Fluorescence-Based Aqueous Phosphate Sensing Using Eu(cpboda)(DMF)2
Abstract
:1. Introduction
2. Method
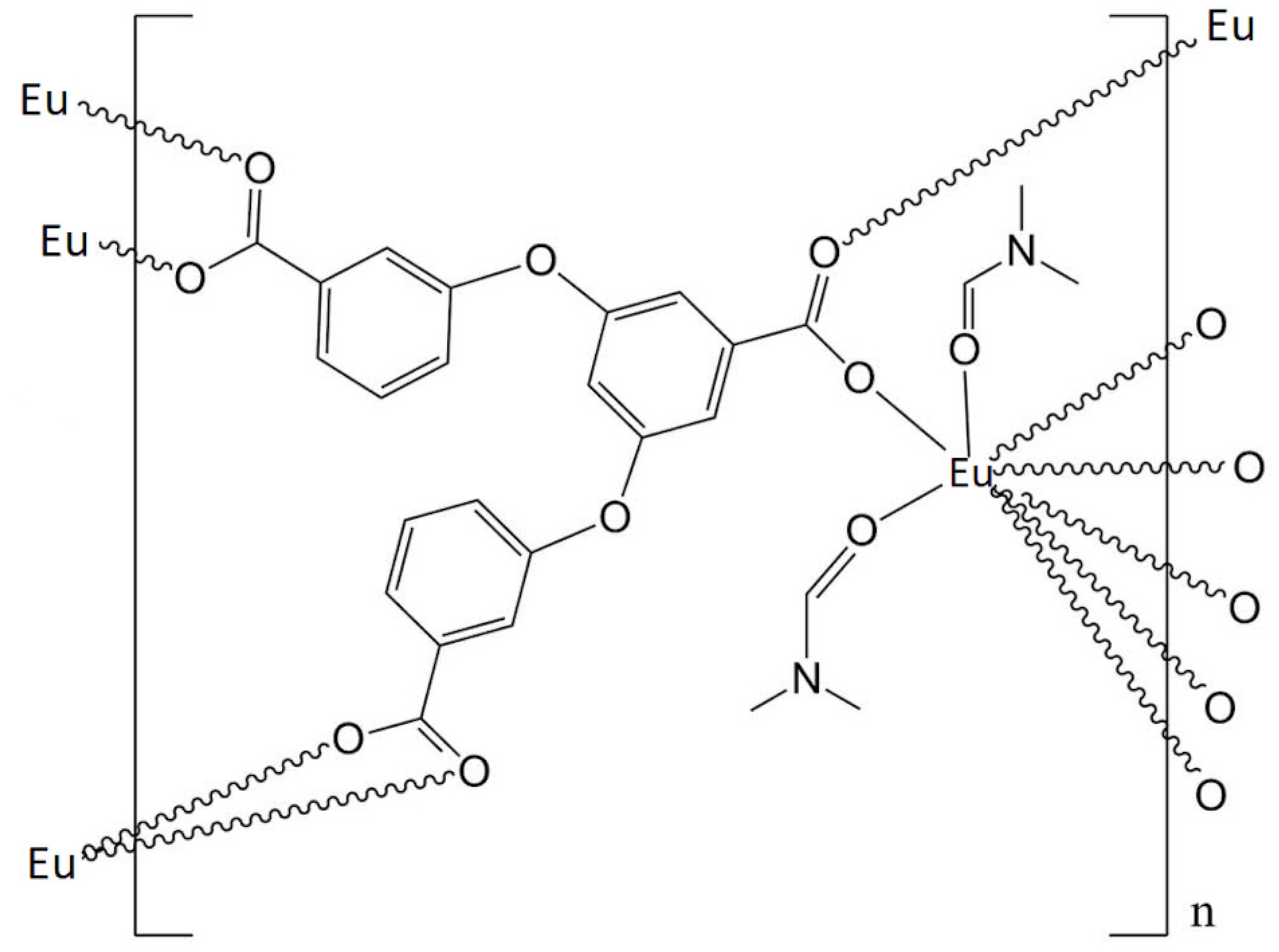
2.1. Eu(cpboda)(DMF Synthesis
2.2. Solutions
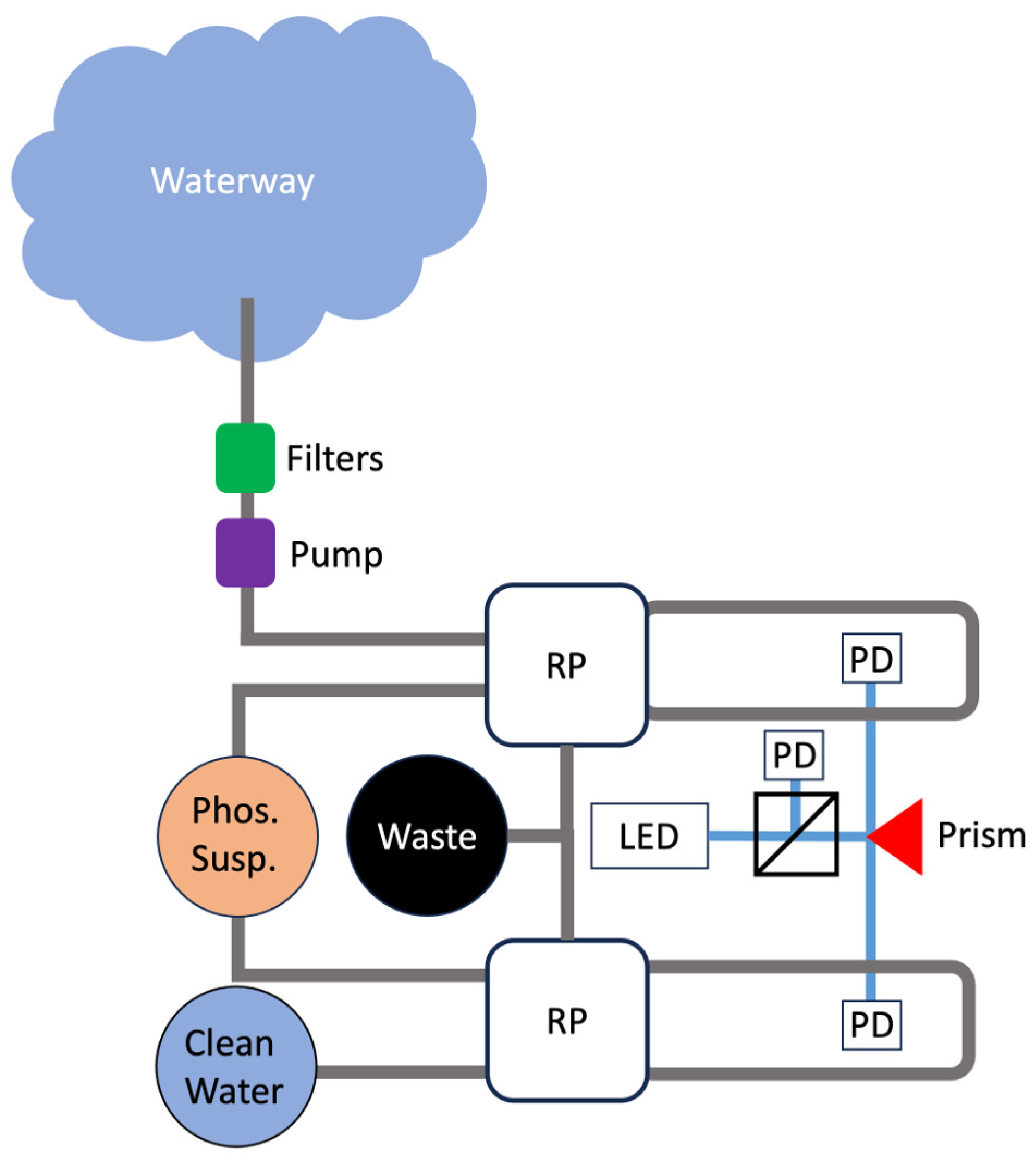
2.3. Fluorescence Spectroscopy
3. Results and Discussions
3.1. Fluorescence Properties
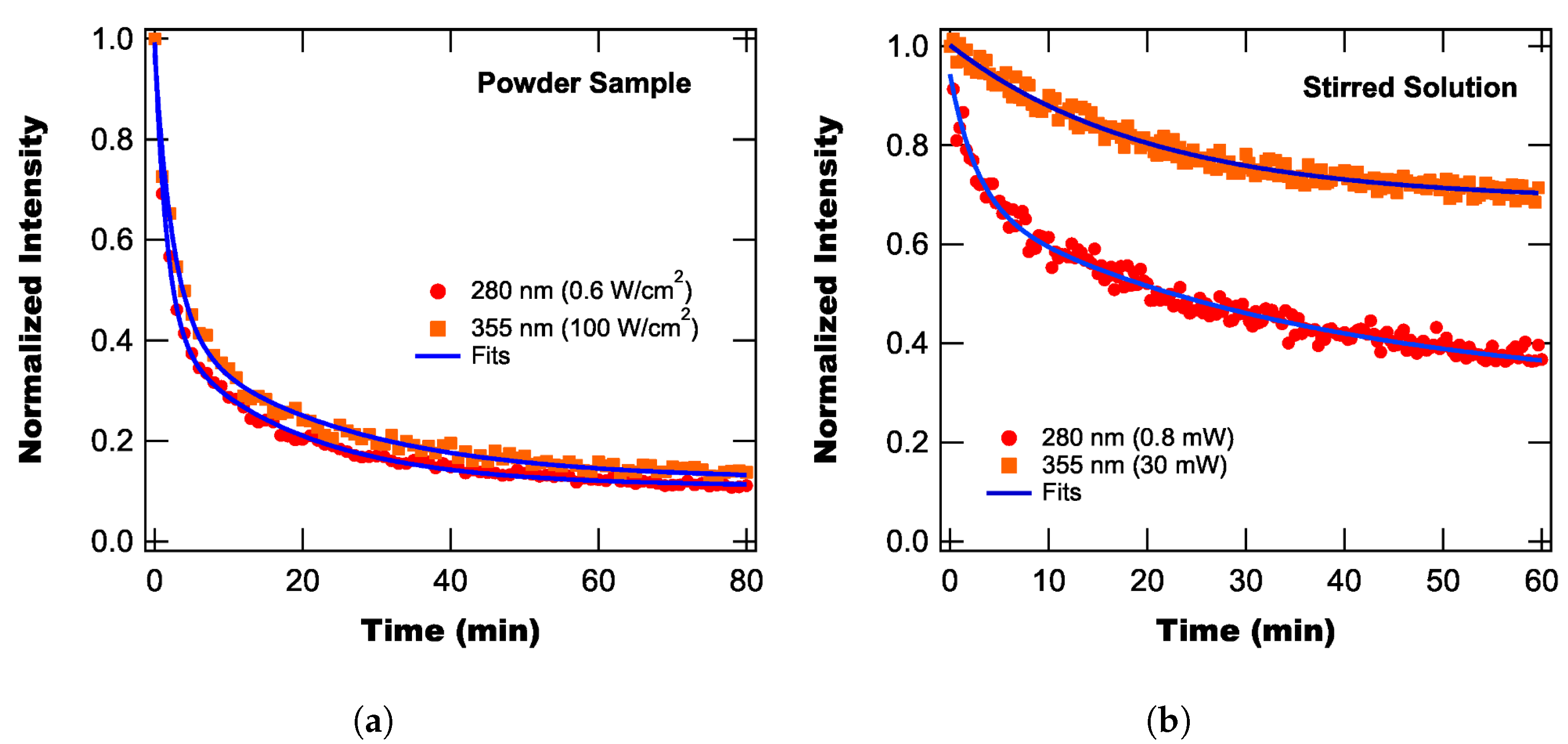
3.2. Photodegradation
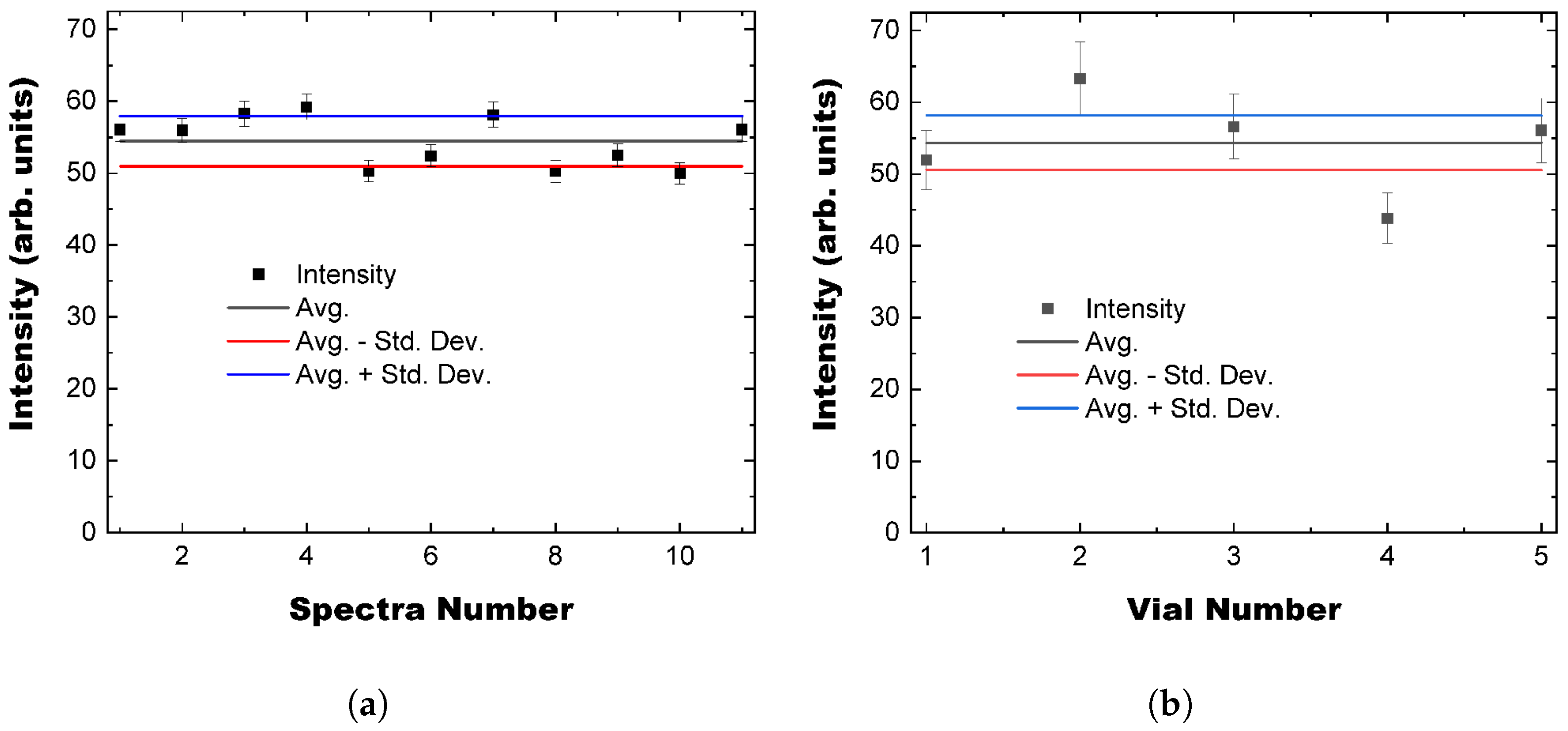
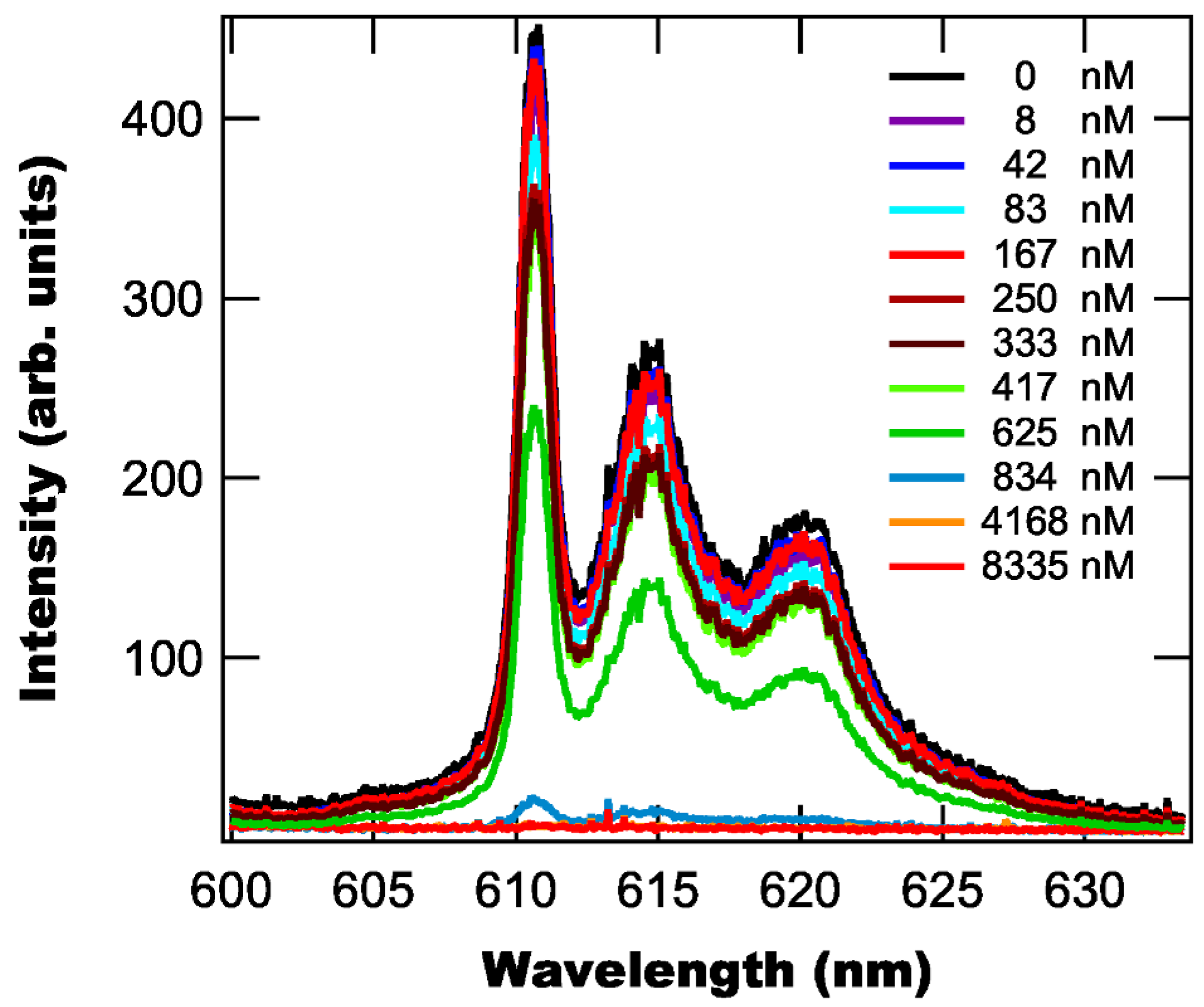
