Design Considerations for Integration of Terahertz Time-Domain Spectroscopy in Microfluidic Platforms
Abstract
:1. Introduction
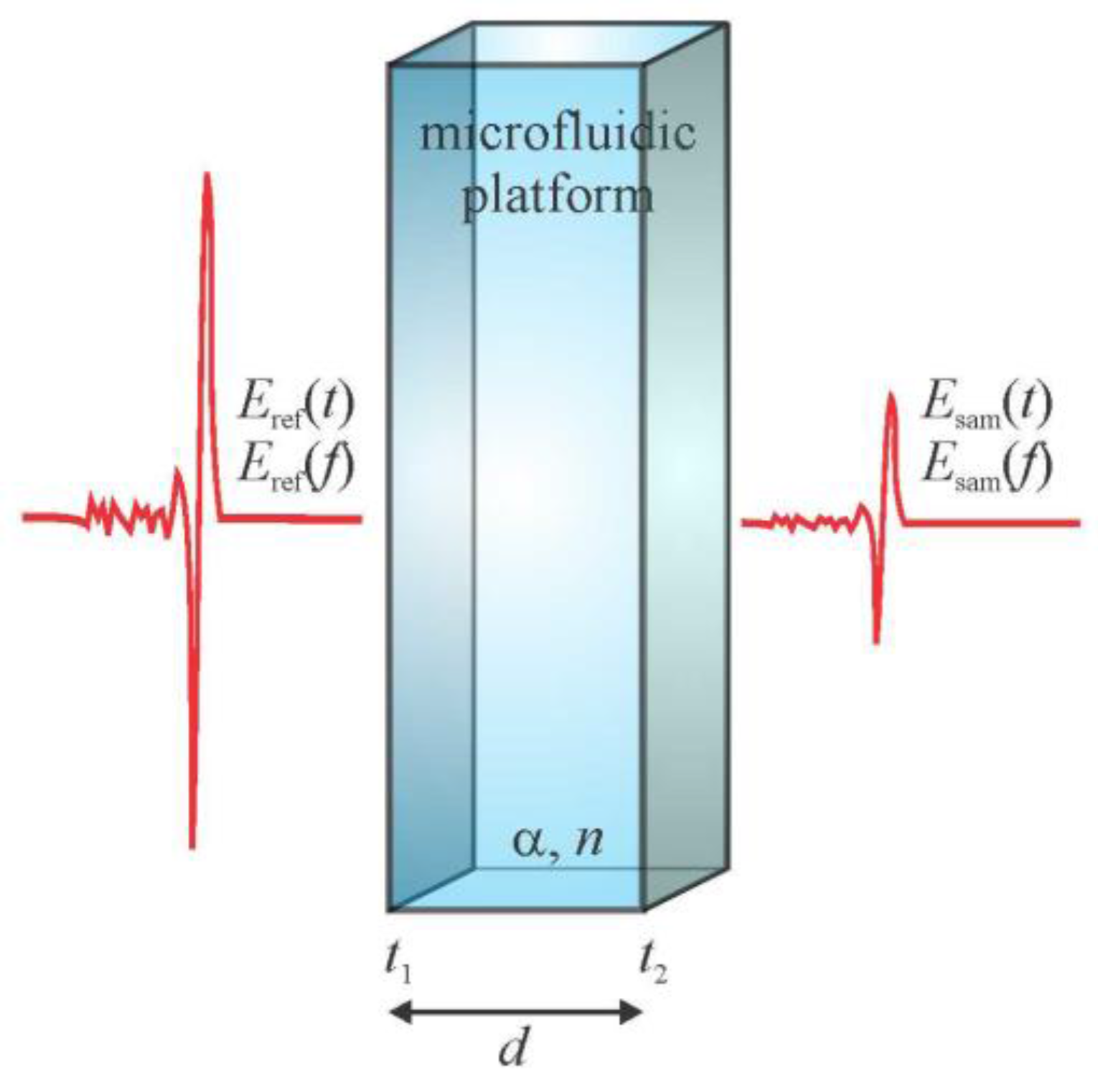
2. Design and Simulation
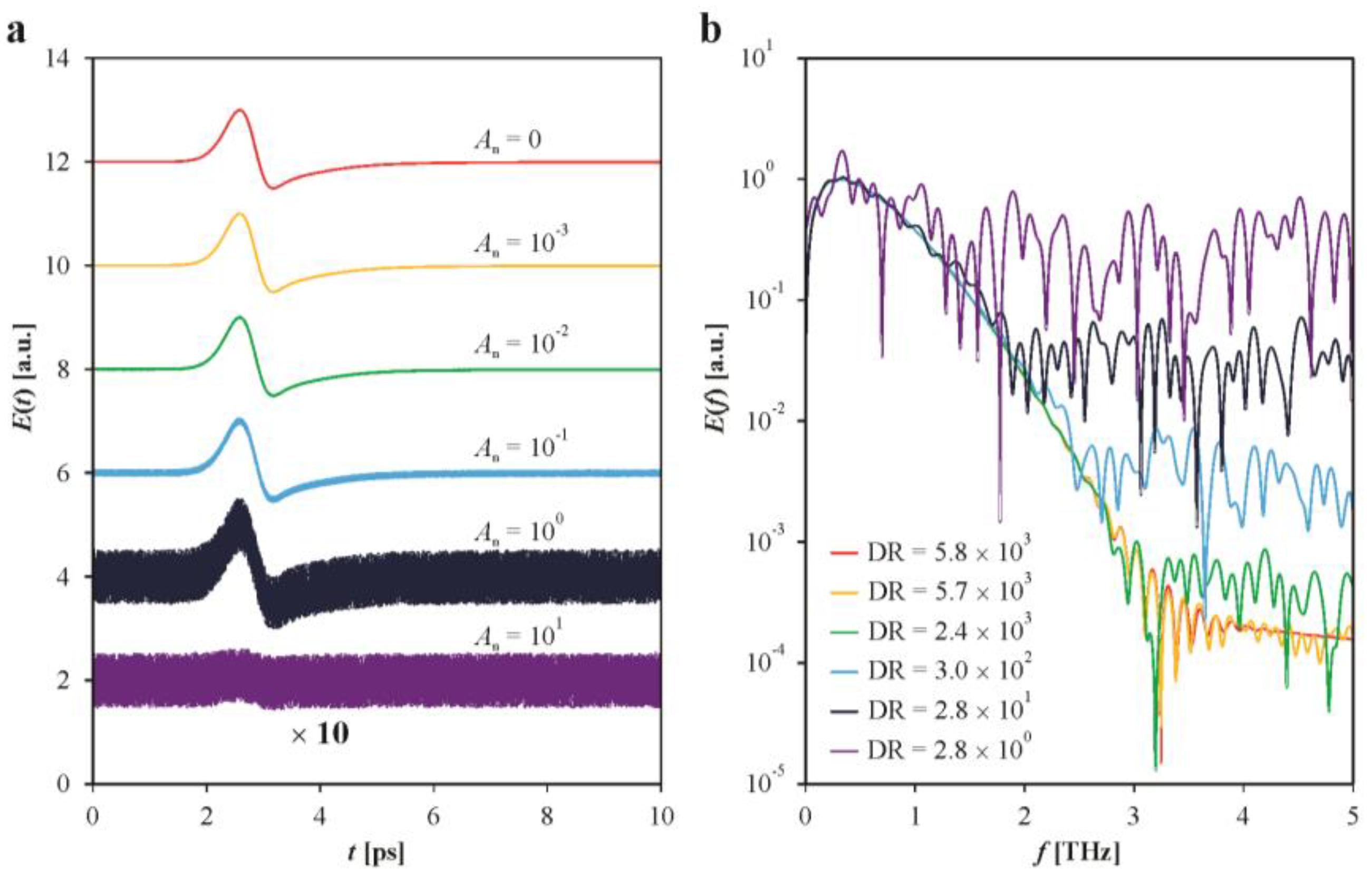
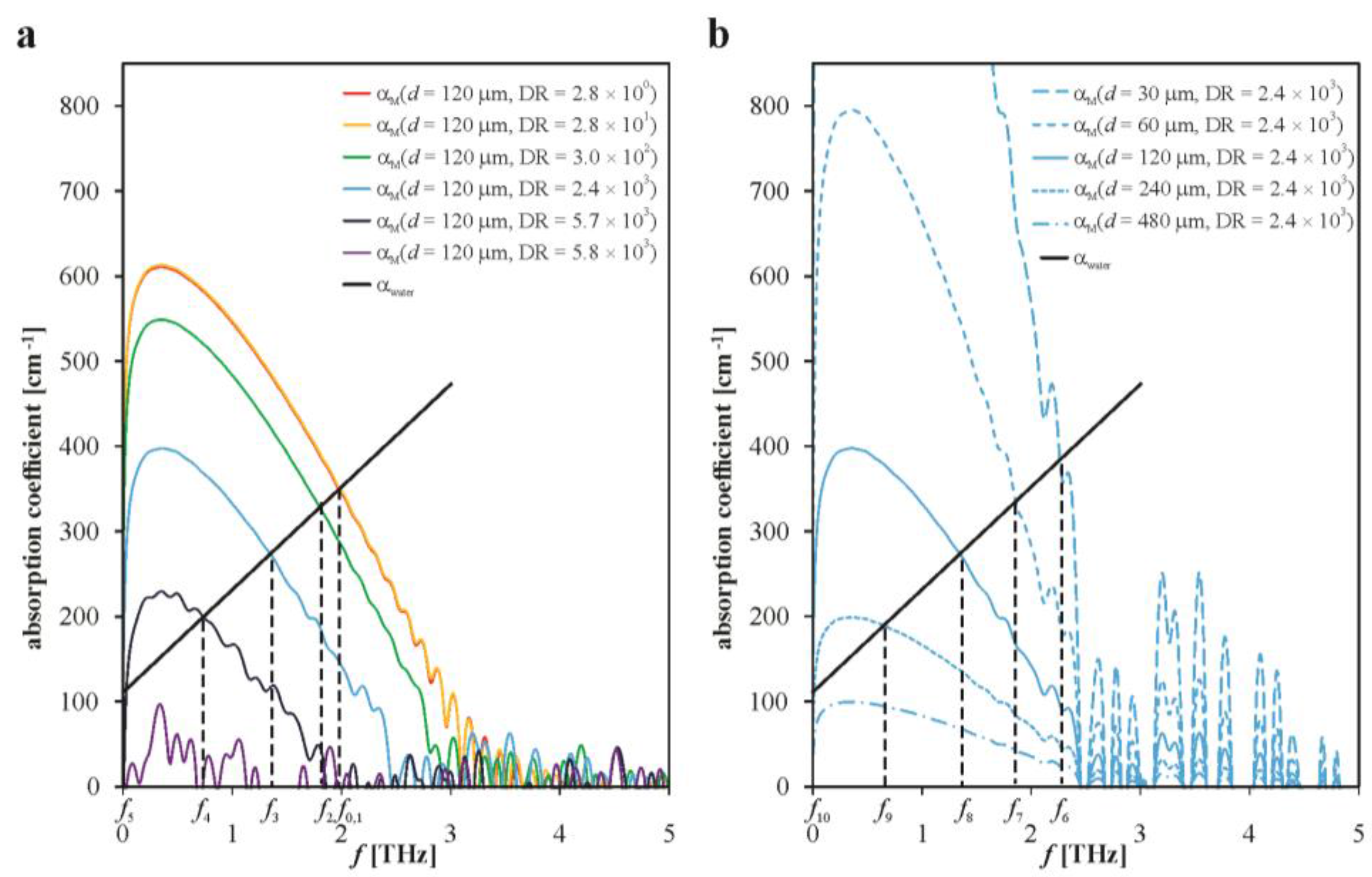
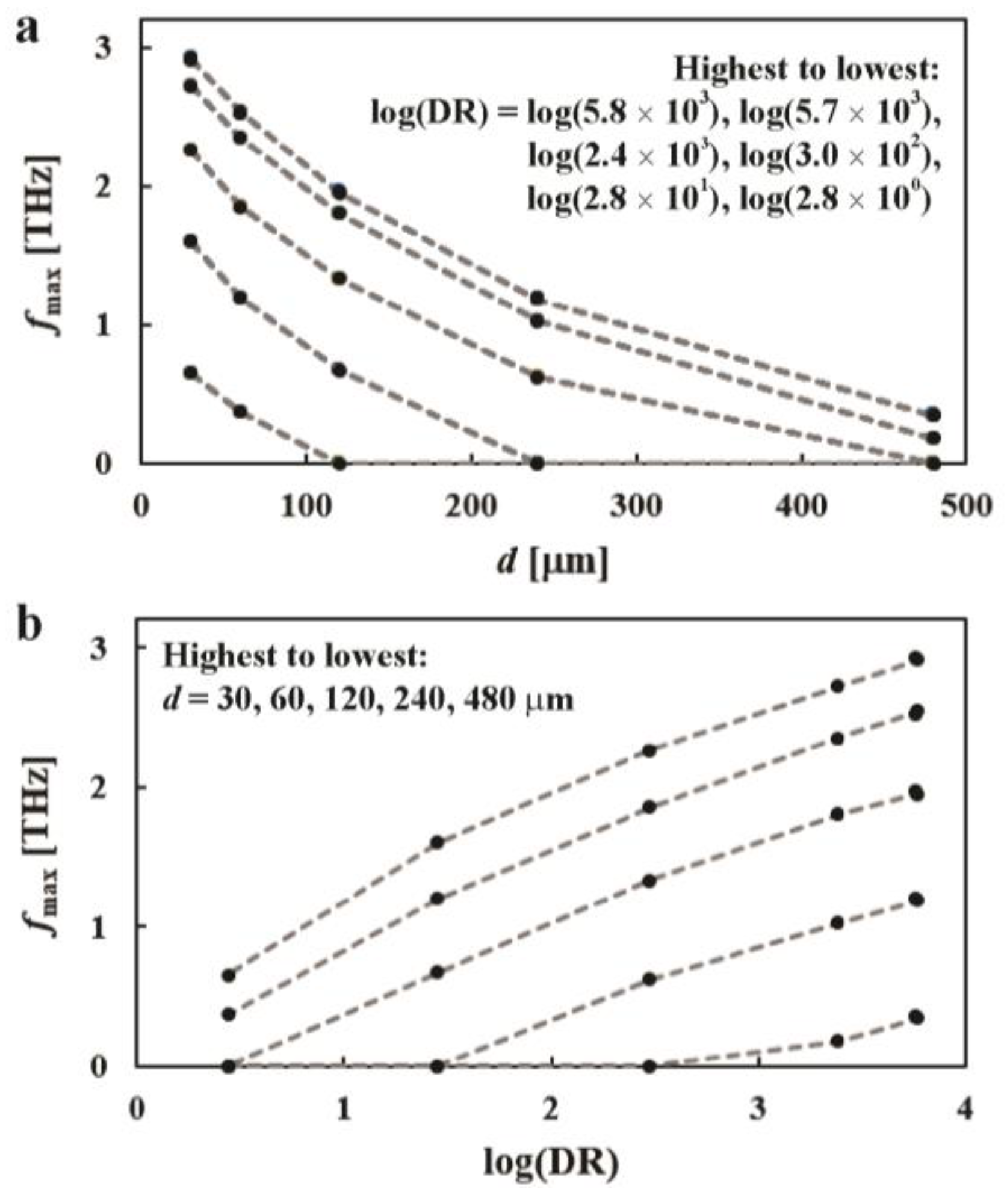
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Jin, X.; Collier, C.M.; Garbowski, J.J.A.; Born, B.; Holzman, J.F. Ultrafast transient responses of optical wireless communication detectors. Appl. Opt. 2013, 52, 5042–5049. [Google Scholar] [CrossRef] [PubMed]
- Haiml, M.; Grange, R.; Keller, U. Optical characterization of semiconductor saturable absorbers. Appl. Phys. B 2004, 79, 331–339. [Google Scholar] [CrossRef]
- Süss, B.; Ringleb, F.; Heberle, J. New ultrarapid-scanning interferometer for FT-IR spectroscopy with microsecond time-resolution. Rev. Sci. Instrum. 2016, 87, 063113. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.M.; Jin, X.; Holzman, J.F. Ultrafast refractometry for characterization of nanocomposite material systems. IEEE Photon. Technol. Lett. 2012, 24, 590–592. [Google Scholar] [CrossRef]
- Correa, D.S.; Almeida, J.M.P.; Almeida, G.F.B.; Cardoso, M.R.; De Boni, L.; Mendonça, C.R. Ultrafast laser pulses for structuring materials at micro/nano scale: From waveguides to superhydrophobic surfaces. Photonics 2017, 4, 8. [Google Scholar] [CrossRef]
- Nefedov, I.; Melnikov, L. Plasmonic terahertz amplification in graphene-based asymmetric hyperbolic metamaterial. Photonics 2015, 2, 594–603. [Google Scholar] [CrossRef]
