Transcranial Photobiomodulation and Chronic Traumatic Brain Injury
Abstract
:1. Introduction
1.1. Background and Purpose of Review
1.2. Physiological Mechanisms of Transcranial Photobiomodulation
1.3. Microvascular Injury as a Potential Target for Transcranial Photobiomodulation in Chronic Traumatic Brain Injury
2. Methods
3. Previous Literature
3.1. Overview of Previous Literature
3.2. Transcranial Photobiomodulation in Chronic Mild TBI
| Mild TBI | |||
|---|---|---|---|
| Title | Naeser et al., 2014 [55] | Naeser et al., 2011 [49] | Naeser et al., 2023 [58] |
| Chronicity | 10 months–8 years. | 2 and 7 years. | 35 to 55 years prior, multiple head traumas took place during their football careers. |
| Mechanism of Injury | Mixed: motor vehicle accidents, concussions, and blast injuries. | Participant 1: Motor vehicle accidentParticipant 2: Multiple concussions with and without loss of consciousness. | Football/contact sports injury. |
| Sample Size | 11 | 2 | 4 |
| Age (years) | 26–62 | 52 and 59 | 55–74 |
| Sex | 6 males | 2 females | 4 males |
| tPBM Delivery | LED | LED | LED (tPBM and iPBM) |
| Duration | 20 min | 5:10 min gradually increased to 12:54 min; 7 min increased to 10 min. | Protocol A: 40 min, 633, and 870 nm tPBM. Protocol B: 20 min, 810 nm, tPBM, and iPBM; 25 min, 633 nm, and iPBM. Protocol C: 10 min tPBM on midline with 5 LED cluster heads, 12 min tPBM, and 5 LED cluster heads, each side of the head. |
| Target Region(s) | Midline from front-to-back hairline and bilaterally over frontal, parietal, and temporal areas. | Bilaterally and over midline sagittal areas. | Protocol A: Whole head. Protocol B: Default mode network and olfactory bulbs. Protocol C: Whole head. |
| tPBM Mode | Continuous | Continuous | Protocol A: Continuous. Protocol B: Pulsed; 40 Hz. Protocol C: Continuous. |
| Wavelength (nm) | 633 and 870 | 633 and 870 | Protocol A: 2 sets of 6 LED cluster heads composed of 9, 633 nm diodes and 52, 870 nm diodes in each LED cluster head. Protocol B: 4, 810 nm single tPBM diodes with 1, 810 nm iPBM, and all pulsed at 40 Hz; 1 iPBM single diode, 633 nm, and continuous. Protocol C: Each LED cluster head: 34, 660 nm, and 35, 850 nm diodes; 5 LED cluster heads on midline, and 5 LED cluster heads on each side. |
| Cortical Irradiance (mW/cm2) | 22.2 | 22.2 and 25.8 | Protocol A: 22.2 mW/cm2 per LED cluster head. Protocol B: 810 nm, default mode network tPBM, 75 mW/cm2, Mesial Prefrontal Cortex; 100 mW/cm2, Precuneus; L and R Angular gyrus; iPBM, 25 mW/cm2. and a separate iPBM, 633 nm 8 mW/cm2. Protocol C: 41 mW/cm2, midline; 35 mW/cm2, sides. |
| Number of Sessions | 18 | Participant 1: Approximately 31 in clinic (following this, participant self-treated at home daily for 5 years). Participant 2: 28. | 18 |
| Outcome | Improved executive function; decreased PTSD symptoms. | Participant 1: Improved self-awareness, and improved inhibition of angry outbursts. Participant 2: Medical disability discontinued; returned to full time work; improved executive function, memory, and inhibition; improved social behavior; and reduced PTSD symptoms. | Improved neuropsychological measures, including executive function, attention, and verbal learning/memory; decreased PTSD, depression, pain, and improved sleep; increased functional connectivity in salience network; increased n- acetyl-aspartate, a measure of mitochondrial oxygenation, in Anterior Cingulate Cortex. |
