Application of Surfactant Micelle-Entrapped Eugenol for Prevention of Growth of the Shiga Toxin-Producing Escherichia coli in Ground Beef
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Revival Procedures
2.2. Preparation of Surfactant for Maximum Non-Inhibitory Concentration (MNIC) Determination
2.3. Preparation of Eugenol-Loaded Micelles and Unencapsulated Eugenol for MIC Determination
2.4. Application of Eugenol-Loaded Micelles to STEC-Inoculated Beef Trimmings and STEC Reductions on Ground Beef Prepared from Treated Trimmings
2.5. Statistical Analysis
3. Results and Discussion
3.1. Minimum Inhibitory Concentration of Eugenol-Loaded SDS Micelles against STEC
3.2. Reduction of STEC on Beef Trimmings by Free and Micelle-Encapsulated Eugenol
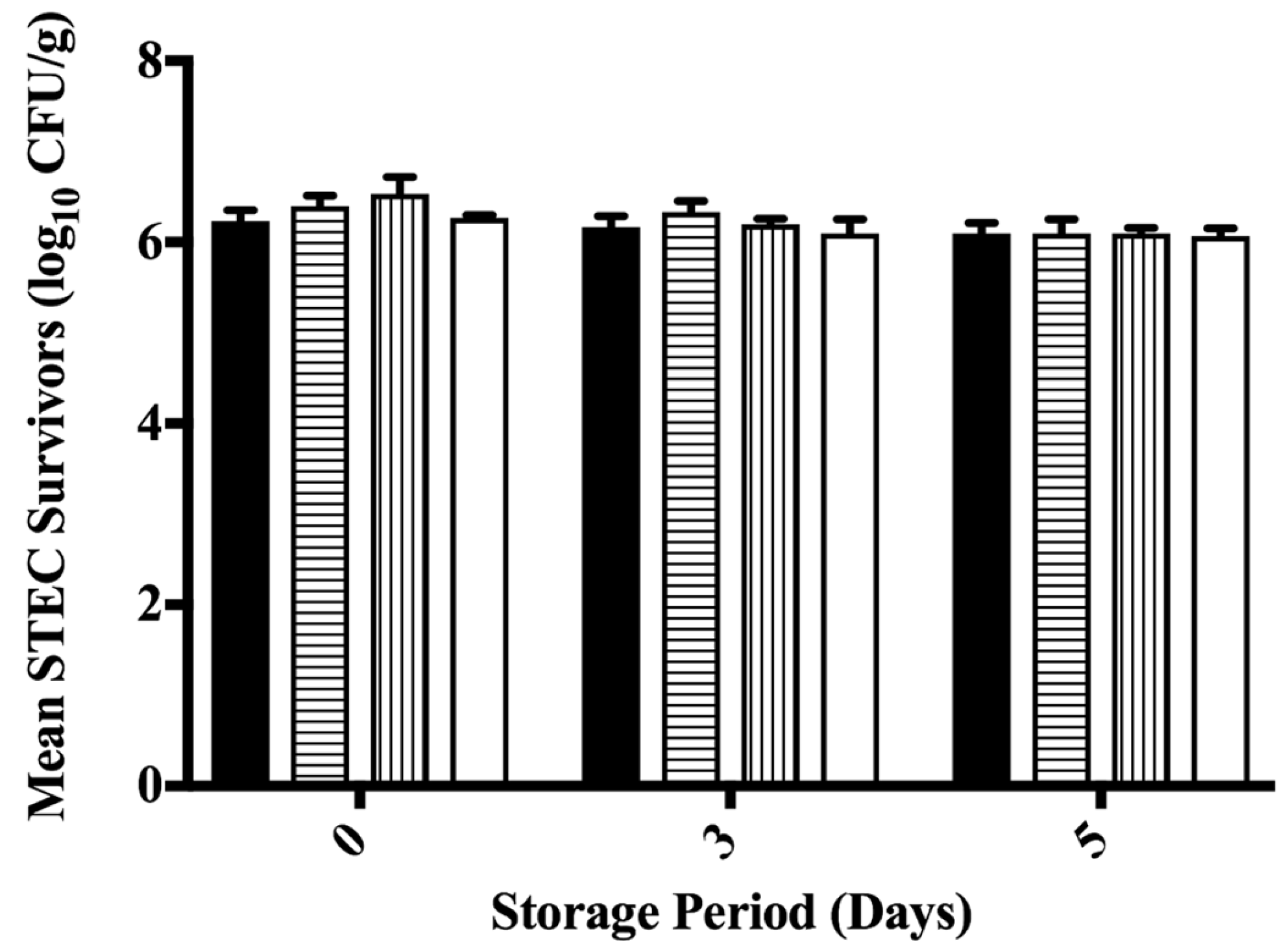
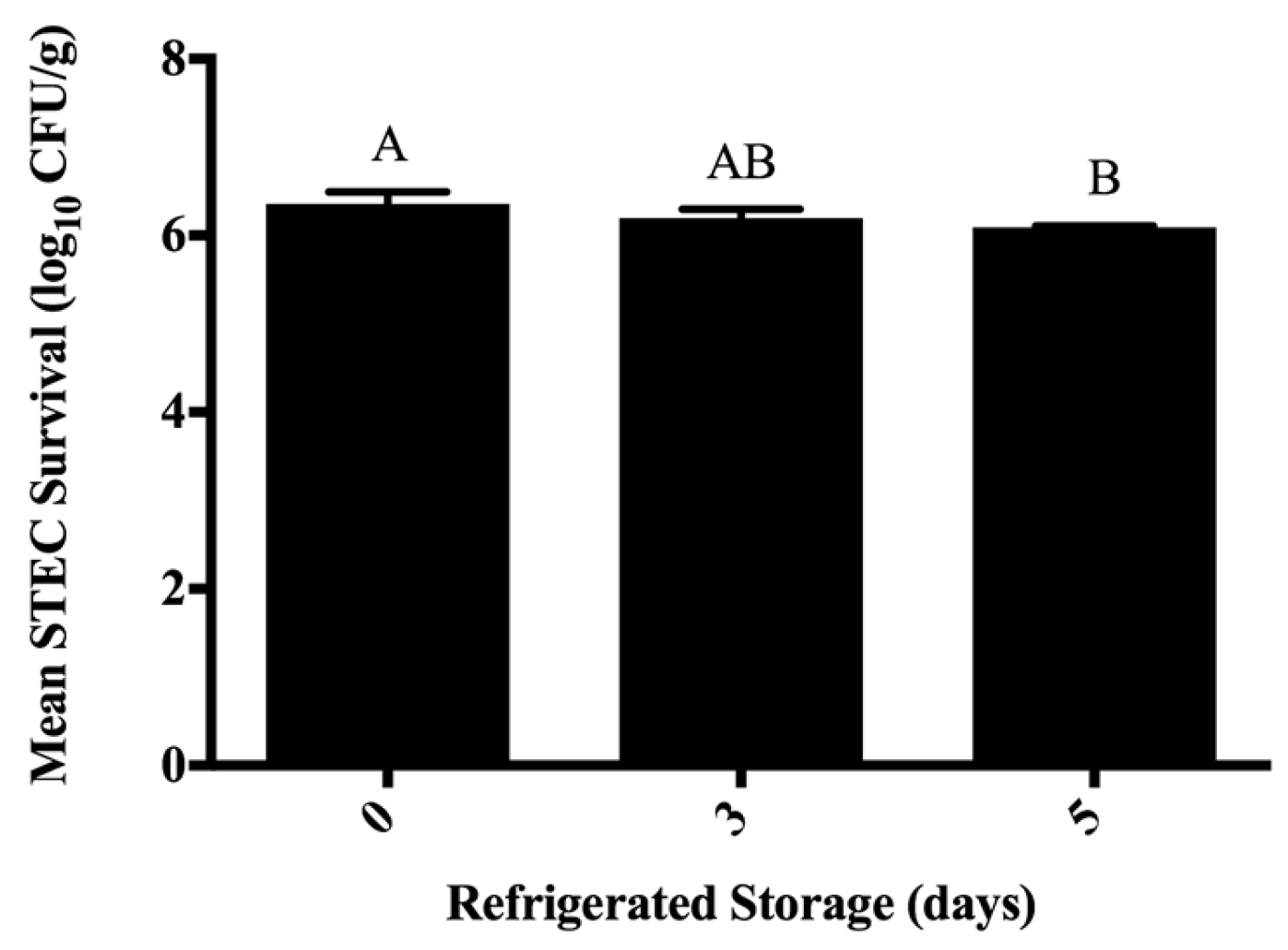
3.3. Inhibition of STEC on Ground Beef Following Antimicrobial Treatment during Refrigerated Storage
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Brooks, J.T.; Sowers, E.G.; Wells, J.G.; Greene, K.D.; Griffin, P.M.; Hoekstra, R.M.; Strockbine, N.A. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983–2002. J. Infect. Dis. 2005, 192, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- General Information: E. coli. Available online: http://www.cdc.gov/ecoli/general/ (accessed on 17 July 2017).
