Therapeutic and Adverse Effects of Lasers in Dentistry: A Systematic Review
Abstract
:1. Introduction
1.1. Laser Classifications
1.1.1. LLLT
1.1.2. CO2 Laser
1.1.3. The Nd: YAG Laser
1.1.4. The Er: YAG Laser
1.1.5. The Argon Laser
1.1.6. The Diode Laser
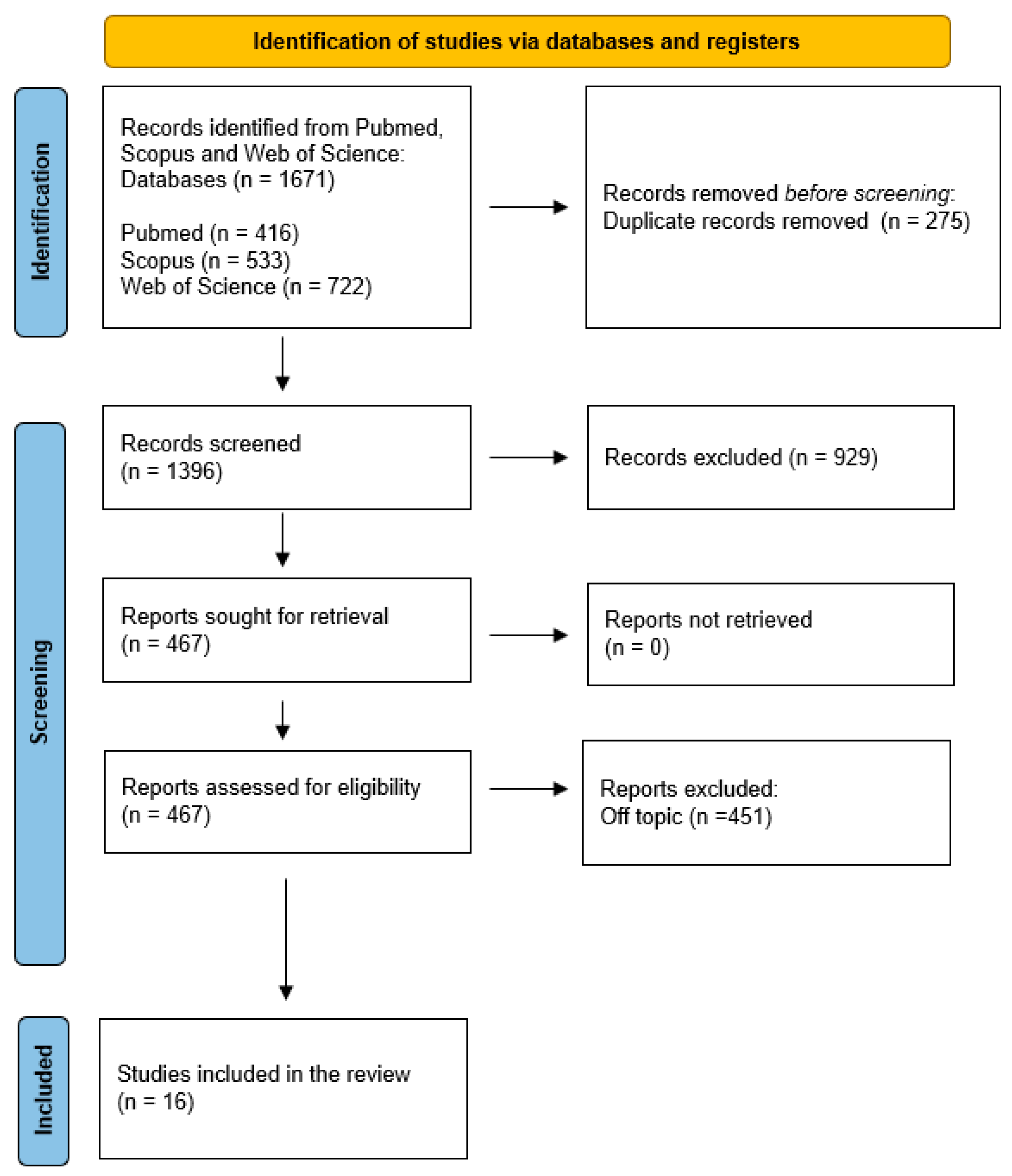
2. Materials and Methods
- Participants: children and adults were included.
- Interventions: actions of the lasers.
- Comparisons: traditional procedures in dentistry.
- Outcomes: efficacy and disadvantages of lasers in dentistry.
- Study: randomized clinical trials, retrospective and observational studies on human teeth.
3. Results
4. Discussion
Current Trends and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATF/CREB | Group of transcription factors |
| ATP | Adenosine triphosphate |
| BRONJ | Bisphosphonate-related osteonecrosis of the jaw |
| cAMP | Cyclic adenosine monophosphate |
| CO2 | Carbone Dioxine |
| Cox | Cytochrome C oxidase |
| DNA | Deoxyribonucleic acid |
| Er: YAG | Erbium-doped yttrium aluminum garnet laser |
| FDIP | Forced Dehydration with Induced Photocoagulation |
| FIB | FEI-Helios Plasma |
| HIF-1 | Hypoxia-inducible factor 1 |
| LASER | Light Amplification by Stimulated Emission of Radiation |
| LED | Light-emitting diode |
| LLLT | Low-level laser treatment |
| MMP | Mitochondrial membrane potential |
| MRONJ | Medication related osteonecrosis of the jaw |
| Nd: YAG | Neodymium yttrium aluminum garnet |
| NF- kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIR | Near infrared |
| NO | Nitric oxide |
| p53 | Tumor protein |
| PBM | Photobiomodulation |
| MUC5B | Oligomeric mucus/gel-forming |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| Redox | Oxidation–reduction process |
| Ref-1 | Multifunctional protein |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| WALT | World Association for Laser Therapy |
| YSGG | Yttrium scandium gallium garnet |
References
- Mier y Teran Armida, M. Lasertherapy and its applications in dentistry. Pract. Odontol. 1989, 10, 9–16. [Google Scholar]
- Inchingolo, A.M.; Malcangi, G.; Ferrara, I.; Viapiano, F.; Netti, A.; Buongiorno, S.; Latini, G.; Azzollini, D.; De Leonardis, N.; de Ruvo, E.; et al. Laser Surgical Approach of Upper Labial Frenulum: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 1302. [Google Scholar] [CrossRef]
- Nammour, S. Laser Dentistry, Current Advantages, and Limits. Photomed. Laser Surg. 2012, 30, 1–4. [Google Scholar] [CrossRef]
- Gholami, L.; Parsamanesh, G.; Shahabi, S.; Jazaeri, M.; Baghaei, K.; Fekrazad, R. The Effect of Laser Photobiomodulation on Periodontal Ligament Stem Cells. Photochem. Photobiol. 2021, 97, 851–859. [Google Scholar] [CrossRef]
- Bordea, I.R.; Hanna, R.; Chiniforush, N.; Grădinaru, E.; Câmpian, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation of the Outcome of Various Laser Therapy Applications in Root Canal Disinfection: A Systematic Review. Photodiagnosis Photodyn. Ther. 2020, 29, 101611. [Google Scholar] [CrossRef]
- Biagi, R.; Cossellu, G.; Sarcina, M.; Pizzamiglio, I.T.; Farronato, G. Laser-Assisted Treatment of Dentinal Hypersensitivity: A Literature Review. Ann. Stomatol. 2015, 6, 75–80. [Google Scholar] [CrossRef]
- Farronato, D.; Mangano, F.; Briguglio, F.; Iorio-Siciliano, V.; Riccitiello, F.; Guarnieri, R. Influence of Laser-Lok Surface on Immediate Functional Loading of Implants in Single-Tooth Replacement: A 2-Year Prospective Clinical Study. Int. J. Periodontics Restor. Dent. 2014, 34, 79–89. [Google Scholar] [CrossRef]
- Romanos, G.E.; Gupta, B.; Yunker, M.; Romanos, E.B.; Malmstrom, H. Lasers Use in Dental Implantology. Implant. Dent. 2013, 22, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.R.; Ciccolella, F.; D’Apolito, G.; Papa, F.; Iuso, A.; Salzo, A.E.; Trentadue, R.; Nardi, G.M.; Scivetti, M.; De Matteo, M.; et al. Effect of Low-Level Laser Irradiation on Osteoblast Proliferation and Bone Formation. J. Biol. Regul. Homeost. Agents 2011, 25, 603–614. [Google Scholar] [PubMed]
- Demirsoy, K.K.; Kurt, G. Use of Laser Systems in Orthodontics. Turk. J. Orthod. 2020, 33, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Comparison between Traditional Surgery, CO2 and Nd:Yag Laser Treatment for Generalized Gingival Hyperplasia in Sturge-Weber Syndrome: A Retrospective Study. J. Investig. Clin. Dent. 2010, 1, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Inchingolo, F.; Postiglione, F.; Petrini, M. The Effects of Erbium-Doped Yttrium Aluminum Garnet Laser (Er: YAG) Irradiation on Sandblasted and Acid-Etched (SLA) Titanium, an In Vitro Study. Materials 2020, 13, 4174. [Google Scholar] [CrossRef]