| Side Effects | None. | None. | None. |
| Mild TBI | |||
|---|---|---|---|
| Title | Liebel et al., 2022 [56] | Vargas et al., 2017 [48] | Chao et al. 2020 [60] |
| Chronicity | Not reported; former athletes. | 12 months or less. | 6 concussions: most recent was 1 month pre-treatment. |
| Mechanism of Injury | Multiple concussions or sub-concussive events. | - | Multiple concussions. |
| Sample Size | 49 | 12 (3 with TBI) | 1 |
| Age (years) | - | 49–90 | 23 |
| Sex | - | 5 males, 7 females. 2 males with TBI; 1 female with TBI. | Male. |
| tPBM Delivery | LED (tPBM and iPBM) | Laser | LED (tPBM and iPBM) |
| Duration | - | 8 min | 20 min |
| Target Region(s) | - | Right forehead | Default mode network |
| tPBM Mode | Pulsed (40 Hz) | Continuous | Pulsed (10 and 40 Hz) |
| Wavelength (nm) | 810 | 1064 | 810 |
| Cortical Irradiance (mW/cm2) | - | 250 | 100 |
| Number of Sessions | Not reported; 8-week duration. | 5 | 38 |
| Outcome | Reduced depression, PTSD, and and adjustment symptoms; improved sleep quality, reaction times, and nondominant hand grip strength. | Improved cognitive scores. | Improved neuropsychological scores and functional connectivity and increased brain volume. |
| Side Effects | None. | None. | Mild headaches noted with the 40 Hz frequency. |
3.3. Transcranial Photobiomodulation in Chronic Mild–Moderate TBI
3.4. Transcranial Photobiomodulation in Chronic Moderate TBI
3.5. Transcranial Photobiomodulation in Chronic Moderate–Severe TBI
3.6. Transcranial Photobiomodulation in Chronic Severe TBI
3.7. Transcranial Photobiomodulation in Chronic TBI across Mixed Severities
4. Discussion
4.1. Summary
4.2. A Potential Target for Chronic TBI Using Transcranial Photobiomodulation
4.3. A Potential Target for Chronic TBI Using Transcranial Photobiomodulation
4.4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Willemse-van Son, A.H.P.; Ribbers, G.M.; Verhagen, A.P.; Stam, H.J. Prognostic factors of long-term functioning and productivity after traumatic brain injury: A systematic review of prospective cohort studies. Clin. Rehabil. 2007, 21, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.J.; McCormick, W.C.; Kagan, S.H. Traumatic Brain Injury in Older Adults: Epidemiology, Outcomes, and Future Implications. J. Am. Geriatr. Soc. 2006, 54, 1590–1595. [Google Scholar] [CrossRef]
- Bigler, E.D. Traumatic brain injury, neuroimaging, and neurodegeneration. Front. Hum. Neurosci. 2013, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Ding, Z.; Chan, A.S. Can transcranial photobiomodulation improve cognitive function? A systematic review of human studies. Ageing Res. Rev. 2023, 83, 101786. [Google Scholar] [CrossRef]
- Dole, M.; Auboiroux, V.; Langar, L.; Mitrofanis, J. A systematic review of the effects of transcranial photobiomodulation on brain activity in humans. Rev. Neurosci. 2023, 34, 671–693. [Google Scholar] [CrossRef]
- Stevens, A.R.; Hadis, M.; Milward, M.; Ahmed, Z.; Belli, A.; Palin, W.; Davies, D.J. Photobiomodulation in Acute Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma 2022, 40, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Sandsmark, D.K.; Bashir, A.; Wellington, C.L.; Diaz-Arrastia, R. Cerebral Microvascular Injury: A Potentially Treatable Endophenotype of Traumatic Brain Injury-Induced Neurodegeneration. Neuron 2019, 103, 367–379. [Google Scholar] [CrossRef]
