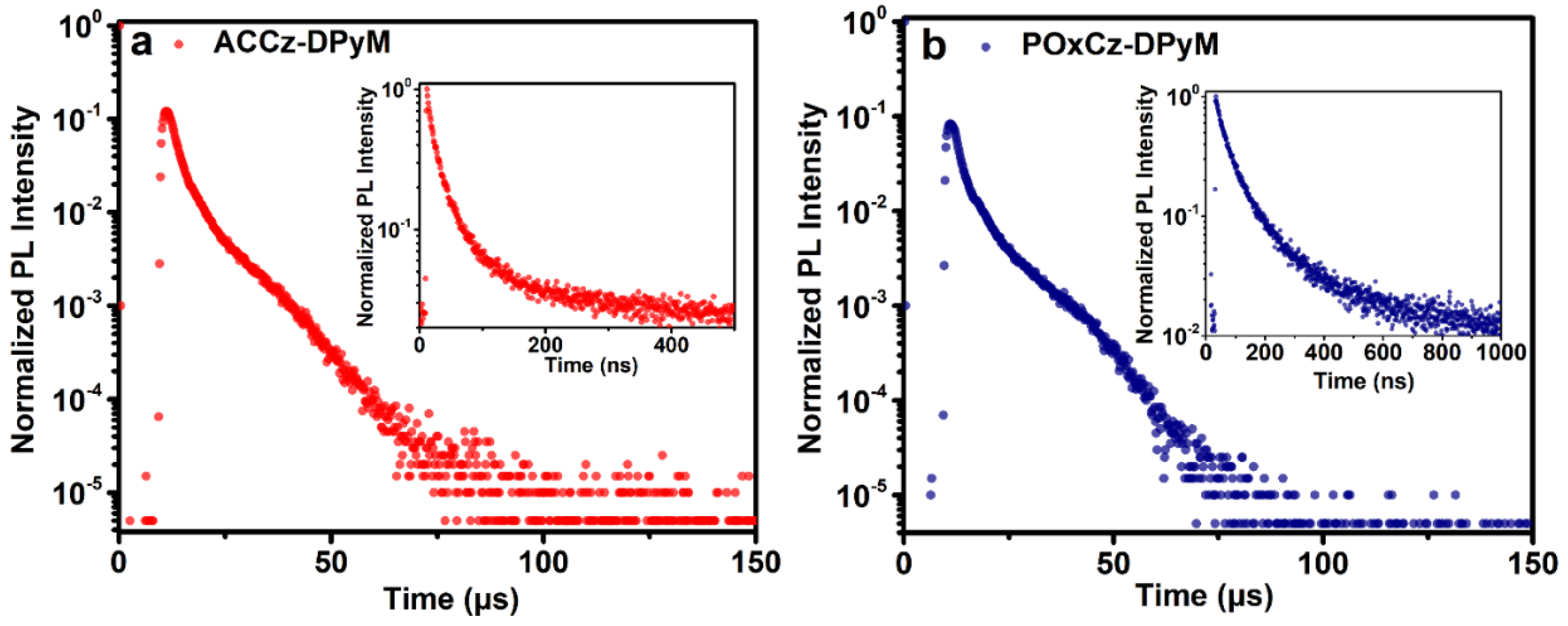
2.4. Photophysical Properties
2.5. Electroluminescence Properties
3. Conclusions
4. Experimental
4.1. General Information
4.1.1. Synthesis of ACCz-DPyM
4.1.2. Synthesis of POxCz-DPyM
4.1.3. Device Fabrication and Measurement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Li, B.; Huang, S.P.; Nomura, H.; Tanaka, H.; Adachi, C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 2014, 8, 326–332. [Google Scholar] [CrossRef]
- Lee, J.; Shizu, K.; Tanaka, H.; Nakanotani, H.; Yasuda, T.; Kaji, H.; Adachi, C. Controlled emission colors and singlet–triplet energy gaps of dihydrophenazine-based thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2015, 3, 2175–2181. [Google Scholar] [CrossRef]
- Li, B.W.; Li, Z.Y.; Hu, T.P.; Zhang, Y.; Wang, Y.; Yi, Y.P.; Guo, F.Y.; Zhao, L.C. Highly efficient blue organic light-emitting diodes from pyrimidine-based thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2018, 6, 2351–2359. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Kim, J.; Lee, D.R.; Han, S.H.; Forrest, S.R.; Lee, J.Y. Nearly 100% horizontal dipole orientation and upconversion efficiency in blue thermally activated delayed fluorescent emitters. Adv. Optical Mater. 2018, 6, 1701340. [Google Scholar] [CrossRef]
- Xiang, S.P.; Lv, X.L.; Sun, S.Q.; Zhang, Q.; Huang, Z.; Guo, R.D.; Gu, H.G.; Liu, S.Y.; Wang, L. To improve the efficiency of thermally activated delayed fluorescence OLEDs by controlling the horizontal orientation through optimizing stereoscopic and linear structures of indolocarbazole isomers. J. Mater. Chem. C 2018, 6, 5812–5820. [Google Scholar] [CrossRef]
- Li, B.; Yang, Z.; Gong, W.Q.; Chen, X.H.; Bruce, D.W.; Wang, S.Y.; Ma, H.L.; Liu, Y.; Zhu, W.G.; Chi, Z.G.; et al. Intramolecular through-space charge transfer based TADF-active multifunctional emitters for high efficiency solution-processed OLED. Adv. Optical Mater. 2021, 9, 2100180. [Google Scholar] [CrossRef]
- Zhan, L.S.; Chen, Z.X.; Gong, S.L.; Xiang, Y.P.; Ni, F.; Zeng, X.; Xie, G.H.; Yang, C.L. A simple organic molecule realizing simultaneous TADF, RTP, AIE, and mechanoluminescence: Understanding the mechanism behind the multifunctional emitter. Angew. Chem. Int. Ed. 2019, 58, 17651–17655. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Cui, L.S.; Kim, J.U.; Nakanotani, H.; Adachi, C. Rational molecular design for deep-blue thermally activated delayed fluorescence emitters. Adv. Funct. Mater. 2018, 28, 1706023. [Google Scholar] [CrossRef]
- Hirata, S.; Sakai, Y.; Masui, K.; Tanaka, H.; Lee, S.Y.; Nomura, H.; Nakamura, N.; Yasumatsu, M.; Nakanotani, H.; Zhang, Q.S.; et al. Highly efficient blue electroluminescence based on thermally activated delayed fluorescence. Nat. Mater. 2015, 14, 330–336. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, S.; Oh, J.; Woo, S.J.; Kumar, A.; Kim, J.J.; Jung, J.; Yoo, S.; Lee, M.H. High-efficiency sky blue to ultradeep blue thermally activated delayed fluorescent diodes based on ortho-carbazole-appended triarylboron emitters: Above 32% external quantum efficiency in blue devices. Adv. Optical Mater. 2018, 6, 1800385. [Google Scholar] [CrossRef]