- Multistate Outbreak of Shiga Toxin-Producing Escherichia coli O157:H7 Infections Linked to Ground Beef (Final Update). Available online: http://www.cdc.gov/ecoli/2014/O157H7-05-14/index.htmL (accessed on 17 July 2017).
- USDA-FSIS. Shiga toxin-producing Escherichia coli in certain raw beef products. Fed. Regist. 2011, 76, 58157–58165. [Google Scholar]
- Hoffmann, S.; Batz, M.B.; Morris, J.G. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J. Food Prot. 2012, 75, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Scharff, R.L. Economic burden from health losses due to foodborne illness in the United States. J. Food Prot. 2012, 75, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Fratamico, P.M.; Gunther, N.W. Shiga toxin-producing Escherichia coli. Adv. Appl. Microbiol. 2014, 86, 145–197. [Google Scholar] [PubMed]
- Arthur, T.M.; Bosilevac, J.M.; Nou, X.; Shackelford, S.D.; Wheeler, T.L.; Kent, M.P.; Jaroni, D.; Pauling, B.; Allen, D.M.; Koohmaraie, M. Escherichia coli O157 prevalence and enumeration of aerobic bacteria, Enterobacteriaceae, and Escherichia coli O157 at various steps in commercial beef processing plants. J. Food Prot. 2004, 67, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Arthur, T.M.; Barkocy-Gallagher, G.A.; Rivera-Betancourt, M.; Koohmaraie, M. Prevalence and characterization of non-O157 Shiga toxin-producing Escherichia coli on carcasses in commercial beef cattle processing plants. Appl. Environ. Microbiol. 2002, 68, 4847–4852. [Google Scholar] [CrossRef] [PubMed]
- Bosilevac, J.M.; Koohmaraie, M. Prevalence and characterization of non-O157 Shiga toxin-producing Escherichia coli isolates from commercial ground beef in the United States. Appl. Environ. Microbiol. 2011, 77, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Geornaras, I.; Yang, H.; Moschonas, G.; Nunnelly, M.C.; Belk, K.E.; Nightingale, K.K.; Woerner, D.R.; Smith, G.C.; Sofos, J.N. Efficacy of chemical interventions against Escherichia coli O157:H7 and multidrug-resistant and antibiotic-susceptible Salmonella on inoculated beef trimmings. J. Food Prot. 2012, 75, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Miller, M.F.; Loneragan, G.H.; Brashears, M.M. Validation of the use of organic acids and acidified sodium chlorite to reduce Escherichia coli O157 and Salmonella Typhimurium in beef trim and ground beef in a simulated processing environment. J. Food Prot. 2006, 69, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Brashears, M.M.; Garmyn, A.J.; Brooks, J.C.; Miller, M.F. Microbiological and organoleptic characteristics of beef trim and ground beef treated with acetic acid, lactic acid, acidified sodium chlorite, or sterile water in a simulated commercial processing environment to reduce Escherichia coli O157:H7 and Salmonella. Meat Sci. 2012, 90, 783–788. [Google Scholar] [PubMed]
- Cutter, C.N. Antimicrobial effect of herb extracts against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella Typhimurium associated with beef. J. Food Prot. 2000, 63, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Surendran Nair, M.; Lau, P.; Belskie, K.; Fancher, S.; Chen, C.-H.; Karumathil, D.P.; Yin, H.-B.; Liu, Y.; Ma, F.; Upadhyaya, I.; et al. Potentiating the heat inactivation of Escherichia coli O157:H7 in ground beef patties by natural antimicrobials. Fron. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ruengvisesh, S.; Loquercio, A.; Castell-Perez, E.; Taylor, T.M. Inhibition of bacterial pathogens in medium and on spinach leaf surfaces using plant-derived antimicrobials loaded in surfactant micelles. J. Food Sci. 2015, 80, M2522–M2529. [Google Scholar] [CrossRef] [PubMed]