3.3. Solution Variance
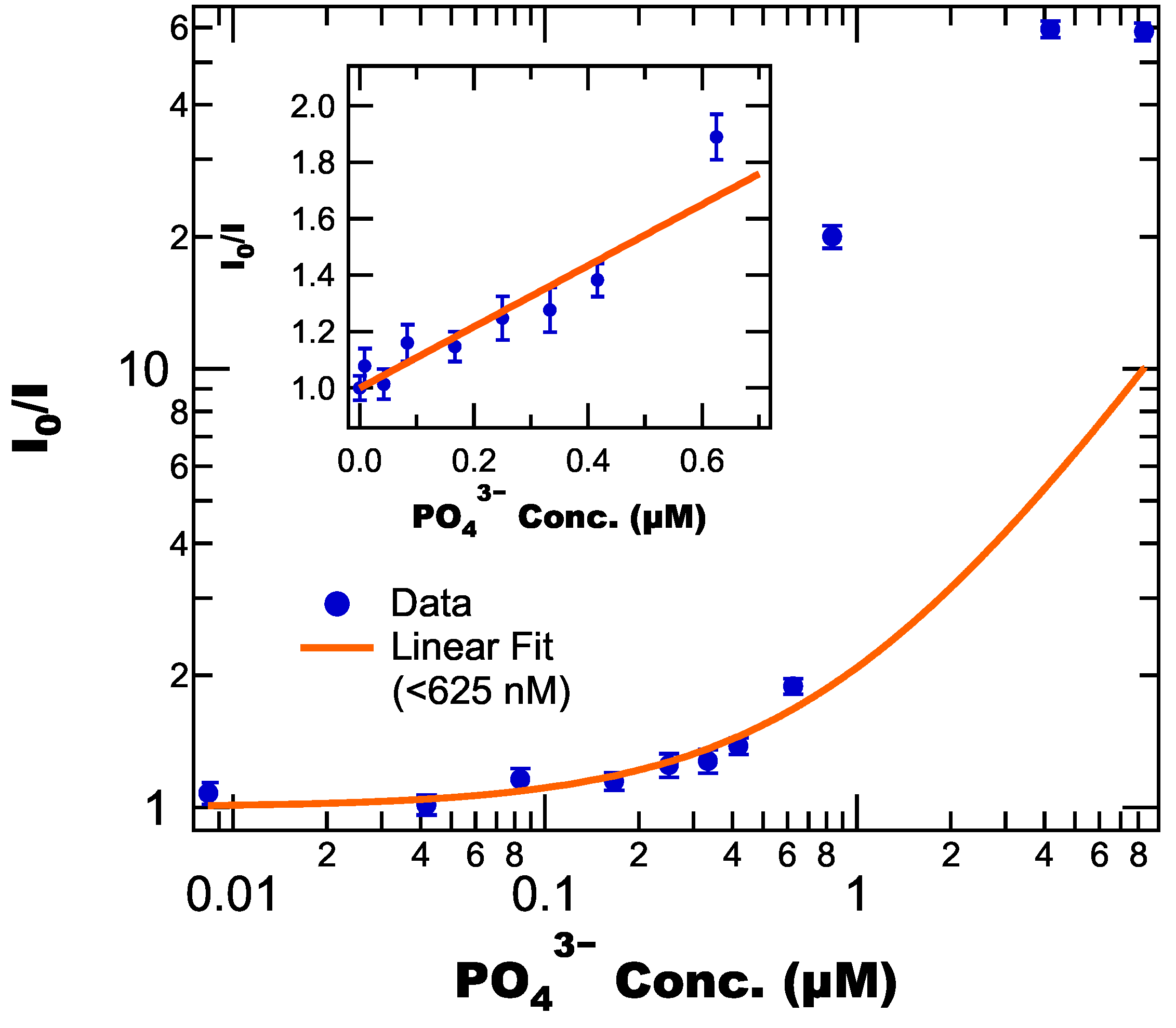
3.4. Effect of on Fluorescence
3.5. Applications
4. Conclusions
5. Disclaimer
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Connecticut Department of Energy & Environmental Protection. Aquatic Life Impacts of Phosphorus. Available online: https://portal.ct.gov/DEEP/Water/Inland-Water-Monitoring/Aquatic-Life-Impacts-of-Phosphorus-Research (accessed on 23 February 2023).