- Auston, D.H.; Cheung, K.P.; Smith, P.R. Picosecond photoconducting Hertzian dipoles. Appl. Phys. Lett. 1984, 45, 284–286. [Google Scholar] [CrossRef]
- Collier, C.M.; Bergen, M.H.; Stirling, T.J.; DeWachter, M.A.; Holzman, J.F. Optimization processes for pulsed terahertz systems. Appl. Opt. 2015, 54, 535–545. [Google Scholar] [CrossRef]
- Petev, M.; Westerberg, N.; Rubino, E.; Moss, D.; Couairon, A.; Légaré, F.; Morandotti, R.; Faccio, D.; Clerici, M. Phase-Insensitive Scattering of Terahertz Radiation. Photonics 2017, 4, 7. [Google Scholar] [CrossRef]
- Omar Clay, G.; Schaffer, C.B.; Kleinfeld, D. Large two-photon absorptivity of hemoglobin in the infrared range of 780–880 nm. J. Chem. Phys. 2017, 126, 025102. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, M.Y.; Ali, A.; Hunter, I.C.; Roberts, N.S. A new method for the precise multiband microwave dielectric measurement using stepped impedance stub. Meas. Sci. Technol. 2016, 27, 117001. [Google Scholar] [CrossRef]
- Shams, H.; Fice, M.J.; Balakier, K.; Renaud, C.C.; van Dijk, F.; Seeds, A.J. Photonic generation for multichannel THz wireless communication. Opt. Express 2014, 22, 23465–23472. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xie, L.; Ying, Y. Rapid analysis of tetracycline hydrochloride solution by attenuated total reflection terahertz time-domain spectroscopy. Food Chem. 2017, 224, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Federici, J.F.; Schulkin, B.; Huang, F.; Gary, D.; Barat, R.; Oliveira, F.; Zimdars, D. THz imaging and sensing for security applications–explosives, weapons and drugs. Semicond. Sci. Technol. 2005, 20, 266–280. [Google Scholar] [CrossRef]