- Tarullo, A.; Laino, L.; Tarullo, A.; Inchingolo, F.; Flace, P.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Podo Brunetti, S.; Cagiano, R. Use of a Diode Laser in an Excisional Biopsy of Two Spoonlike Neoformations on the Tongue Tip. Acta Biomed. 2011, 82, 63–68. [Google Scholar]
- Verma, S.K.; Maheshwari, S.; Singh, R.K.; Chaudhari, P.K. Laser in Dentistry: An Innovative Tool in Modern Dental Practice. Natl. J. Maxillofac. Surg. 2012, 3, 124–132. [Google Scholar] [CrossRef] [Green Version]
- De Santis, D.; Bertossi, D.; Zanotti, G.; Rossetto, A.; Farronato, G.; Gelpi, F.; Marconcini, S.; Covani, U. Nd-YAP Laser Assisted Frenulectomy: A Case Series on 23 Patients. Minerva Stomatol. 2013, 62, 27–36. [Google Scholar] [PubMed]
- Azma, E.; Safavi, N. Diode Laser Application in Soft Tissue Oral Surgery. J. Lasers Med. Sci. 2013, 4, 206–211. [Google Scholar]
- Borzabadi-Farahani, A. The Adjunctive Soft-Tissue Diode Laser in Orthodontics. Compend. Contin. Educ. Dent. 2017, 38, e18–e31. [Google Scholar] [PubMed]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-Level Light/Laser Therapy Versus Photobiomodulation Therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Josephs, S.F.; Alexandrescu, D.T.; Ramos, F.; Bogin, V.; Gammill, V.; Dasanu, C.A.; De Necochea-Campion, R.; Patel, A.N.; Carrier, E.; et al. Lasers, Stem Cells, and COPD. J. Transl. Med. 2010, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R. Mechanisms and Applications of the Anti-Inflammatory Effects of Photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.J.; Arany, P.R.; Baxter, G.D.; Lanzafame, R.J. Light-Emitting Diode Therapy and Low-Level Light Therapy Are Photobiomodulation Therapy. Photobiomodulation Photomed. Laser Surg. 2019, 37, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Kalhori, K.A.M.; Vahdatinia, F.; Jamalpour, M.R.; Vescovi, P.; Fornaini, C.; Merigo, E.; Fekrazad, R. Photobiomodulation in Oral Medicine. Photobiomodulation Photomed. Laser Surg. 2019, 37, 837–861. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; et al. Management of Medication-Related Osteonecrosis of the Jaw (MRONJ) Using Leukocyte- and Platelet-Rich Fibrin (L-PRF) and Photobiomodulation: A Retrospective Study. J. Clin. Med. 2020, 9, 3505. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.B.B.; Camilotti, R.S.; Ponte, M.E. Efficacy of Laser Therapy in the Management of Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ): A Systematic Review. Lasers Med. Sci. 2016, 31, 1261–1272. [Google Scholar] [CrossRef]
- Wibawa, A.; Sucharitakul, J.; Dansirikul, R.; Pisarnturakit, P.; Pisarnturakit, P.; Bhuridej, P.; Arirachakaran, P. Low-Level Laser Therapy to the Major Salivary Glands Increases Salivary Flow and MUC5B Protein Secretion in Diabetic Patients with Hyposalivation: A Preliminary Study. Makara J. Health Res. 2018, 22, 14–21. [Google Scholar] [CrossRef]
- Vale, F.A.; Moreira, M.S.; de Almeida, F.C.S.; Ramalho, K.M. Low-Level Laser Therapy in the Treatment of Recurrent Aphthous Ulcers: A Systematic Review. Sci. World J. 2015, 2015, 150412. [Google Scholar] [CrossRef] [Green Version]
- Honarmand, M.; Farhadmollashahi, L.; Vosoughirahbar, E. Comparing the Effect of Diode Laser against Acyclovir Cream for the Treatment of Herpes Labialis. J. Clin. Exp. Dent. 2017, 9, e729–e732. [Google Scholar] [CrossRef] [Green Version]
- Falaki, F.; Nejat, A.H.; Dalirsani, Z. The Effect of Low-Level Laser Therapy on Trigeminal Neuralgia: A Review of Literature. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 1–5. [Google Scholar] [CrossRef]
- Gholami, L.; Asefi, S.; Hooshyarfard, A.; Sculean, A.; Romanos, G.E.; Aoki, A.; Fekrazad, R. Photobiomodulation in Periodontology and Implant Dentistry: Part 1. Photobiomodulation Photomed. Laser Surg. 2019, 37, 739–765. [Google Scholar] [CrossRef]
- Gholami, L.; Asefi, S.; Hooshyarfard, A.; Sculean, A.; Romanos, G.E.; Aoki, A.; Fekrazad, R. Photobiomodulation in Periodontology and Implant Dentistry: Part 2. Photobiomodulation Photomed. Laser Surg. 2019, 37, 766–783. [Google Scholar] [CrossRef] [Green Version]