- Kim, D.H.; D’Aléo, A.; Chen, X.K.; Sandanayaka, A.D.S.; Yao, D.D.; Zhao, L.; Komino, T.; Zaborova, E.; Canard, G.; Tsuchiya, Y.; et al. High-efficiency electroluminescence and amplified spontaneous emission from a thermally activated delayed fluorescent near-infrared emitter. Nat. Photon. 2018, 12, 98–104. [Google Scholar] [CrossRef]
- Chan, C.Y.; Tanaka, M.; Nakanotani, H.; Adachi, C. Efficient and stable sky-blue delayed fluorescence organic light-emitting diodes with CIE below 0.4. Nat. Commun. 2018, 9, 5036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.S.; Li, J.; Shizu, K.; Huang, S.P.; Hirata, S.; Miyazaki, H.; Adachi, C. Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes. J. Am. Chem. Soc. 2012, 134, 14706–14709. [Google Scholar] [CrossRef]
- Li, C.S.; Ren, Z.J.; Sun, X.L.; Li, H.H.; Yan, S.K. Deep-blue thermally activated delayed fluorescence polymers for nondoped solution-processed organic light-emitting diodes. Macromolecules 2019, 52, 2296–2303. [Google Scholar] [CrossRef]
- Li, C.S.; Harrison, A.K.; Liu, Y.C.; Zhao, Z.N.; Zeng, C.; Dias, F.B.; Ren, Z.J.; Yan, S.; Bryce, M.R. Asymmetrical-dendronized TADF emitters for efficient non-doped solution-processed OLEDs by eliminating degenerate excited states and creating solely thermal equilibrium routes. Angew. Chem. Int. Ed. 2022, 61, e202115140. [Google Scholar]
- Li, C.S.; Xu, Y.W.; Liu, Y.C.; Ren, Z.J.; Ma, Y.G.; Yan, S.K. Highly efficient white-emitting thermally activated delayed fluorescence polymers: Synthesis, non-doped white OLEDs and electroluminescent mechanism. Nano Energy 2019, 65, 104057. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Wu, H.; Wang, K.; Yu, J.; Ou, X.M.; Zhang, X.H. Recent progress in thermally activated delayed fluorescence emitters for nondoped organic light-emitting diodes. Chem. Sci. 2022, 13, 3625–3651. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Harrison, A.K.; Liu, Y.C.; Zhao, Z.N.; Dias, F.B.; Zeng, C.; Yan, S.K.; Bryce, M.R.; Ren, Z.J. TADF dendronized polymer with vibrationally enhanced direct spin-flip between charge-transfer states for efficient non-doped solution-processed OLEDs. Chem. Eng. J. 2022, 435, 134924. [Google Scholar] [CrossRef]
- Sun, D.M.; Duda, E.; Fan, X.C.; Saxena, R.; Zhang, M.; Bagnich, S.; Zhang, X.H.; Köhler, A.; Zysman-Colman, E. Thermally activated delayed fluorescent dendrimers that underpin high-efficiency host-free solution-processed organic light-emitting diodes. Adv. Mater. 2022, 34, 2110344. [Google Scholar] [CrossRef]
- Sun, D.M.; Saxena, R.; Fan, X.C.; Athanasopoulos, S.; Duda, E.; Zhang, M.; Bagnich, S.; Zhang, X.H.; Zysman-Colman, E.; Köhler, A. Regiochemistry of Donor Dendrons Controls thePerformance of Thermally Activated Delayed FluorescenceDendrimer Emitters for High Efficiency Solution-ProcessedOrganic Light-Emitting Diodes. Adv. Sci. 2022, 9, 2201470. [Google Scholar] [CrossRef]