- Hernandez-Serrano, A.I.; Corzo-Garcia, S.C.; Garcia-Sanchez, E.; Alfaro, M.; Castro-Camus, E. Quality control of leather by terahertz time-domain spectroscopy. Appl. Opt. 2014, 53, 7872–7876. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; He, Y.; Ung, B.S.; Pickwell-MacPherson, E. The growth of biomedical terahertz research. J. Phys. D Appl. Phys. 2014, 47, 374009. [Google Scholar] [CrossRef]
- Shumyatsky, P.; Alfano, R.R. Terahertz sources. J. Biomed. Opt. 2011, 16, 033001. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Fan, S.; Sun, Y.; Pickwell-MacPherson, E. The potential of terahertz imaging for cancer diagnosis: A review of investigations to date. Quant. Imag. Med. Surg. 2012, 2, 33–45. [Google Scholar] [CrossRef]
- Markelz, A.; Whitmire, S.; Hillebrecht, J.; Birge, R. THz time domain spectroscopy of biomolecular conformational modes. Phys. Med. Biol. 2002, 47, 3797–3805. [Google Scholar] [CrossRef] [PubMed]
- Rogenbuck, A.; Schmitz, H.; Deninger, A.; Mayorga, I.C.; Hemberger, J.; Güsten, R.; Grüninger, M. Coherent broadband continuous-wave terahertz spectroscopy on solid-state samples. New J. Phys. 2010, 12, 043017. [Google Scholar] [CrossRef]
- Falconer, R.J.; Markelz, A. Terahertz spectroscopic analysis of peptides and proteins. J. Infrared Millim. Terahertz Waves 2012, 33, 973–988. [Google Scholar] [CrossRef]