- Gaggi, N.L.; Ware, J.B.; Dolui, S.; Brennan, D.; Torrellas, J.; Wang, Z.; Whyte, J.; Diaz-Arrastia, R.; Kim, J.J. Temporal dynamics of cerebral blood flow during the first year after moderate-severe traumatic brain injury: A longitudinal perfusion MRI study. NeuroImage Clin. 2023, 37, 103344. [Google Scholar] [CrossRef]
- Amyot, F.; Kenney, K.; Moore, C.; Haber, M.; Turtzo, L.C.; Shenouda, C.; Silverman, E.; Gong, Y.; Qu, B.X.; Harburg, L.; et al. Imaging of cerebrovascular function in chronic traumatic brain injury. J. Neurotrauma 2018, 35, 1116–1123. [Google Scholar] [CrossRef]
- Hay, J.R.; Johnson, V.E.; Young, A.M.H.; Smith, D.H.; Stewart, W. Blood-brain barrier disruption Is an early event that may persist for many years after traumatic brain injury in humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar] [PubMed]
- Dmochowski, G.M.; Shereen, A.D.; Berisha, D.; Dmochowski, J.P. Near-Infrared Light Increases Functional Connectivity with a Non-thermal Mechanism. Cereb. Cortex Commun. 2020, 1, tgaa004. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Chen, A.C.-H.; Carroll, J.D.; Hamblin, M.R. Biphasic Dose Response in Low Level Light Therapy. Dose-Response 2009, 7, 358–383. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Mechanisms of Low-Power Laser Light Action on Cellular Level; Karu, T.I., Lubart, R., Eds.; SPIE: Amsterdam, The Netherlands, 2000; pp. 1–17. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Kolyakov, S.F.; Afanasyeva, N.I. Absorption measurements of a cell monolayer relevant to phototherapy: Reduction of cytochrome c oxidase under near IR radiation. J. Photochem. Photobiol. B 2005, 81, 98–106. [Google Scholar] [CrossRef]
- Wong-Riley, M.T.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation Directly Benefits Primary Neurons Functionally Inactivated by Toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.P.; Lo, S.C.L.; Siu, F.K.W.; So, K.-F. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg. Med. 2002, 31, 283–288. [Google Scholar] [CrossRef]
- Fujimaki, Y.; Shimoyama, T.; Liu, Q.; Umeda, T.; Nakaji, S.; Sugawara, K. Low-Level Laser Irradiation Attenuates Production of Reactive Oxygen Species by Human Neutrophils. J. Clin. Laser Med. Surg. 2003, 21, 165–170. [Google Scholar] [CrossRef]
- Liang, H.L.; Whelan, H.T.; Eells, J.T.; Wong-Riley, M.T.T. Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience 2008, 153, 963–974. [Google Scholar] [CrossRef]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Regulation of miRNA Expression by Low-Level Laser Therapy (LLLT) and Photodynamic Therapy (PDT). Int. J. Mol. Sci. 2013, 14, 13542–13558. [Google Scholar] [CrossRef]
- Greco, M.; Vacca, R.A.; Moro, L.; Perlino, E.; Petragallo, V.A.; Marra, E.; Passarella, S. Helium-Neon laser irradiation of hepatocytes can trigger increase of the mitochondrial membrane potential and can stimulate c-fos expression in a Ca2+-dependent manner. Lasers Surg. Med. 2001, 29, 433–441. [Google Scholar] [CrossRef]