- Pathak, S.K.; Liu, H.; Zhou, C.J.; Xie, G.H.; Yang, C.L. Triazatruxene based star-shaped thermally activated delayed fluorescence emitters: Modulating the performance of solution-processed non-doped OLEDs via side-group engineering. J. Mater. Chem. C 2021, 9, 7363–7373. [Google Scholar] [CrossRef]
- Park, I.S.; Komiyama, H.; Yasuda, T. Pyrimidine-based twisted donor–acceptor delayed fluorescence molecules: A new universal platform for highly efficient blue electroluminescence. Chem. Sci. 2017, 8, 953–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.J.; Kukhta, N.A.; Ward, J.S.; Danos, A.; Batsanov, A.S.; Bryce, M.R.; Dias, F.B. Balancing charge-transfer strength and triplet states for deep-blue thermally activated delayed fluorescence with an unconventional electron rich dibenzothiophene acceptor. J. Mater. Chem. C 2019, 7, 13224–13234. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Jeon, S.O.; Chung, Y.S.; Numata, M.; Lee, H.; Lee, E.K.; Kwon, E.S.; Sim, M.; Choi, H.; Lee, J.Y. An excited state managing molecular design platform of blue thermally activated delayed fluorescence emitters by p-linker engineering. J. Mater. Chem. C 2020, 8, 1736–1745. [Google Scholar] [CrossRef]
- Zeng, X.; Pan, K.C.; Lee, W.K.; Gong, S.L.; Ni, F.; Xiao, X.; Zeng, W.X.; Xiang, Y.P.; Zhan, L.S.; Zhang, Y.; et al. High-efficiency pure blue thermally activated delayed fluorescence emitters with a preferentially horizontal emitting dipole orientation via a spiro-linked double D–A molecular architecture. J. Mater. Chem. C 2019, 7, 10851–10859. [Google Scholar] [CrossRef]
- Rajamalli, P.; Senthilkumar, N.; Huang, P.Y.; Wu, C.C.R.; Lin, H.W.; Cheng, C.H. New molecular design concurrently providing superior pure blue, thermally activated delayed fluorescence and optical out-coupling efficiencies. J. Am. Chem. Soc. 2017, 139, 10948–10951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.S.; Nomura, H.; Geng, Y.; Kim, J.U.; Nakanotani, H.; Adachi, C. Controlling singlet-triplet energy splitting for deep-blue thermally activated delayed fluorescence emitters. Angew. Chem. Int. Ed. 2017, 56, 1571–1575. [Google Scholar] [CrossRef]
- Luo, J.J.; Gong, S.L.; Gu, Y.; Chen, T.H.; Li, Y.F.; Zhong, C.; Xie, G.H.; Yang, C.L. Multi-carbazole encapsulation as a simple strategy for the construction of solution-processed, non-doped thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2016, 4, 2442–2446. [Google Scholar] [CrossRef]
- Li, Y.F.; Xie, G.H.; Gong, S.L.; Wu, K.L.; Yang, C.L. Dendronized delayed fluorescence emitters for non-doped, solution-processed organic light-emitting diodes with high efficiency and low efficiency roll-off simultaneously: Two parallel emissive channels. Chem. Sci. 2016, 7, 5441–5447. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Ban, X.X.; Sun, K.Y.; Ma, Z.M.; Mei, Y.N.; Jiang, W.; Lin, B.P.; Sun, Y.M. Thermally activated delayed fluorescence materials based on benzophenone derivative as emitter for efficient solution-processed non-doped green OLED. Dyes Pigm. 2016, 133, 380–386. [Google Scholar] [CrossRef]