- Collier, C.M.; Wiltshire, M.; Nichols, J.; Born, B.; Landry, E.L.; Holzman, J.F. Nonlinear dual-phase multiplexing in digital microfluidic architectures. Micromachines 2011, 2, 369–384. [Google Scholar] [CrossRef]
- Nichols, J.; Collier, C.M.; Landry, E.L.; Wiltshire, M.; Born, B.; Holzman, J.F. On-chip digital microfluidic architectures for enhanced actuation and sensing. J. Biomed. Opt. 2012, 17, 067005. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Landry, E.L.; Born, B.; Wiltshire, M.; Collier, C.M.; Holzman, J.F. Optical sensing for on-chip digital microfluidics. In Proceedings of the SPIE Photonics West, San Francisco, CA, USA, 14 February 2012. [Google Scholar]
- Tang, Q.; Liang, M.; Lu, Y.; Wong, P.K.; Wilmink, G.J.; Zhang, D.D.; Xin, H. Microfluidic devices for terahertz spectroscopy of live cells toward lab-on-a-chip applications. Sensors 2016, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- George, P.A.; Hui, W.; Rana, F.; Hawkins, B.G.; Smith, A.E.; Kirby, B.J. Microfluidic devices for terahertz spectroscopy of biomolecules. Opt. Express 2008, 16, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Kindt, J.T.; Schmuttenmaer, C.A. Far-infrared dielectric properties of polar liquids probed by femtosecond terahertz pulse spectroscopy. J. Phys. Chem. 1996, 100, 10373–10379. [Google Scholar] [CrossRef]