- Wang, X.; Tian, F.; Reddy, D.D.; Nalawade, S.S.; Barrett, D.W.; Gonzalez-Lima, F.; Liu, H. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J. Cereb. Blood Flow Metab. 2017, 37, 3789–3802. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.M.; Marklund, N.; Lebold, D.; Thompson, H.J.; Pitkanen, A.; Maxwell, W.L.; Longhi, L.; Laurer, H.; Maegele, M.; Neugebauer, E.; et al. Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience 2005, 136, 971–989. [Google Scholar] [CrossRef] [PubMed]
- Kenney, K.; Haber, M.; Amyot, F.; Davis, C.; Pronger, A.; Moore, C.; Diaz-Arrastia, R. Cerebral Microvascular Injury in Traumatic Brain Injury. J. Neurol. Neuromed. 2016, 6, 40–46. [Google Scholar]
- Soustiel, J.F.; Glenn, T.C.; Shik, V.; Boscardin, J.; Mahamid, E.; Zaaroor, M. Monitoring of cerebral blood flow and metabolism in traumatic brain injury. J. Neurotrauma 2005, 22, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Whyte, J.; Patel, S.; Europa, E.; Slattery, J.; Coslett, H.B.; Detre, J.A. A Perfusion fMRI Study of the Neural Correlates of Sustained-Attention and Working-Memory Deficits in Chronic Traumatic Brain Injury. Neurorehabil. Neural Repair 2012, 26, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Obrist, W.D.; Langfitt, T.W.; Jaggi, J.L.; Cruz, J.; Gennarelli, T.A. Cerebral blood flow and metabolism in comatose patients with acute head injury: Relationship to intracranial hypertension. J. Neurosurg. 1984, 61, 241–253. [Google Scholar] [CrossRef]
- Ding, K.; Tarumi, T.; Tomoto, T.; Mccolloster, M.; Le, T.; Dieppa, M.; Diaz-Arrastia, R.; Bell, K.; Madden, C.; Cullum, C.M.; et al. Impaired cerebral blood flow regulation in chronic traumatic brain injury. Brain Res. 2020, 1743, 146924. [Google Scholar] [CrossRef]
- Ware, J.B.; Dolui, S.; Duda, J.; Gaggi, N.L.; Choi, R.; Detre, J.; Whyte, J.; Diaz-Arrastia, R.; Kim, J.J. Relationship of Cerebral Blood Flow to Cognitive Function and Recovery in Early Chronic Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2180–2187. [Google Scholar] [CrossRef]
- Hlatky, R.; Contant, C.F.; Diaz-Marchan, P.; Valadka, A.B.; Robertson, C.S. Significance of a reduced cerebral blood flow during the first 12 hours after traumatic brain injury. Neurocrit. Care 2004, 1, 69–83. [Google Scholar] [CrossRef]
- Benedictus, M.R.; Leeuwis, A.E.; Binnewijzend, M.A.; Kuijer, J.P.; Scheltens, P.; Barkhof, F.; van der Flier, W.M.; Prins, N.D. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. Eur. Radiol. 2017, 27, 1169–1175. [Google Scholar] [CrossRef]
- Chao, L.L.; Buckley, S.T.; Kornak, J.; Schuff, N.; Madison, C.; Yaffe, K.; Miller, B.L.; Kramer, J.H.; Weiner, M.W. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cejudo, J.; Wisniewski, T.; Marmar, C.; Zetterberg, H.; Blennow, K.; de Leon, M.J.; Fossati, S. Traumatic Brain Injury and Alzheimer’s Disease: The Cerebrovascular Link. EBioMedicine 2018, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Shively, S.; Scher, A.I.; Perl, D.P.; Diaz-Arrastia, R. Dementia resulting from traumatic brain injury: What is the pathology? Arch. Neurol. 2012, 69, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Xekardaki, A.; Rodriguez, C.; Montandon, M.L.; Toma, S.; Tombeur, E.; Herrmann, F.R.; Zekry, D.; Lovblad, K.O.; Barkhof, F.; Giannakopoulos, P.; et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 2015, 274, 490–499. [Google Scholar] [CrossRef]
- Hwang, J.; Castelli, D.M.; Gonzalez-Lima, F. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med. Sci. 2016, 31, 1151–1160. [Google Scholar] [CrossRef]