- Griffiths, N.A.; Sebestyen, S.D. Dynamic vertical profiles of peat porewater chemistry in a northern peatland. Wetlands 2016, 36, 1119–1130. [Google Scholar] [CrossRef]
- USA Department of Energy. Earth and Environmental Systems Sciences Division, Strategic Plan. Available online: https://science.osti.gov/-/media/ber/pdf/workshop-reports/2018_CESD_Strategic_Plan.pdf (accessed on 23 February 2023).
- Pellerin, B.A.; Stauffer, B.A.; Young, D.A.; Sullivan, D.J.; Bricker, S.B.; Walbridge, M.R.; Clyde, G.A.; Shaw, D.M. Emerging tools for continuous nutrient monitoring networks: Sensors advancing science and water resources protection. J. Am. Water Resour. Assoc. 2016, 52, 993–1008. [Google Scholar] [CrossRef]
- USA Environmental Protection Agency. Methods for Evaluating Wetland Condition: #18 Biogeochemical Indicators. Available online: https://www.epa.gov/sites/default/files/documents/wetlands_18biogeochemical.pdf (accessed on 23 February 2023).
- USA Environmental Protection Agency. National Aquatic Resource Surveys. Available online: https://www.epa.gov/national-aquatic-resource-surveys/indicators-phosphorus (accessed on 23 February 2023).
- USA Environmental Protection Agency. Nutrient Pollution. Available online: https://www.epa.gov/nutrientpollution/issue (accessed on 23 February 2023).