- American Academy of Periodontology Statement on the Efficacy of Lasers in the Non-Surgical Treatment of Inflammatory Periodontal Disease. J. Periodontol. 2011, 82, 513–514. [CrossRef]
- He, M.; Zhang, B.; Shen, N.; Wu, N.; Sun, J. A Systematic Review and Meta-Analysis of the Effect of Low-Level Laser Therapy (LLLT) on Chemotherapy-Induced Oral Mucositis in Pediatric and Young Patients. Eur. J. Pediatr. 2018, 177, 7–17. [Google Scholar] [CrossRef]
- Mayer, L.; Gomes, F.V.; Carlsson, L.; Gerhardt-Oliveira, M. Histologic and Resonance Frequency Analysis of Peri-Implant Bone Healing After Low-Level Laser Therapy: An In Vivo Study. Int. J. Oral. Maxillofac. Implant. 2015, 30, 1028–1035. [Google Scholar] [CrossRef]
- Rajaei Jafarabadi, M.; Rouhi, G.; Kaka, G.; Sadraie, S.H.; Arum, J. The Effects of Photobiomodulation and Low-Amplitude High-Frequency Vibration on Bone Healing Process: A Comparative Study. Lasers Med. Sci. 2016, 31, 1827–1836. [Google Scholar] [CrossRef]
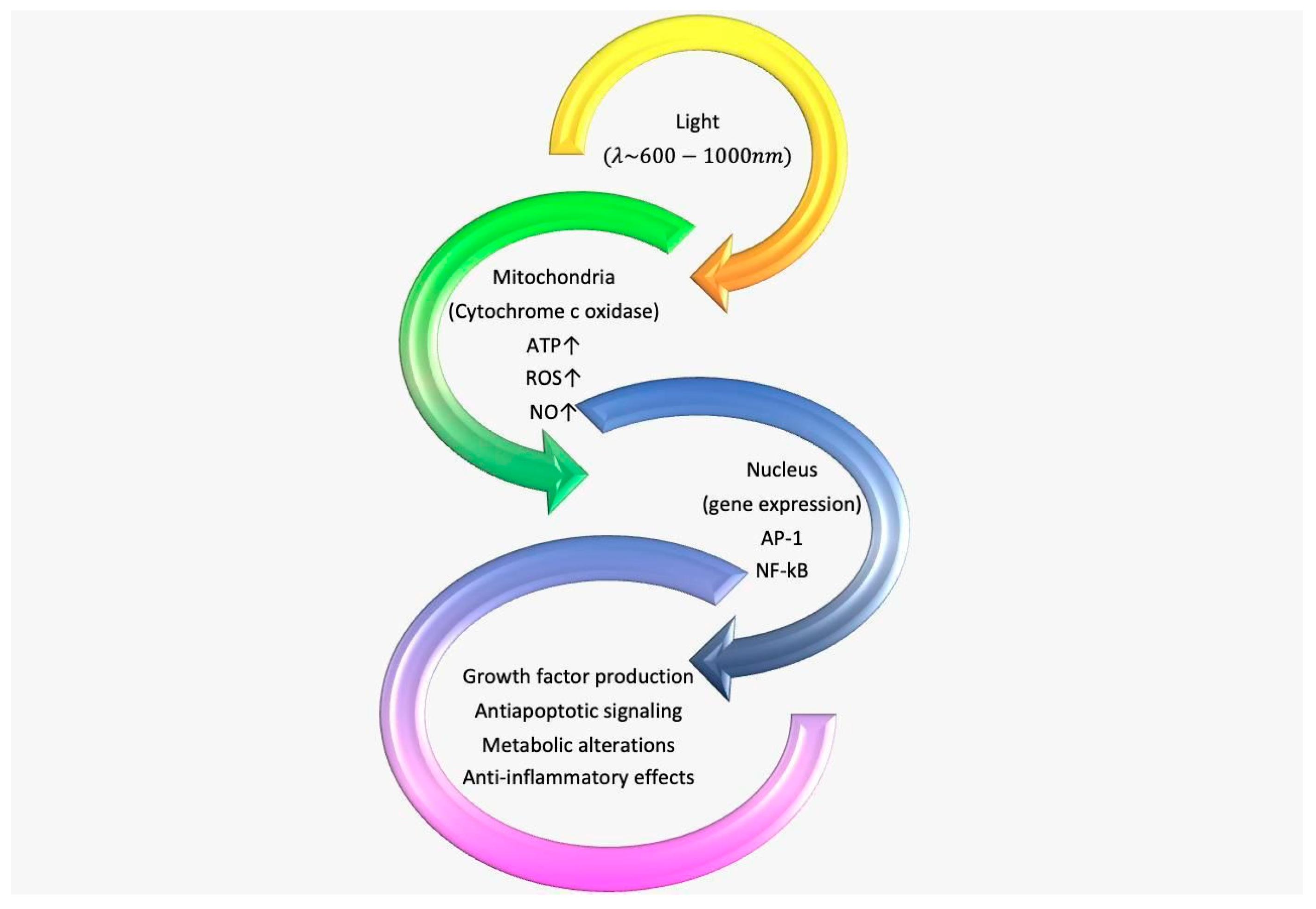
- Karu, T.I. Multiple Roles of Cytochrome c Oxidase in Mammalian Cells under Action of Red and IR-A Radiation. IUBMB Life 2010, 62, 607–610. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, F.; Wei, Y.; Chen, W.R.; Chen, Q.; Xing, D. Cancer Phototherapy via Selective Photoinactivation of Respiratory Chain Oxidase to Trigger a Fatal Superoxide Anion Burst. Antioxid. Redox Signal. 2014, 20, 733–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-Level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Fatima Zanirato Lizarelli, R.; Bagnato, V.S. Based Orofacial Rehabilitation and Harmonization. In Lasers in Oral and Maxillofacial Surgery; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Cotler, H.B.; Chow, R.T.; Hamblin, M.R.; Carroll, J. The Use of Low Level Laser Therapy (LLLT) for Musculoskeletal Pain. MOJ Orthop. Rheumatol. 2015, 2, 00068. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Cell Biology: Power Games. Nature 2006, 443, 901–903. [Google Scholar] [CrossRef]
- Pannala, V.R.; Camara, A.K.S.; Dash, R.K. Modeling the Detailed Kinetics of Mitochondrial Cytochrome c Oxidase: Catalytic Mechanism and Nitric Oxide Inhibition. J. Appl. Physiol. 2016, 121, 1196–1207. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Colavitti, R.; Rovira, I.I.; Finkel, T. Redox-Dependent Transcriptional Regulation. Circ. Res. 2005, 97, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Alaluf, S.; Muir-Howie, H.; Hu, H.-H.; Evans, A.; Green, M.R. Atmospheric Oxygen Accelerates the Induction of a Post-Mitotic Phenotype in Human Dermal Fibroblasts: The Key Protective Role of Glutathione. Differentiation 2000, 66, 147–155. [Google Scholar] [CrossRef]
- Pastore, D.; Greco, M.; Petragallo, V.; Passarella, S. Increase in <--H+/e- Ratio of the Cytochrome c Oxidase Reaction in Mitochondria Irradiated with Helium-Neon Laser. Biochem. Mol. Biol. Int. 1994, 34, 817–826. [Google Scholar] [PubMed]
- Knappe, V.; Frank, F.; Rohde, E. Principles of Lasers and Biophotonic Effects. Photomed. Laser Surg. 2004, 22, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jacques, S.L.; Zheng, L. MCML—Monte Carlo Modeling of Light Transport in Multi-Layered Tissues. Comput. Methods Programs Biomed. 1995, 47, 131–146. [Google Scholar] [CrossRef]
- Taylor, D.N.; Winfield, T.; Wynd, S. Low-Level Laser Light Therapy Dosage Variables vs Treatment Efficacy of Neuromusculoskeletal Conditions: A Scoping Review. J. Chiropr. Med. 2020, 19, 119–127. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic Dose Response in Low Level Light Therapy—An Update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef]
- Tam, S.Y.; Tam, V.C.W.; Ramkumar, S.; Khaw, M.L.; Law, H.K.W.; Lee, S.W.Y. Review on the Cellular Mechanisms of Low-Level Laser Therapy Use in Oncology. Front. Oncol. 2020, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Tumilty, S.; Munn, J.; McDonough, S.; Hurley, D.A.; Basford, J.R.; David Baxter, G.; Longo, L. The Dose That Works: Low Level Laser Treatment of Tendinopathy. AIP Conf. Proc. 2010, 1226, 170–178. [Google Scholar]
- Hamblin, M.R.; Nelson, S.T.; Strahan, J.R. Photobiomodulation and Cancer: What Is the Truth? Photomed. Laser Surg. 2018, 36, 241–245. [Google Scholar] [CrossRef]
- Bjordal, J.M. Low Level Laser Therapy (LLLT) and World Association for Laser Therapy (WALT) Dosage Recommendations. Photomed. Laser Surg. 2012, 30, 61–62. [Google Scholar] [CrossRef]