- Marion, D.W.; Darby, J.; Yonas, H. Acute regional cerebral blood flow changes caused by severe head injuries. J. Neurosurg. 1991, 74, 407–414. [Google Scholar] [CrossRef]
- Stamatakis, E.A.; Tyler, L.K. Identifying lesions on structural brain images—Validation of the method and application to neuropsychological patients. Brain Lang. 2005, 94, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Jullienne, A.; Obenaus, A.; Ichkova, A.; Savona-Baron, C.; Pearce, W.J.; Badaut, J. Chronic cerebrovascular dysfunction after traumatic brain injury: Cerebrovascular Dysfunction After TBI. J. Neurosci. Res. 2016, 94, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-W.; Wu, J.-H.; Hsieh, C.-H.; Wang, Q.-F.; Chen, J.-H. Different Brain Network Activations Induced by Modulation and Nonmodulation Laser Acupuncture. Evid. Based Complement. Alternat. Med. 2011, 2011, 951258. [Google Scholar] [CrossRef]
- Hipskind, S.G.; Grover, F.L.; Fort, T.R.; Helffenstein, D.; Burke, T.J.; Quint, S.A.; Bussiere, G.; Stone, M.; Hurtado, T. Pulsed Transcranial Red/Near-Infrared Light Therapy Using Light-Emitting Diodes Improves Cerebral Blood Flow and Cognitive Function in Veterans with Chronic Traumatic Brain Injury: A Case Series. Photomed. Laser Surg. 2018, pho.2018.4489. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Morries, L.D. SPECT Perfusion Imaging Demonstrates Improvement of Traumatic Brain Injury with Transcranial Near-infrared Laser Phototherapy. Adv. Mind Body Med. 2015, 29, 27–33. [Google Scholar] [PubMed]
- Morries, L.; Cassano, P.; Henderson, T.A. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015, 11, 2159. [Google Scholar] [CrossRef]
- Vargas, E.; Barrett, D.W.; Saucedo, C.L.; Huang, L.D.; Abraham, J.A.; Tanaka, H.; Haley, A.P.; Gonzalez-Lima, F. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med. Sci. 2017, 32, 1153–1162. [Google Scholar] [CrossRef]
- Naeser, M.A.; Saltmarche, A.; Krengel, M.H.; Hamblin, M.R.; Knight, J.A. Improved Cognitive Function after Transcranial, Light-Emitting Diode Treatments in Chronic, Traumatic Brain Injury: Two Case Reports. Photomed. Laser Surg. 2011, 29, 351–358. [Google Scholar] [CrossRef]
- Henderson, T.A.; Morries, L.D. Multi-Watt Near-Infrared Phototherapy for the Treatment of Comorbid Depression: An Open-Label Single-Arm Study. Front. Psychiatry 2017, 8, 187. [Google Scholar] [CrossRef]
- Poiani, G.D.C.R.; Zaninotto, A.L.; Carneiro, A.M.C.; Zangaro, R.A.; Salgado, A.S.I.; Parreira, R.B.; de Andrade, A.; Teixeira, M.J.; Paiva, W.S. Photobiomodulation using low-level laser therapy (LLLT) for patients with chronic traumatic brain injury: A randomized controlled trial study protocol. Trials 2018, 19, 17. [Google Scholar] [CrossRef]
- Carneiro, A.M.C.; Poiani, G.C.; Zaninnoto, A.L.; Lazo Osorio, R.; Oliveira, M.L.; Paiva, W.S.; Zângaro, R.A. Transcranial Photobiomodulation Therapy in the Cognitive Rehabilitation of Patients with Cranioencephalic Trauma. Photobiomodulation Photomed. Laser Surg. 2019, 37, 657–666. [Google Scholar] [CrossRef]