- Water Research Center. Phosphates in the Environment. Available online: https://www.knowyourh2o.com/outdoor-4/phosphates-in-the-environment (accessed on 23 February 2023).
- Reddy, K.R.; Kadlec, R.H.; Flaig, E.; Gale, P.M. Phosphorus retention in streams and wetlands: A review. Crit. Rev. Environ. Sci. Technol. 1999, 29, 83–146. [Google Scholar] [CrossRef]
- USA Geological Survey. Phosphorus and Water. Available online: https://www.usgs.gov/special-topic/water-science-school/science/phosphorus-and-water?qt-science_center_objects=0#qt-science_center_objects (accessed on 23 February 2023).
- Watson, S.J.; Cade-Menun, B.J.; Needoba, J.A.; Peterson, T.D. Phosphorus forms in sediments of a river-dominated estuary. Front. Mar. Sci. 2018, 5, 302. [Google Scholar] [CrossRef]
- Brenner, J.; Porter, W.; Phillips, J.R.; Childs, J.; Yang, X.; Mayes, M.A. Phosphorus sorption on tropical soils with relevance to earth system model needs. Soil Res. 2019, 57, 17. [Google Scholar] [CrossRef]
- USA Department of Energy. Research Priorities to Incorporate Terrestrial-Aquatic Interfaces in Earth System Models. Available online: https://ess.science.energy.gov/wp-content/uploads/2020/12/TAI_Workshop2016-1.pdf (accessed on 23 February 2023).
- Dunne, E.; Clark, M.; Mitchell, J.; Jawitz, J.; Reddy, K. Soil phosphorus flux from emergent marsh wetlands and surrounding grazed pasture uplands. Ecol. Eng. 2010, 36, 1392–1400. [Google Scholar] [CrossRef]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of u.s. freshwaters: Analysis of potential economic damages. Environ. Sci. Technol. 2008, 43, 12–19. [Google Scholar] [CrossRef]
- Mueller, D.K.; Helsel, D.R. Nutrients in the Nation’s Waters—Too Much of a Good Thing? Available online: https://pubs.usgs.gov/circ/circ1136/ (accessed on 23 February 2023).
- Patel, V.; Kruse, P.; Selvaganapathy, P.R. Review-solid state sensors for phosphate detection in environmental and medical diagnostics. J. Electrochem. Soc. 2022, 169, 077505. [Google Scholar] [CrossRef]
- Zhu, X.; Ma, J. Recent advances in the determination of phosphate in environmental water samples: Insights from practical perspectives. Trends Anal. Chem. 2020, 127, 115908. [Google Scholar] [CrossRef]
- Yang, D.-D.; Lu, L.-P.; Zhu, M.-L. A design for detecting phosphate ions in aqueous solution by luminescent tb-coordination polymer. Inorg. Chim. Acta 2021, 515, 120030. [Google Scholar] [CrossRef]
- Kröckel, L.; Lehmann, H.; Wieduwilt, T.; Schmidt, M.A. Fluorescence detection for phosphate monitoring using reverse injection analysis. Talanta 2014, 125, 107–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, S.; Mao, S.; Wu, X.; Li, Z.; Tao, W.; Jenkinson, I.R. Highly sensitive and selective fluorescent detection of phosphate in water environment by a functionalized coordination polymer. Water Res. 2019, 163, 114883. [Google Scholar] [CrossRef]