- Patel, C.K.N. Interpretation of CO2 Optical Maser Experiments. Phys. Rev. Lett. 1964, 12, 684. [Google Scholar] [CrossRef]
- Coluzzi, D.J. An Overview of Laser Wavelengths Used in Dentistry. Dent. Clin. N. Am. 2000, 44, 753–765. [Google Scholar] [CrossRef]
- Lippert, B.M.; Werner, J.A.; Rudert, H. Tissue Effects of CO2 Laser and Nd: YAG Laser. In Advances in Oto-Rhino-Laryngology; Rudert, H., Werner, J.A., Eds.; S. Karger AG: Basel, Switzerland, 1995; Volume 49, pp. 1–4. ISBN 978-3-8055-6087-0. [Google Scholar]
- Chomette, G.; Auriol, M.; Labrousse, F.; Vaillant, J.M. The effect of CO2 laser radiation on the morphological changes of mucocutaneous wound healing in oral surgery. A histo-enzymologic and ultrastructural study. Rev. Stomatol. Chir. Maxillofac. 1991, 92, 1–7. [Google Scholar] [PubMed]
- Holsinger, F.C.; Prichard, C.N.; Shapira, G.; Weisberg, O.; Torres, D.S.; Anastassiou, C.; Harel, E.; Fink, Y.; Weber, R.S. Use of the Photonic Band Gap Fiber Assembly CO2 Laser System in Head and Neck Surgical Oncology. Laryngoscope 2006, 116, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Devaiah, A.K.; Shapshay, S.M.; Desai, U.; Shapira, G.; Weisberg, O.; Torres, D.S.; Wang, Z. Surgical Utility of a New Carbon Dioxide Laser Fiber: Functional and Histological Study. Laryngoscope 2005, 115, 1463–1468. [Google Scholar] [CrossRef]
- Pons, Y.; Gauthier, J.; Clément, P.; Conessa, C. Ultrasonic Partial Glossectomy. Head Neck Oncol. 2009, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Hanby, D.F.; Gremillion, G.; Zieske, A.W.; Loehn, B.; Whitworth, R.; Wolf, T.; Kakade, A.C.; Walvekar, R.R. Harmonic Scalpel versus Flexible CO2 Laser for Tongue Resection: A Histopathological Analysis of Thermal Damage in Human Cadavers. World J. Surg. Oncol. 2011, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Slot, D.E.; Kranendonk, A.A.; Paraskevas, S.; Van der Weijden, F. The Effect of a Pulsed Nd:YAG Laser in Non-Surgical Periodontal Therapy. J. Periodontol. 2009, 80, 1041–1056. [Google Scholar] [CrossRef] [Green Version]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and Peri-Implant Wound Healing Following Laser Therapy. Periodontology 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, R.; Lv, X.; Qu, C. Efficacy of a Combined Er:YAG Laser and Nd:YAG Laser in Non-Surgical Treatment for Severe Periodontitis. Lasers Med. Sci. 2022, 37, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, M.; Köseoğlu, S.; Taşdemir, İ.; Erbak Yılmaz, H.; Savran, L.; Sütçü, R. Combined Application of Er:YAG and Nd:YAG Lasers in Treatment of Chronic Periodontitis. A Split-Mouth, Single-Blind, Randomized Controlled Trial. J. Periodontal Res. 2017, 52, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ashnagar, S.; Gianfilippo, R.D.; Arnett, M.; Kinney, J.; Wang, H. Laser-assisted Regenerative Surgical Therapy for Peri-implantitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2021, 92, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Namour, M.; Verspecht, T.; El Mobadder, M.; Teughels, W.; Peremans, A.; Nammour, S.; Rompen, E. Q-Switch Nd:YAG Laser-Assisted Elimination of Multi-Species Biofilm on Titanium Surfaces. Materials 2020, 13, 1573. [Google Scholar] [CrossRef] [Green Version]
- Namour; El Mobadder; Magnin; Peremans; Verspecht; Teughels; Lamard; Nammour; Rompen Q-Switch Nd:YAG Laser-Assisted Decontamination of Implant Surface. Dent. J. 2019, 7, 99. [CrossRef] [Green Version]
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Appl. Sci. 2020, 10, 5084. [Google Scholar] [CrossRef]
- Harashima, T.; Kinoshita, J.-I.; Kimura, Y.; Brugnera, A.; Zanin, F.; Pecora, J.D.; Matsumoto, K. Morphological Comparative Study on Ablation of Dental Hard Tissues at Cavity Preparation by Er:YAG and Er,Cr:YSGG Lasers. Photomed. Laser Surg. 2005, 23, 52–55. [Google Scholar] [CrossRef]
- De Benedittis, M.; Petruzzi, M.; Pastore, L.; Inchingolo, F.; Serpico, R. Nd:YAG Laser for Gingivectomy in Sturge-Weber Syndrome. J. Oral. Maxillofac. Surg. 2007, 65, 314–316. [Google Scholar] [CrossRef]
- Soft Tissue Conditions and Marginal Bone Levels of Implants with a Laser-Microtextured Collar: A 5-Year, Retrospective, Controlled Study—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25331762/ (accessed on 6 March 2023).