- Nawashiro, H.; Wada, K.; Nakai, K.; Sato, S. Focal Increase in Cerebral Blood Flow after Treatment with Near-Infrared Light to the Forehead in a Patient in a Persistent Vegetative State. Photomed. Laser Surg. 2012, 30, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Rindner, E.S.; Haroon, J.M.; Jordan, K.G.; Mahdavi, K.D.; Surya, J.R.; Zielinski, M.A.; Habelhah, B.; Venkatraman, V.; Becerra, S.A.; Chan, L.; et al. Transcranial Infrared Laser Stimulation for the Treatment of Traumatic Brain Injury: A Case Series. J. Lasers Med. Sci. 2022, 13, e65. [Google Scholar] [CrossRef]
- Naeser, M.A.; Zafonte, R.; Krengel, M.H.; Martin, P.I.; Frazier, J.; Hamblin, M.R.; Knight, J.A.; Meehan, W.P., 3rd; Baker, E.H. Significant Improvements in Cognitive Performance Post-Transcranial, Red/Near-Infrared Light-Emitting Diode Treatments in Chronic, Mild Traumatic Brain Injury: Open-Protocol Study. J. Neurotrauma 2014, 31, 1008–1017. [Google Scholar] [CrossRef]
- Liebel, S.W.; Johnson, P.K.; Lindsey, H.M.; Russell, H.A.; Hovenden, E.S.; Velez, C.; Carr, L.S.; Wilde, E.A.; Tate, D.F. A-25 Transcranial Photobiomodulation Treatment Effects in Former Athletes with Repetitive Head Hits. Arch. Clin. Neuropsychol. 2022, 37, 1066. [Google Scholar] [CrossRef]
- Chao, L.L. Effects of Home Photobiomodulation Treatments on Cognitive and Behavioral Function, Cerebral Perfusion, and Resting-State Functional Connectivity in Patients with Dementia: A Pilot Trial. Photobiomodul. Photomed. Laser Surg. 2019, 37, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Naeser, M.A.; Martin, P.I.; Ho, M.D.; Krengel, M.H.; Bogdanova, Y.; Knight, J.A.; Hamblin, M.R.; Fedoruk, A.E.; Poole, L.G.; Cheng, C.; et al. Transcranial Photobiomodulation Treatment: Significant Improvements in Four Ex-Football Players with Possible Chronic Traumatic Encephalopathy. J. Alzheimers Dis. Rep. 2023, 7, 77–105. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.L. Improvements in Gulf War Illness Symptoms after Near-Infrared Transcranial and Intranasal Photobiomodulation: Two Case Reports. Mil. Med. 2019, 184, e568–e574. [Google Scholar] [CrossRef]
- Chao, L.L.; Barlow, C.; Karimpoor, M.; Lim, L. Changes in Brain Function and Structure After Self-Administered Home Photobiomodulation Treatment in a Concussion Case. Front. Neurol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Bogdanova, Y.; Martin, P.; Ho, M.; Krengel, M.; Ho, V.; Yee, M.; Knight, J.; Hamblin, M.; Naeser, M. LED Therapy Improves Sleep and Cognition in Chronic Moderate TBI: Pilot Case Studies. Arch. Phys. Med. Rehabil. 2014, 95, e77. [Google Scholar] [CrossRef]
- Oron, A.; Oron, U.; Streeter, J.; de Taboada, L.; Alexandrovich, A.; Trembovler, V.; Shohami, E. Low-Level Laser Therapy Applied Transcranially to Mice following Traumatic Brain Injury Significantly Reduces Long-term Neurological Deficits. J. Neurotrauma 2007, 24, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Vatansever, F.; Huang, L.; Hamblin, M.R. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J. Biomed. Opt. 2014, 19, 108003. [Google Scholar] [CrossRef] [PubMed]
- Figueiro Longo, M.G.; Tan, C.O.; Chan, S.T.; Welt, J.; Avesta, A.; Ratai, E.; Mercaldo, N.D.; Yendiki, A.; Namati, J.; Chico-Calero, I.; et al. Effect of Transcranial Low-Level Light Therapy vs Sham Therapy Among Patients with Moderate Traumatic Brain Injury: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2017337. [Google Scholar] [CrossRef]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed. Laser Surgery 2017, 35, 432–441. [Google Scholar] [CrossRef]