- Yang, J.; Dai, Y.; Zhu, X.; Wang, Z.; Li, Y.; Zhuang, Q.; Shi, J.; Gu, J. Metal–organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J. Mater. Chem. 2015, 3, 7445–7452. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.-S.; Zhao, B. A water-stable lanthanide-organic framework as a recyclable luminescent probe for detecting pollutant phosphorus anions. Chem. Commun. 2015, 51, 10280–10283. [Google Scholar] [CrossRef]
- Jiang, S.-Q.; Zhou, Z.-Y.; Zhuo, S.-P.; Shan, G.-G.; Xing, L.-B.; Wang, H.-N.; Su, Z.-M. Rational design of a highly sensitive and select turn-on fluorescent sensor for detection. Dalton Trans. 2015, 44, 20830–20833. [Google Scholar] [CrossRef]
- Ji, G.; Gao, X.; Zheng, T.; Guan, W.; Liu, H.; Liu, Z. Postsynthetic metalation metal-organic framework as a fluorescent probe for the ultrasensitive and reversible detection of ions. Inorg. Chem. 2018, 57, 10525–10532. [Google Scholar] [CrossRef]
- Asha, K.S.; Bhattacharjee, R.; Mandal, S. Complete transmetalation in a metal-organic framework by metal ion metathesis in a single crystal for selective sensing of phosphate ions in aqueous media. Angew. Chem. Int. Ed. 2016, 55, 11528–11532. [Google Scholar] [CrossRef]
- Zhao, S.; Xiao, J.; Zheng, T.; Liu, M.; Wu, H.; Liu, Z. Highly Selective and Sensitive Detection of Ions in Aqueous Solution by a Luminescent Terbium Metal–Organic Framework. ASC Omega 2019, 4, 16378–16384. [Google Scholar] [CrossRef]
- Das, A.; Das, S.; Trivedi, V.; Biswas, S. A dual functional MOF-based fluorescent sensor for intracellular phosphate and extracellular 4-nitrobenzaldehyde. Dalton Trans. 2019, 48, 1332–1343. [Google Scholar] [CrossRef]
- Li, P.; Dong, L.; Jin, H.; Yang, J.; Tu, Y.; Wang, C.; He, Y. Fluorescence detection of phosphate in an aqueous environment by an aluminum-based metal-organic framework with amido functionalized ligands. Front. Environ. Sci. Eng. 2022, 16, 159. [Google Scholar] [CrossRef]
- Rao, P.C.; Mandal, S. Europium-based metal–organic framework as a dual luminescence sensor for the selective detection of the phosphate anion and fe3+ ion in aqueous media. Inorg. Chem. 2018, 57, 11855–11858. [Google Scholar]
- Wu, H.; Tong, C. A specific turn-on fluorescent sensing for ultrasensitive and selective detection of phosphate in environmental samples based on antenna effect-improved FRET by surfactant. ACS Sens. 2018, 3, 1539–1545. [Google Scholar] [CrossRef]
- Zhao, D.; Wan, X.; Song, H.; Hao, L.; Su, Y.; Lv, Y. Metal-organic frameworks (MOFs) combined with ZnO quantum dots as a fluorescent sensing platform for phosphate. Sens. Actuators D Chem. 2014, 197, 50–57. [Google Scholar] [CrossRef]
- Naskar, K.; Bhanja, A.K.; Paul, S.; Pal, K.; Sinha, C. Trace quantity detection of H2 by fluorescent metal–organic framework (f-MOF) and bioimaging study. Cryst. Growth Des. 2020, 20, 6453–6460. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Basics of Lathanide Photophysics. In Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects; Hanninen, P., Harma, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Yang, D.-D.; Lu, L.-P.; Zhu, M.-L. A new family of lanthanide coordination polymers based on 3,3’-[(5-carboxylato-1,3-phenylene)bis(oxy)]dibenzoate: Synthesis, crystal structures and magnetic and luminescence properties. Acta Crystallogr. Sect. Struct. Chem. 2020, 76, 763–770. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Ciric, A.; Stojadinovic, S.; Sekulic, M.; Dramicanin, M.D. JOES: An application software for Judd-Ofelt analysis from Eu3+ emission spectra. J. Lumin. 2019, 205, 351–356. [Google Scholar] [CrossRef]