- Cheon, H.; Yang, H.; Lee, S.-H.; Kim, Y.A.; Son, J.-H. Terahertz molecular resonance of cancer DNA. Sci. Rep. 2016, 6, 37103. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, P.C.; Shen, Y.C.; Davies, A.G.; Linfield, E.H. Terahertz time-domain spectroscopy of glucose and uric acid. J. Biol. Phys. 2003, 29, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.-J.; Esteva-Font, C.; Verkman, A.S. Droplet-based microfluidics platform for measurement of rapid erythrocyte water transport. Lab Chip 2015, 15, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.R.; Aoki, M.; Mochizuki, K.; Asahi, T.; Hosako, I.; Hiromoto, N. Randon error estimation in refractive index measured with the terahertz time domain spectroscopy. IEICE Electron. Express 2009, 6, 1690–1696. [Google Scholar] [CrossRef]
- Jarrahi, M. Terahertz radiation-band engineering through spatial beam-shaping. IEEE Photon. Technol. Lett. 2009, 21, 830–832. [Google Scholar] [CrossRef]
- Rodriguez, G.; Taylor, A.J. Screening of the bias field in terahertz generation from photoconductors. Opt. Lett. 1996, 21, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.U.; Fischer, B.M. Dynamic range in terahertz time-domain transmission and reflection spectroscopy. Opt. Lett. 2005, 30, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.-K.; Hsieh, T.-H.; Lin, D.-Y. General digital microfluidic platform manipulating dielectric and conductive droplets by dielectrophoresis and electrowetting. Lab Chip 2009, 9, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Duvillaret, L.; Garet, F.; Coutaz, J.-L. Influence of noise on the characterization of materials by terahertz time-domain spectroscopy. J. Opt. Soc. Am. B 2000, 17, 452–461. [Google Scholar] [CrossRef]