- Gaysinsky, S.; Taylor, T.M.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Antimicrobial efficacy of eugenol microemulsions in milk against Listeria monocytogenes and Escherichia coli O157:H7. J. Food Prot. 2007, 70, 2631–2637. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; McClements, D.J. Mass transport phenomena in oil-in-water emulsions containing surfactant micelles: Solubilization. Langmuir 2000, 16, 5879–5883. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, P.; Doyle, M.P. Inactivation of Salmonella and Escherichia coli O157:H7 on lettuce and poultry skin by combinations of levulinic acid and sodium dodecyl sulfate. J. Food Prot. 2009, 72, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, S.J.; Story, R.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C.; Goodridge, L. A membrane filtration method for determining minimum inhibitory concentrations of essential oils. Agric. Food Anal. Bacteriol. 2012, 2, 88–93. [Google Scholar]
- Brandt, A.L.; Castillo, A.; Harris, K.B.; Keeton, J.T.; Hardin, M.D.; Taylor, T.M. Synergistic inhibition of Listeria monocytogenes in vitro through the combination of octanoic acid and acidic calcium sulfate. J. Food Prot. 2011, 74, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, K.R.; Taylor, T.M.; Griffin, D.; Castillo, A.; Marx, D.B.; Smith, L. Growth of Shiga toxin-producing Escerichia coli (STEC) and impacts of chilling and post-inoculation storage on STEC attachment to beef surfaces. Food Microbiol. 2014, 44, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Title 9, U.S. Code of Federal Regulations, §319.15: Miscellaneous Beef Products. Available online: http://www.ecfr.gov/ (accessed on 23 December 2016).
- Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Growth inhibition of Escherichia coli O157:H7 and Listeria monocytogenes by carvacrol and eugenol encapsulated in surfactant micelles. J. Food Prot. 2005, 68, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Antimicrobial properties of lauric arginate alone or in combination with essential oils in tryptic soy broth and 2% reduced fat milk. Int. J. Food Microbiol. 2013, 166, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Blaszyk, M.; Holley, R.A. Interaction of monolaurin, eugenol and sodium citrate on growth of common meat spoilage and pathogenic organisms. Int. J. Food Microbiol. 1998, 39, 175–183. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Bao, Y.; He, Y.; Feng, F.; Zheng, X. Characterization and syergistic antimicrobial activities of food-grade dilution-stable microemulsions against Bacillus subtilis. Food Res. Int. 2008, 41, 495–499. [Google Scholar] [CrossRef]
- Hsu, S.T.; Breukink, E.; de Kruijff, B.; Kapstein, R.; Bonvin, A.M.J.J.; van Nuland, N.A.J.J. Mapping the targeted membrane pore formation mechanism by solution NMR: The nisin Z and lipid II interaction in SDS micelles. Biochemistry 2002, 41, 7670–7676. [Google Scholar] [CrossRef] [PubMed]