- Anderson, B.R.; Gese, N.; Eilers, H. Spectroscopic properties of Eu:Y(acac)3(DPEPO) and characterization of its photo- and thermal- degradation. J. Lumin. 2022, 251, 119183. [Google Scholar] [CrossRef]
- Anderson, B.R.; Gese, N.; Nawani, P.; Eilers, H. Aqueous Phosphate Detection using Eu(acac)3. Appl. Phys. B 2023, 129, hl188. [Google Scholar] [CrossRef]
- Saeed, M.A.; Powell, D.R.; Hossain, M.A. Fluorescent detection of phosphate anion by a highly selective chemosensor in water. Tetrahedron Lett. 2010, 51, 4904–4907. [Google Scholar] [CrossRef]
- Pierre, V.C.; Wilharm, R.K. Design Principles and Applications of Selective Lanthanide-Based Receptors for Inorganic Phosphate. Front. Chem. 2022, 10, 821020. [Google Scholar] [CrossRef] [PubMed]
- Shiery, R.C.; Cooper, K.A.; Cantu, D.C. Computational Prediction of All Lanthanide Aqua Ion Acidity Constants. Inorg. Chem. 2021, 60, 10257–10266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Zhu, G.; Du, B.; Yu, B.; Wang, C. Water-stable europium(III) and terbium(III)-metal organic frameworks as fluorescent sensors to detect ions, antibiotics and pesticides in aqueous solutions. J. Mol. Struct. 2022, 1251, 132009. [Google Scholar] [CrossRef]
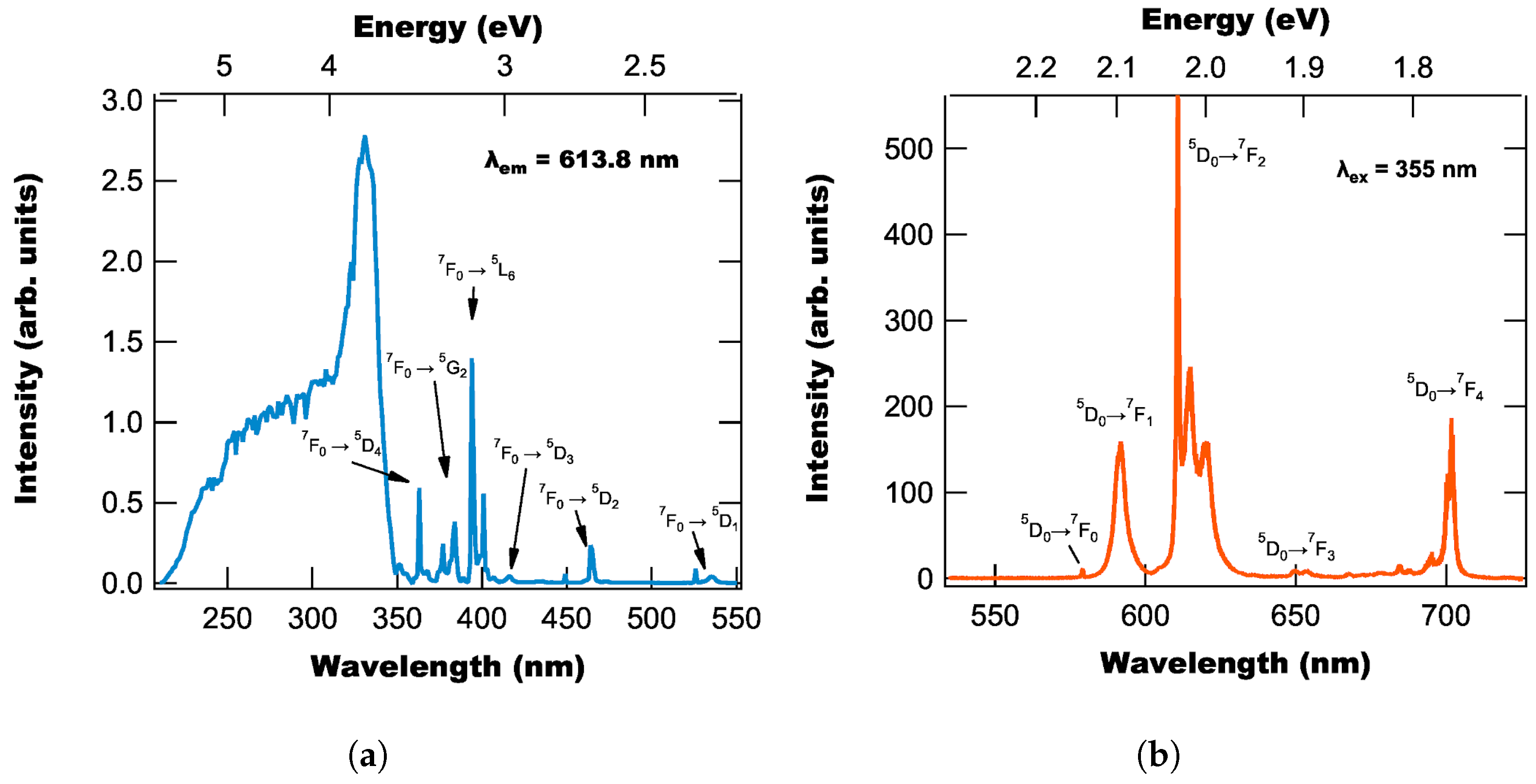
| Transition | Peak Locations | Transition | Peak Locations | ||
|---|---|---|---|---|---|
| (eV) | (nm) | eV | nm | ||
|
2.316 2.338 2.358 | 535.36 530.33 528.82 | 1.761 | 704.12 | ||
| 1.767 | 701.88 | ||||
| 1.771 | 700.34 | ||||
| 1.784 | 694.97 | ||||
| 1.803 | 687.69 | ||||
| 1.811 | 684.53 | ||||
| 2.617 2.670 | 472.80 464.47 | 1.829 | 678.04 | ||
| 1.858 | 667.66 | ||||
| 1.897 | 653.63 | ||||
| 1.909 | 649.56 | ||||
| 2.980 | 416.13 | 2.000 | 620.15 | ||
| 2.026 | 617.53 | ||||
| 2.030 | 616.30 | ||||
| 2.017 | 615.08 | ||||
| 3.094 | 400.74 | 2.094 | 592.04 | ||
| 3.144 | 394.42 | 2.100 | 590.44 | ||
| 3.233 | 383.58 | 2.141 | 579.08 | ||
| 3.291 | 376.74 | ||||
| 3.415 | 363.14 | ||||
| Parameter | Value |
|---|---|
| ( c) | 5.58 |
| ( c) | 3.46 |
| () | 54.0 |
| () | 185 |
| () | 56.3 |
| (ms) | 3.45 |
| 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, B.R.; Gese, N.; Nawani, P.; Eilers, H. Fluorescence-Based Aqueous Phosphate Sensing Using Eu(cpboda)(DMF)2. Photonics 2024, 11, 250. https://doi.org/10.3390/photonics11030250
Anderson BR, Gese N, Nawani P, Eilers H. Fluorescence-Based Aqueous Phosphate Sensing Using Eu(cpboda)(DMF)2. Photonics. 2024; 11(3):250. https://doi.org/10.3390/photonics11030250
Chicago/Turabian StyleAnderson, Benjamin R., Natalie Gese, Pranav Nawani, and Hergen Eilers. 2024. "Fluorescence-Based Aqueous Phosphate Sensing Using Eu(cpboda)(DMF)2" Photonics 11, no. 3: 250. https://doi.org/10.3390/photonics11030250