- Van Exter, M.; Grischkowsky, D. Characterization of an optoelectronic terahertz beam system. IEEE Trans. Microw. Theory Tech. 1990, 38, 1684–1691. [Google Scholar] [CrossRef]
- Wang, T.; Klarskov, P.; Jepsen, P.U. Ultrabroadband THz time-domain spectroscopy of a free-flowing water film. IEEE Trans. Terahertz Sci. Technol. 2014, 4, 425–431. [Google Scholar] [CrossRef]
- Venkatesh, M.; Rao, K.S.; Abhilash, T.S.; Tewari, S.P.; Chaudhary, A.K. Optical characterization of GaAs photoconductive antennas for efficient generation and detection of Terahertz radiation. Opt. Mater. 2014, 36, 596–601. [Google Scholar] [CrossRef]
- Ng, A.H.C.; Chamberlain, M.D.; Situ, H.; Lee, V.; Wheeler, A.R. Digital microfluidic immunocytochemistry in single cells. Nat. Commun. 2015, 6, 7513. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hujazy, R.; Collier, C.M. Design Considerations for Integration of Terahertz Time-Domain Spectroscopy in Microfluidic Platforms. Photonics 2018, 5, 5. https://doi.org/10.3390/photonics5010005
Al-Hujazy R, Collier CM. Design Considerations for Integration of Terahertz Time-Domain Spectroscopy in Microfluidic Platforms. Photonics. 2018; 5(1):5. https://doi.org/10.3390/photonics5010005
Chicago/Turabian StyleAl-Hujazy, Rasha, and Christopher M. Collier. 2018. "Design Considerations for Integration of Terahertz Time-Domain Spectroscopy in Microfluidic Platforms" Photonics 5, no. 1: 5. https://doi.org/10.3390/photonics5010005