- Guarnieri, R.; Serra, M.; Bava, L.; Grande, M.; Farronato, D.; Iorio-Siciliano, V. The Impact of a Laser-Microtextured Collar on Crestal Bone Level and Clinical Parameters under Various Placement and Loading Protocols. Int. J. Oral. Maxillofac. Implants 2014, 29, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Mangano, F.; Bazzoli, M.; Tettamanti, L.; Farronato, D.; Maineri, M.; Macchi, A.; Mangano, C. Custom-Made, Selective Laser Sintering (SLS) Blade Implants as a Non-Conventional Solution for the Prosthetic Rehabilitation of Extremely Atrophied Posterior Mandible. Lasers Med. Sci. 2013, 28, 1241–1247. [Google Scholar] [CrossRef]
- Botsali, A.; Beksac, B.; Gahramanov, İ.; Caliskan, E. Erbium:YAG Laser Augments the Penetration of Cryotherapy. Lasers Med. Sci. 2022, 37, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Delgado, M.; Garces, F.; Machado, M.; Ferreira, F.; Martins, M.; Salazar, F.; Pacheco, J. A Histological Evaluation of the Surgical Margins from Human Oral Fibrous-Epithelial Lesions Excised with CO2 Laser, Diode Laser, Er:YAG Laser, Nd:YAG Laser, Electrosurgical Scalpel and Cold Scalpel. Med. Oral. 2019, 24, e271–e280. [Google Scholar] [CrossRef]
- Nazemisalman, B.; Farsadeghi, M.; Sokhansanj, M. Types of Lasers and Their Applications in Pediatric Dentistry. J. Lasers Med. Sci. 2015, 6, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, J.; Yu, Q.; Li, H.; Lin, M.; Mustapha, A.; Hong, L.; Wang, Y. Oral Bacterial Deactivation Using a Low-Temperature Atmospheric Argon Plasma Brush. J. Dent. 2011, 39, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Libonati, A.; Marzo, G.; Klinger, F.G.; Farini, D.; Gallusi, G.; Tecco, S.; Mummolo, S.; De Felici, M.; Campanella, V. Embryotoxicity Assays for Leached Components from Dental Restorative Materials. Reprod. Biol. Endocrinol. 2011, 9, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saydjari, Y.; Kuypers, T.; Gutknecht, N. Laser Application in Dentistry: Irradiation Effects of Nd:YAG 1064 Nm and Diode 810 Nm and 980 Nm in Infected Root Canals-A Literature Overview. Biomed. Res. Int. 2016, 2016, 8421656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanos, G.; Nentwig, G.H. Diode Laser (980 Nm) in Oral and Maxillofacial Surgical Procedures: Clinical Observations Based on Clinical Applications. J. Clin. Laser Med. Surg. 1999, 17, 193–197. [Google Scholar] [CrossRef]
- Saleh, H.M.; Saafan, A.M. Excision Biopsy of Tongue Lesions by Diode Laser. Photomed. Laser Surg. 2007, 25, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Farronato, D.; Fumagalli, D.; Asa’ad, F.; Rasperini, G. Decontamination of Customized Laser-Microtextured Titanium Abutments: A Comparative in Vitro Study of Different Cleaning Procedures. Int. J. Periodontics Restor. Dent. 2018, 38, e87–e95. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Onoda, C.; Sugiyama, S.; Noro, A.; Makiishi, T.; Ishikawa, T. Clinical evaluation of Ga-Al-As semiconductor laser diode (UNI-LASER) irradiation in treatment of solitary aphtha, erosion and hypersensitive dentin. Shikwa Gakuho 1987, 87, 295–303. [Google Scholar]
- Derikvand, N.; Chinipardaz, Z.; Ghasemi, S.; Chiniforush, N. The Versatility of 980 Nm Diode Laser in Dentistry: A Case Series. J. Lasers Med. Sci. 2016, 7, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Polizzi, A.; Cavalcanti, R.; Ronsivalle, V.; Chaurasia, A.; Spagnuolo, G.; Isola, G. Impact of Laser Therapy on Periodontal and Peri-Implant Diseases. Photobiomodulation Photomed. Laser Surg. 2022, 40, 454–462. [Google Scholar] [CrossRef]
- Merigo, E.; Rocca, J.-P.; Pinheiro, A.L.B.; Fornaini, C. Photobiomodulation Therapy in Oral Medicine: A Guide for the Practitioner with Focus on New Possible Protocols. Photobiomodulation Photomed. Laser Surg. 2019, 37, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Petrini, M.; Inchingolo, F.; Lorusso, F.; Amuso, D. A New Technique for the Treatment of Nasal Telangiectasia Using Atmospheric Plasma (Voltaic Arc Dermabrasion): Postoperative Pain Assessment by Thermal Infrared Imaging. J. Cosmet. Dermatol. 2020, 19, 2912–2918. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tarullo, A.; Cagiano, R.; Resta, G.; Dipalma, G.; Inchingolo, A.M.; Tarullo, A.; Scacco, S.; Marrelli, M.; Corti, L.; et al. Successful Use of a Topical Mixture with Ozolipoile in the Treatment of Actinic Ulcers. Clin. Cosmet. Investig. Dermatol. 2015, 8, 147–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Ventura, A.; Lanteri, V.; Farronato, G.; Gaffuri, F.; Beretta, M.; Lanteri, C.; Cossellu, G. Three-Dimensional Evaluation of Rapid Maxillary Expansion Anchored to Primary Molars: Direct Effects on Maxillary Arch and Spontaneous Mandibular Response. Eur. J. Paediatr. Dent. 2019, 20, 38–42. [Google Scholar] [CrossRef]
- Mangano, F.G.; Caprioglio, A.; Levrini, L.; Farronato, D.; Zecca, P.A.; Mangano, C. Immediate Loading of Mandibular Overdentures Supported by One-Piece, Direct Metal Laser Sintering Mini-Implants: A Short-Term Prospective Clinical Study. J. Periodontol. 2015, 86, 192–200. [Google Scholar] [CrossRef]
- Kripal, K.; Sirajuddin, S.; Rafiuddin, S.; Mp, R.; Chungkham, S. Iatrogenic Damage to the Periodontium Caused by Laser: An Overview. Open Dent. J. 2015, 9, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Cobb, C.M. Lasers in Periodontics: A Review of the Literature. J. Periodontol. 2006, 77, 545–564. [Google Scholar] [CrossRef] [Green Version]
- Coluzzi, D.J. Lasers and Soft Tissue Curettage: An Update. Compend. Contin. Educ. Dent. 2002, 23, 1104–1111. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Explanation of the 2011 OCEBM Levels of Evidence—Centre for Evidence-Based Medicine (CEBM), University of Oxford. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/explanation-of-the-2011-ocebm-levels-of-evidence (accessed on 6 March 2023).