- Wong, M.Y.; Zysman-Colman, E. Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, K.; Albrecht, K.; Nakayama, A.; Yamamoto, K.; Fujita, K. Highly efficient thermally activated delayed fluorescence organic light-emitting diodes with fully solution-processed organic multilayered architecture: Impact of terminal substitution on carbazole-benzophenone dendrimer and interfacial engineering. ACS Appl. Mater. Interfaces 2018, 10, 33343–33352. [Google Scholar] [CrossRef]
- Gong, S.L.; Luo, J.J.; Wang, Z.; Li, Y.F.; Chen, T.H.; Xie, G.H.; Yang, C.L. Tuning emissive characteristics and singlet-triplet energy splitting of fluorescent emitters by encapsulation group modification: Yellow TADF emitter for solution-processed OLEDs with high luminance and ultraslow efficiency roll-off. Dyes Pigm. 2017, 139, 593–600. [Google Scholar] [CrossRef]
- Rajamalli, P.; Chen, D.Y.; Li, W.B.; Samuel, I.D.W.; Cordes, D.B.; Slawin, A.M.Z.; Zysman-Colman, E. Enhanced thermally activated delayed fluorescence through bridge modification in sulfone-based emitters employed in deep blue organic light-emitting diodes. J. Mater. Chem. C 2019, 7, 6664–6671. [Google Scholar] [CrossRef] [Green Version]


| Compound | λabs a (nm) | λPL a/λPL b (nm) | ΦPL b/% | c (V) | HOMO d/HOMO e (eV) | LUMO f/LUMO e (eV) | kRISC g (s−1) | ΔEST h/ΔEST e (eV) | τp/τd i [ns]/[μs] | Td/Tg j (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| ACCz-DPyM | 366 | 541/503 | 66.2 | 0.18 | −4.98/−4.95 | −2.20/−2.45 | 4.5 × 105 | 0.04/0.005 | 39.8/7.2 | 434/254 |
| POxCz-DPyM | 321 | 590/541 | 58.2 | 0.28 | −5.08/−4.72 | −2.51/−2.53 | 2.9 × 105 | 0.04/0.004 | 98.9/8.7 | 527/168 |
| Device | λELmax (nm) | Von (V) | Lmax (cd m−2) | CEmax (cd A−1) | EQEmax (%) | CIE (x, y) |
|---|---|---|---|---|---|---|
| I | 510 | 4.0 | 6209 | 9.9 | 6.6 | 0.28, 0.48 |
| II | 560 | 3.4 | 3248 | 15.9 | 6.5 | 0.43, 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zhang, C.; Yan, H.; Chai, Y.; Zhou, D. Highly Efficient Solution-Processed Bluish-Green Thermally Activated Delayed Fluorescence Compounds Using Di(pyridin-3-yl)methanone as Acceptor. Photonics 2023, 10, 456. https://doi.org/10.3390/photonics10040456
He Y, Zhang C, Yan H, Chai Y, Zhou D. Highly Efficient Solution-Processed Bluish-Green Thermally Activated Delayed Fluorescence Compounds Using Di(pyridin-3-yl)methanone as Acceptor. Photonics. 2023; 10(4):456. https://doi.org/10.3390/photonics10040456
Chicago/Turabian StyleHe, Yuting, Cheng Zhang, Hao Yan, Yongshuai Chai, and Deyun Zhou. 2023. "Highly Efficient Solution-Processed Bluish-Green Thermally Activated Delayed Fluorescence Compounds Using Di(pyridin-3-yl)methanone as Acceptor" Photonics 10, no. 4: 456. https://doi.org/10.3390/photonics10040456