- Naeser, M.A.; Ho, M.D.; Martin, P.I.; Hamblin, M.R.; Koo, B.-B. Increased Functional Connectivity within Intrinsic Neural Networks in Chronic Stroke Following Treatment with Red/Near-Infrared Transcranial Photobiomodulation: Case Series with Improved Naming in Aphasia. Photobiomodul. Photomed. Laser Surg. 2020, 38, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Zhou, L.; Ozsahin, I.; Wang, X.H.; Garetti, J.; Zetterberg, H.; Blennow, K.; Jamison, K.; de Leon, M.J.; Li, Y.; et al. Glymphatic clearance estimated using diffusion tensor imaging along perivascular spaces is reduced after traumatic brain injury and correlates with plasma neurofilament light, a biomarker of injury severity. Brain Commun. 2023, 5, fcad134. [Google Scholar] [CrossRef]
- Formolo, D.A.; Yu, J.; Lin, K.; Tsang, H.W.H.; Ou, H.; Kranz, G.S.; Yau, S.Y. Leveraging the glymphatic and meningeal lymphatic systems as therapeutic strategies in Alzheimer’s disease: An updated overview of nonpharmacological therapies. Mol. Neurodegener. 2023, 18, 26. [Google Scholar] [CrossRef]
- Salehpour, F.; Khademi, M.; Bragin, D.E.; DiDuro, J.O. Photobiomodulation Therapy and the Glymphatic System: Promising Applications for Augmenting the Brain Lymphatic Drainage System. Int. J. Mol. Sci. 2022, 23, 2975. [Google Scholar] [CrossRef]
- Holmes, E.; Barrett, D.W.; Saucedo, C.L.; O’Connor, P.; Liu, H.; Gonzalez-Lima, F. Cognitive Enhancement by Transcranial Photobiomodulation Is Associated with Cerebrovascular Oxygenation of the Prefrontal Cortex. Front. Neurosci. 2019, 13, 1129. [Google Scholar] [CrossRef]

| Mild–Moderate TBI | |||
|---|---|---|---|
| Title | Hipskind et al., 2019 [45] | Morries et al., 2015 [47] | Henderson et al., 2017 [50] |
| Chronicity | 18 months. | 9.3 years average. | 3 months–4 years. |
| Mechanism of Injury | Mixed: motor vehicle accidents, blasts, and concussions. | Mixed: motor vehicle accidents, abuse, blast, and hypoxic encephalopathy. | Undefined, but all had comorbid depression. |
| Sample Size | 12 | 10 | 39 |
| Age (years) | 31–56 | - | 40.5 ± 16.9 |
| Sex | 12 males | 4 males; 6 females | 19 males; 20 females |
| tPBM Delivery | LED | Laser | Laser |
| Duration | 20 min. | 8–10 min per area; 2–3 areas per subject. | 9–12 min duration per site, 30 min total. |
| Target Region(s) | One pad with embedded LEDs circled the skull and the other covered the top of the head. | Frontal and temporal regions. | Bilateral forehead and temporal regions. |
| tPBM Mode | Pulsed | Continuous or pulsed | - |
| Wavelength (nm) | 629 and 950 | 810 and 980 | 810 and 980 |
| Cortical Irradiance (mW/cm2) | Average power density of 6.4 mW/cm2, average energy density of 7.7 J/cm2, and a peak power density of 18.3 mW/cm2. | 7.5 | - |
| Number of Sessions | 18 | 10 or 20 (varied between subjects). | 8–34 (varied between subjects). |
| Outcome | Increased regional CBF. | Improved cognitive function; decreased anxiety/depression/irritability. | Decreased depression (QIDS). |
| Side Effects | None. | None. | Headache and fatigue were noted after the first few treatments but dissipated with continued treatment. |
| Moderate TBI | |||
|---|---|---|---|
| Title | Henderson et al., 2015 [46] | Chao, 2019 [59] | Bogdanova et al., 2014 [61] |
| Chronicity | 31 years | - | - |