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A Randomized Controlled Clinical and Histopathological Trial Comparing Excisional Biopsies of Oral Fibrous Hyperplasias Using CO2 and Er:YAG Laser. Lasers Med. Sci. 2017, 32, 573–581. [Google Scholar] [CrossRef]
- Saibene, A.M.; Rosso, C.; Castellarin, P.; Vultaggio, F.; Pipolo, C.; Maccari, A.; Ferrari, D.; Abati, S.; Felisati, G. Managing Benign and Malignant Oral Lesions with Carbon Dioxide Laser: Indications, Techniques, and Outcomes for Outpatient Surgery. Surg. J. 2019, 5, e69–e75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikov, T.; Pecheva, E.; Montgomery, P.; Antoni, F.; Leong-Hoi, A.; Petrov, T. Precise Femtosecond Laser Ablation of Dental Hard Tissue: Preliminary Investigation on Adequate Laser Parameters. J. Phys. Conf. Ser. 2017, 794, 012036. [Google Scholar] [CrossRef]
- Lauritano, D.; Lucchese, A.; Gabrione, F.; Di Stasio, D.; Silvestre Rangil, J.; Carinci, F. The Effectiveness of Laser-Assisted Surgical Excision of Leukoplakias and Hyperkeratosis of Oral Mucosa: A Case Series in a Group of Patients. Int. J. Environ. Res. Public. Health 2019, 16, 210. [Google Scholar] [CrossRef] [Green Version]
- Bahammam, M.A. Treatment of a Gingival Injury from a Cosmetic Laser Burn: A Case Report. Compend. Contin. Educ. Dent. 2018, 39, 238–243. [Google Scholar]
- Fabian Falkenstein; Norbert Gutknecht; René Franzen Analysis of Laser Transmission and Thermal Effects on the Inner Root Surface during Periodontal Treatment with a 940-Nm Diode Laser in an in Vitro Pocket Model. J. Biomed. Opt. 2014, 19, 128002. [CrossRef]
- Franzen, R.; Rashidisangsary, B.; Ozturan, S.; Vanweersch, L.; Gutknecht, N. Intrapulpal Temperature Changes during Root Surface Irradiation with Dual-Wavelength Laser (2780 and 940 Nm): In Vitro Study. J. Biomed. Opt. 2015, 20, 018002. [Google Scholar] [CrossRef]
- Tonin, M.H.; Brites, F.C.; Mariano, J.R.; Freitas, K.M.S.; Ortiz, M.A.L.; Salmeron, S. Low-Level Laser and Antimicrobial Photodynamic Therapy Reduce Peri-Implantitis-Related Microorganisms Grown In Vitro. Eur. J. Dent. 2022, 16, 161–166. [Google Scholar] [CrossRef]
- Monzavi, A.; Fekrazad, R.; Chinipardaz, Z.; Shahabi, S.; Behruzi, R.; Chiniforush, N. Effect of Various Laser Wavelengths on Temperature Changes During Periimplantitis Treatment: An in Vitro Study. Implant. Dent. 2018, 27, 311–316. [Google Scholar] [CrossRef]
- Hoedke, D.; Enseleit, C.; Gruner, D.; Dommisch, H.; Schlafer, S.; Dige, I.; Bitter, K. Effect of Photodynamic Therapy in Combination with Various Irrigation Protocols on an Endodontic Multispecies Biofilm Ex Vivo. Int. Endod. J. 2018, 51, e23–e34. [Google Scholar] [CrossRef] [Green Version]
- Sobral, M.F.P.; Cassoni, A.; Tenis, C.A.; Steagall, W.; Brugnera Junior, A.; Bagnato, V.S.; Botta, S.B. Longitudinal, Randomized, and Parallel Clinical Trial Comparing a Violet Light-Emitting Diodes System and In-Office Dental Bleaching: 6-Month Follow-Up. Photobiomodulation Photomed. Laser Surg. 2021, 39, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.E.V.; Tompson, B.; Gong, S.-G. The Effect of Light Emitting Diode Phototherapy on Rate of Orthodontic Tooth Movement: A Split Mouth, Controlled Clinical Trial. J. Orthod. 2015, 42, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Saluja, M. Comparative Morphologic Evaluation and Occluding Effectiveness of Nd: YAG, CO 2 and Diode Lasers on Exposed Human Dentinal Tubules: An Invitro SEM Study. JCDR 2016, 10, ZC66–ZC70. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.R.; Ehteshami, A.; Zallaghi, F.; Chiniforush, N.; Moradi, Z. Microleakage in Class V Composite Restorations after Desensitizing Surface Treatment with Er:YAG and CO2 Lasers. Laser Ther. 2016, 25, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Aljdaimi, A.; Devlin, H.; Dickinson, M.; Burnett, T.; Slater, T.J.A. Micron-Scale Crack Propagation in Laser-Irradiated Enamel and Dentine Studied with Nano-CT. Clin. Oral. Investig. 2019, 23, 2279–2285. [Google Scholar] [CrossRef] [Green Version]
- Kuhn-Dall’Magro, A.; Zamboni, E.; Fontana, T.; Dogenski, L.C.; De Carli, J.P.; Dall’Magro, E.; Fornari, F. Low-Level Laser Therapy in the Management of Oral Mucositis Induced by Radiotherapy: A Randomized Double-Blind Clinical Trial. J. Contemp. Dent. Pract. 2022, 23, 31–36. [Google Scholar]
- Romeo, U.; Russo, C.; Palaia, G.; Lo Giudice, R.; Del Vecchio, A.; Visca, P.; Migliau, G.; De Biase, A. Biopsy of Different Oral Soft Tissues Lesions by KTP and Diode Laser: Histological Evaluation. Sci. World J. 2014, 2014, 761704. [Google Scholar] [CrossRef]
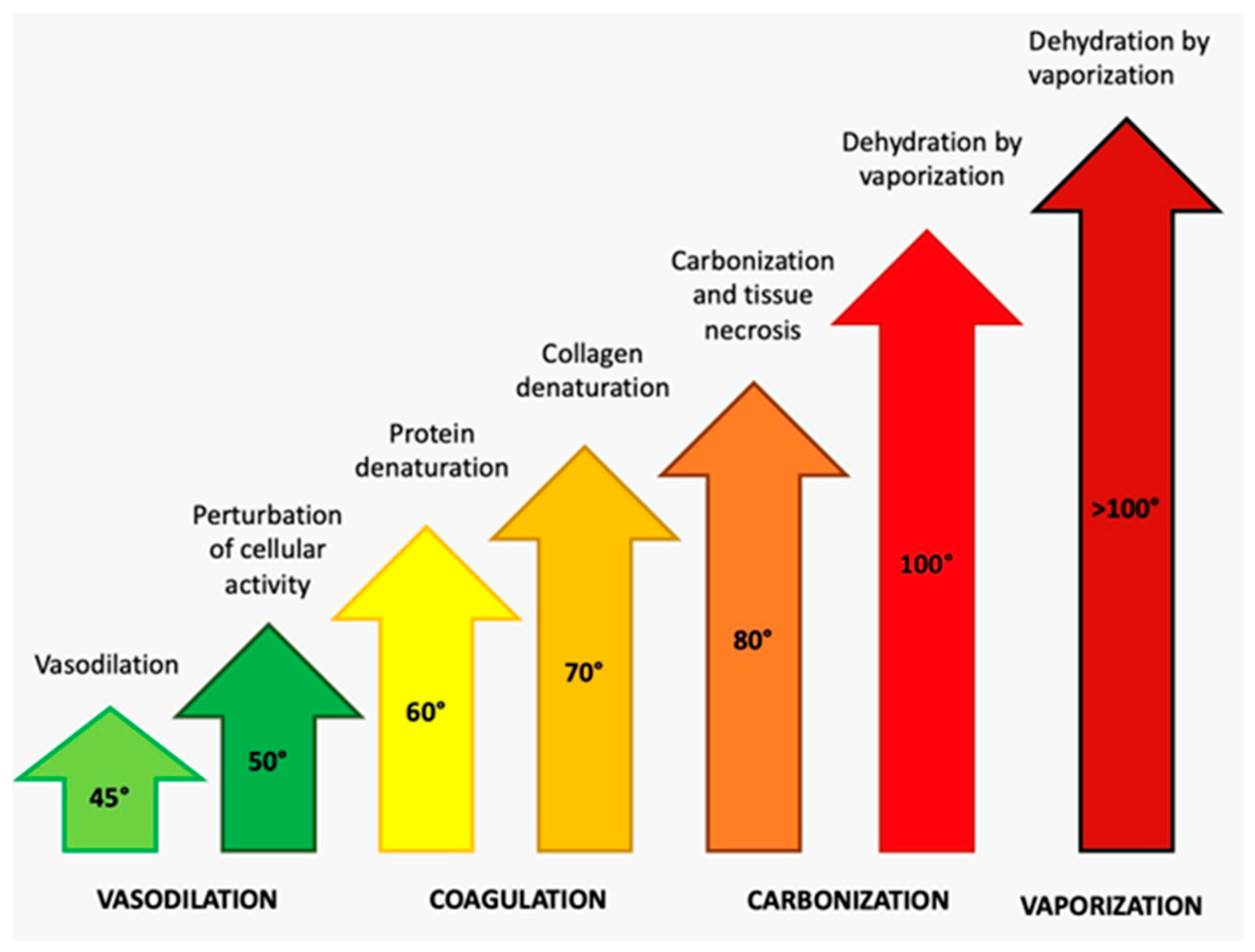
- Launay, Y.; Mordon, S.; Cornil, A.; Brunetaud, J.M.; Moschetto, Y. Thermal Effects of Lasers on Dental Tissues. Lasers Surg. Med. 1987, 7, 473–477. [Google Scholar] [CrossRef]
- Mortazavi, H.; Baharvand, M.; Mokhber-Dezfuli, M.; Rostami, N.; Doost-Hoseini, M.; Alavi-Chafi, O.; Nourshad, S. Lasers in Dentistry: Is It Really Safe? Dent. Hypotheses 2016, 7, 123–127. [Google Scholar] [CrossRef]
- Fu, W.; Wo, C. The Use of Laser in Dentistry: A Narrative Review. J. Biol. Regul. Homeost. Agents 2021, 35, 11–18. [Google Scholar]


| Authors (Year) | Type of the Study | Aim of the Study | Materials | Results |
|---|---|---|---|---|
| Valerie, G.A., et al., 2017 [97] | Randomized controlled clinical and histopathological trial | Compare excisional biopsies using CO2 and Er: YAG laser | 32 Patients with f.h. in the buccal mucosa | Intraoperative bleeding 100% excisions with Er: YAG and 56% with CO2 laser |
| Saibene, A.M., et al., 2019 [98] | Study in vivo | To evaluate the outcomes of laser surgery while providing a protocol for CO2 laser surgery | 78 patients treated with laser for benign and malignant lesions | 5 patients complained of marginal pain; 3 patients had post-surgery bleeding |
| Hikov, T., et al., 2017 [99] | Study in vivo | To evaluate the possibility of introducing a femtosecond laser system in restorative dentistry | Patients’ molars | The FELS used in this work is promising for an efficient and controlled cavity preparation |
| Lauritano, D., et al., 2018 [100] | Case series in a group of patients | Setting up a laser- assisted protocol for the surgical excision of malignant lesions | A specially designed medical record was used. The diode laser was used | Our findings demonstrate a statistically significant reduction in the size of the lesion |
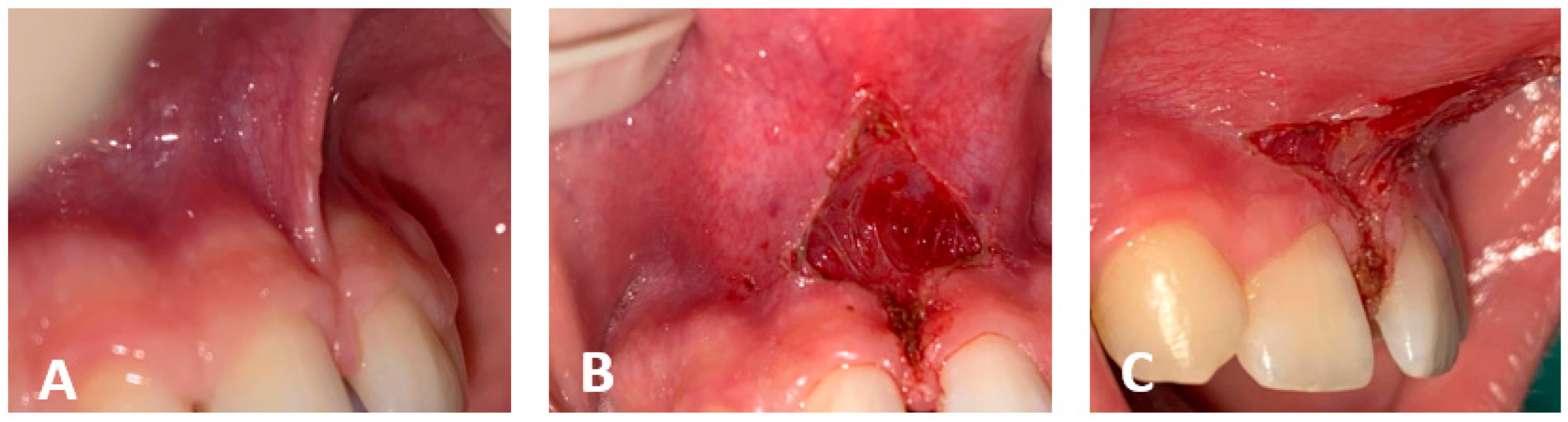
| Bahammam, M.A., et al., 2018 [101] | Case report | To raise an awareness regarding the potential complications of laser gingival depigmentation | One case report | A possible treatment approach for such complications |
| Falkenstein, F., et al., 2014 [102] | Study in vitro | To demonstrate that laser-assisted treatment can produce thermal damage to pulp tissues | Extracted upper and lower human incisors stored in 0.9% saline solution | Middle-third portion of the root seems to be the most at-risk area for pulp damage |
| Franzen, R., et al., 2015 [103] | Study in vitro | To demonstrate that laser-assisted treatment can produce thermal damage to pulp tissues | 30 single-rooted human teeth | Er, Cr:YSGG and a diode 2 W during irradiation show thermal rises on average of 1.68 ± 0.98 °C in the pulp chamber |
| Tonin, M.H., et al., 2022 [104] | Study in vitro | To show that the increasing dose of Er: YAG can produce damage of implant surface | Implants’ surfaces | Modification of implant surface as adverse effect may not allow the correct formation of oxide layer that is important for wettability of implant and for the differentiation of osteoblasts |
| Monzavi, A., et al., 2018 [105] | Study in vitro | To investigate and compare temperature change during implant decontamination with different laser types ([Co2]/Nd: YAG/Er: YAG/PDT | Sixty implants were inserted into a bone block cut from a sheep’s mandible | Temperature changes over 10 °C occurred at the apical point of the implants with the Co2, Nd: YAG and diode laser irradiation; however, only the Co2 laser reached statistical significance in this regard |