| Mechanism of Injury | Motor vehicle accident. | Mild TBI defined by Ohio State University TBI Identification Method. | - |
| Sample Size | 1 | 2 (1 with TBI) | 2 |
| Age (years) | - | 55–66 | - |
| Sex | 1 male | 2 males | 1 male; 1 female |
| tPBM Delivery | Laser | LED | LED |
| Duration | - | 20 min | - |
| Target Region(s) | - | Default mode network | - |
| tPBM Mode | - | Pulse (10 Hz) | - |
| Wavelength (nm) | 810 and 980 | 810 | - |
| Cortical Irradiance (mW/cm2) | - | 25, 75, and 100 (among different LEDs). | - |
| Number of Sessions | 20 | 36 | 18 |
| Outcome | Improved cognition, quality of life, and blood flow (via SPECT). | Improved sleep, cognition, and mood. Decreased pain and fatigue. | Improved sleep and cognition; decreased depression and PTSD symptoms. |
| Side Effects | None. | None. | None. |
| Moderate-Severe TBI | |
|---|---|
| Title | Poiani et al., 2018 [51] |
| Chronicity | 6 months |
| Mechanism of Injury | Closed TBI (undefined) |
| Sample Size | 36 |
| Age (years) | - |
| Sex | - |
| tPBM Delivery | LED |
| Duration | 30 min |
| Target Region(s) | Whole head |
| tPBM Mode | - |
| Wavelength (nm) | 632 |
| Cortical Irradiance (mW/cm2) | 830 over 400 cm2 |
| Number of Sessions | 18 |
| Outcome | - |
| Side Effects | - |
| Severe TBI | ||
|---|---|---|
| Title | Carneiro et al., 2019 [52] | Nawashiro et al., 2012 [53] |
| Chronicity | 4 months–4 years | 8 months |
| Mechanism of Injury | Motor vehicle accident; plane drop | Fall from fighting |
| Sample Size | 10 | 1 |
| Age (years) | 37.8 ± 10.2 | 40 |
| Sex | 9 Male; 1 Female | Male |
| tPBM Delivery | LED | LED |
| Duration | 30 min | 30 min |
| Target Region(s) | Whole head | Bilateral forehead |
| tPBM Mode | - | Continuous |
| Wavelength (nm) | 630 | 850 |
| Cortical Irradiance (mW/cm2) | 25.73 | 11.4 |
| Number of Sessions | 18 | 146 |
| Outcome | Improved CBF | Increased regional CBF |
| Side Effects | None | None |
| Mixed TBI Severities | |
|---|---|
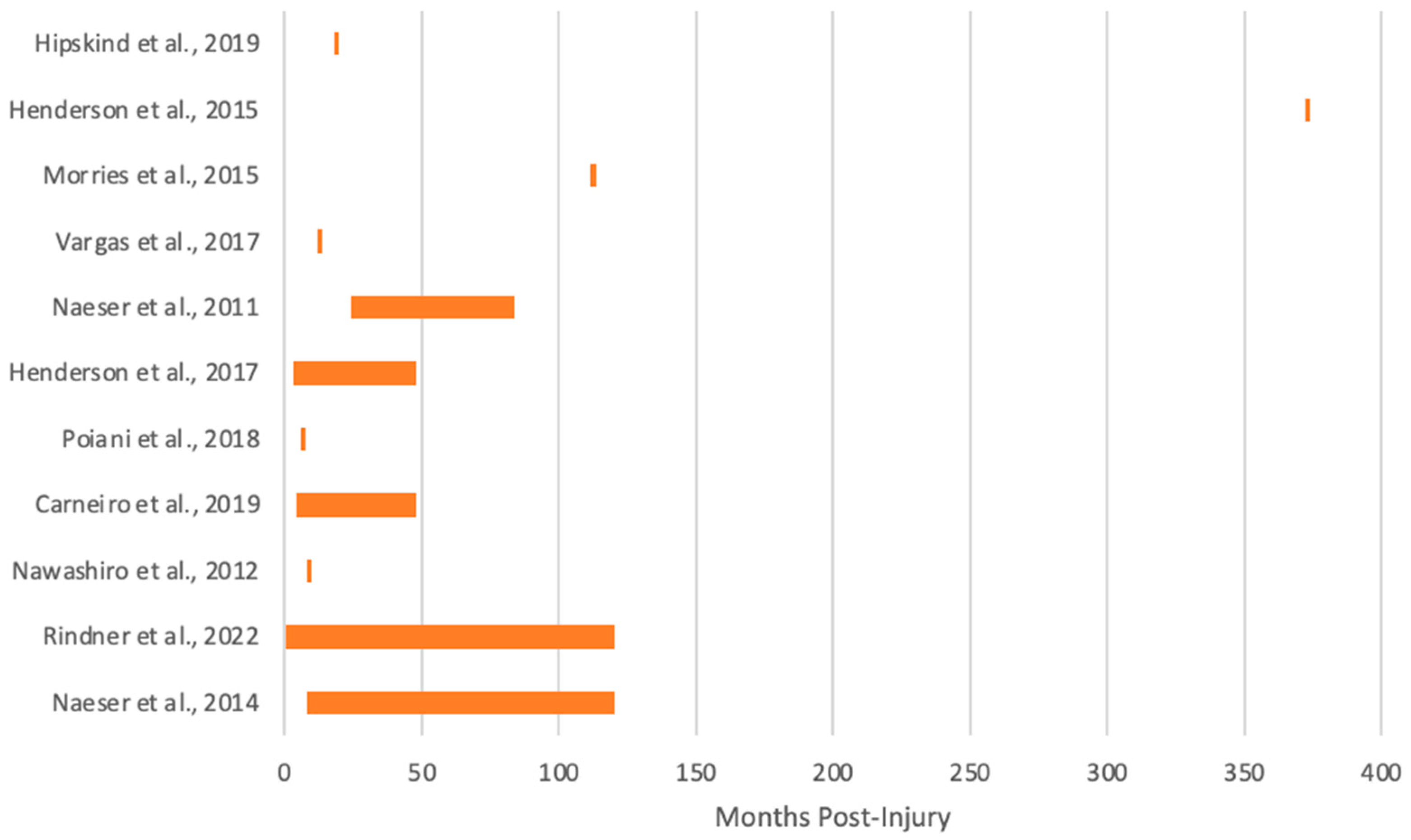
| Title | Rindner et al., 2022 [54] |
| Chronicity | 9 days–10 years |
| Mechanism of Injury | Motor vehicle accident; sports concussion(s) with andand without loss of consciousness |
| Sample Size | 11 |
| Age (years) | 17–53 |
| Sex | 9 male; 2 female |
| tPBM Delivery | Laser |
| Duration | 20 min |
| Target Region(s) | Broadman Area 10 |
| tPBM Mode | Continuous |
| Wavelength (nm) | 1064 |
| Cortical Irradiance (mW/cm2) | 250 |
| Number of Sessions | 8 |
| Outcome | Better quality of life |
| Side Effects | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaggi, N.L.; Roy, N.L.; Song, X.; Peterson, A.L.; Iosifescu, D.V.; Diaz-Arrastia, R.; Cassano, P.; Kim, J.J. Transcranial Photobiomodulation and Chronic Traumatic Brain Injury. Photonics 2024, 11, 260. https://doi.org/10.3390/photonics11030260
Gaggi NL, Roy NL, Song X, Peterson AL, Iosifescu DV, Diaz-Arrastia R, Cassano P, Kim JJ. Transcranial Photobiomodulation and Chronic Traumatic Brain Injury. Photonics. 2024; 11(3):260. https://doi.org/10.3390/photonics11030260
Chicago/Turabian StyleGaggi, Naomi L., Nathaniel Lewis Roy, Xiaotong Song, Anna Leigh Peterson, Dan V. Iosifescu, Ramon Diaz-Arrastia, Paolo Cassano, and Junghoon J. Kim. 2024. "Transcranial Photobiomodulation and Chronic Traumatic Brain Injury" Photonics 11, no. 3: 260. https://doi.org/10.3390/photonics11030260