| Hoedke, D., et al., 2018 [106] | Study in vitro | To show the removal of bacteria biofilm inside the root canals of extracted teeth through photodynamic therapy using phenothiazine chloride as photosensitizer and a diode laser with a wavelength 660 nm in combination with irrigants | 160 extracted human single-rooted teeth | Effective method for the reduction in bacterial biofilm inside the root canals of extracted teeth |
| Ferreira Pires Sobral, M., et al., 2021 [107] | Longitudinal randomized and parallel clinical trial | To compare a violet light-emitting diode system and in-office dental bleaching | Sixty volunteers selected for group one bleaching with 35% hydrogen peroxide gel and for group two using bleaching using a LED | Violet light alone caused repigmentation after dental bleaching |
| Chung, S.E.V., et al., 2015 [108] | Study in vitro | To demonstrate that Er: YAG laser favors bacteria elimination in infected root canals together with sodium hypochlorite irrigation | The adverse reactions observed in endodontics with Er: YAG laser include the risk of thermal injury to periodontal tissues, even if it not a temperature that causes the necrosis of periodontal ligaments | |
| Saluja, M., et al., 2016 [109] | Study in vitro | To show that Nd: YAG, CO2 and diode lasers can be used also for treatment of dentinal hypersensitivity through the occlusion of human dental tubules | 24 extracted teeth | Risk of the expansion of dental pulp through damage |
| Mozaffari, H.R., et al., 2016 [110] | Study in vitro | To demonstrate that laser for dentinal hypersensitivity can also interfere with composite restoration | 60 extracted teeth | The application of glutaraldehyde desensitizer and the CO2 laser to the surface in order to complete restoration does not increase microleakage in the enamel or dentin damage tooth surface treatment, while Er: YAG laser significantly increased microleakage at the dentin margins |
| Aljdaimi, A., et al., 2019 [111] | Study in vitro | To compare the impact of Er: YAG laser irradiation on enamel using an FIB | Coronal sections of sound enamel and dentine were machined to 50 μm thickness using an FIB | Microcracks in the peritubular dentine ran orthogonally to the tubules, whereas fractures in the intertubular area ran parallel to the tubules. The average depth of these fissures was around 10 m below the surface. As a result of preferential ablation of the less mineralized intertubular dentine on the dentine surface, a tubule-associated uneven topography was created. |
| Kuhn-Dal Magro, A., et al., 2022 [112] | Randomized double-blind clinical trial | The effect of LLLT associated with therapeutic measures from the oral mucositis | 80 subjects treated for oral mucositis with LLLT | The LLLT was effective for OM lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malcangi, G.; Patano, A.; Trilli, I.; Piras, F.; Ciocia, A.M.; Inchingolo, A.D.; Mancini, A.; Hazballa, D.; Di Venere, D.; Inchingolo, F.; et al. Therapeutic and Adverse Effects of Lasers in Dentistry: A Systematic Review. Photonics 2023, 10, 650. https://doi.org/10.3390/photonics10060650
Malcangi G, Patano A, Trilli I, Piras F, Ciocia AM, Inchingolo AD, Mancini A, Hazballa D, Di Venere D, Inchingolo F, et al. Therapeutic and Adverse Effects of Lasers in Dentistry: A Systematic Review. Photonics. 2023; 10(6):650. https://doi.org/10.3390/photonics10060650
Chicago/Turabian StyleMalcangi, Giuseppina, Assunta Patano, Irma Trilli, Fabio Piras, Anna Maria Ciocia, Alessio Danilo Inchingolo, Antonio Mancini, Denisa Hazballa, Daniela Di Venere, Francesco Inchingolo, and et al. 2023. "Therapeutic and Adverse Effects of Lasers in Dentistry: A Systematic Review" Photonics 10, no. 6: 650. https://doi.org/10.3390/photonics10060650