- Conner, D.E.; Kotrola, J.S.; Mikel, W.B.; Tamblyn, K.C. Effects of acetic-lactic acid treatments applied to beef trim on populations of Escherichia coli O157:H7 and Listeria monocytogenes in ground beef. J. Food Prot. 1997, 60, 1560–1563. [Google Scholar] [CrossRef]
- Ellebracht, E.A.; Castillo, A.; Lucia, L.M.; Miller, R.K.; Acuff, G.R. Reduction of pathogens using hot water and lactic acid on beef trimmings. J. Food Sci. 1999, 64, 1094–1099. [Google Scholar] [CrossRef]
- Fouladkhah, A.; Geornaras, I.; Yang, H.; Belk, K.E.; Nightingale, K.K.; Woerner, D.R.; Smith, G.C.; Sofos, J.N. Sensitivity of Shiga toxin-producing Escherichia coli, multidrug-resistant Salmonella, and antibiotic-susceptible Salmonella to lactic acid on inoculated beef trimmings. J. Food Prot. 2012, 75, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.J.; Miller, M.F.; Parks, A.R.; Loneragan, G.H.; Garmyn, A.J.; Thompson, L.D.; Echeverry, A.; Brashears, M.M. Validation comparing the effectiveness of a lactic acid dip with a lactic acid spray for reducing Escherichia coli O157:H7, Salmonella, and non-O157 Shiga toxigenic Escherichia coli on beef trim and ground beef. J. Food Prot. 2012, 75, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Muthukumarasamy, P.; Han, J.H.; Holley, R.A. Bactericidal effects of Lactobacillus reuteri and allyl isothiocyanate on Escherichia coli O157:H7 in refrigerated ground beef. J. Food Prot. 2003, 66, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.; Ushimaru, P.I.; Probst, I.D.S.; Fernandes, A., Jr. Essential oils against foodborne pathogens and spoilage bacteria in minced meat. Foodborne Pathog. Dis. 2009, 6, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.R.; Baskaran, S.A.; Ramanathan, R.; Johny, A.K.; Charles, A.S.; Valipe, S.R.; Mattson, T.; Schreiber, D.; Juneja, V.K.; Mancini, R.; et al. Enhancing the thermal destruction of Escherichia coli O157:H7 in ground beef patties by trans-cinnamaldehyde. Food Microbiol. 2010, 27, 841–844. [Google Scholar] [CrossRef] [PubMed]
| STEC Serotype | Isolate 1 | Source | Free Eugenol MIC (% w/v) | Micelle-Eugenol MIC (% w/v) 2 |
|---|---|---|---|---|
| O157:H7 | USDA-FSIS-380-94 | Salami | 0.5 | 0.125 |
| O26:H11 | H30 | Clinical | 0.5 | 0.125 |
| O121:H19 | CDC 97-3068 | Human Stool | 0.5 | 0.125 |
| Treatment 1 | STEC Survivors (log10 CFU/g) 2 | p > F | Pooled Standard Error |
|---|---|---|---|
| Sterile Water (25 °C) | 6.5 ± 0.40 | 0.902 | 0.294 |
| 2× FreeEug | 6.5 ± 0.36 | ||
| Micelles | 6.4 ± 0.36 | ||
| 2% LA | 6.4 ± 0.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolen, T.N.; Ruengvisesh, S.; Taylor, T.M. Application of Surfactant Micelle-Entrapped Eugenol for Prevention of Growth of the Shiga Toxin-Producing Escherichia coli in Ground Beef. Foods 2017, 6, 69. https://doi.org/10.3390/foods6080069
Tolen TN, Ruengvisesh S, Taylor TM. Application of Surfactant Micelle-Entrapped Eugenol for Prevention of Growth of the Shiga Toxin-Producing Escherichia coli in Ground Beef. Foods. 2017; 6(8):69. https://doi.org/10.3390/foods6080069
Chicago/Turabian StyleTolen, Tamra N., Songsirin Ruengvisesh, and Thomas M. Taylor. 2017. "Application of Surfactant Micelle-Entrapped Eugenol for Prevention of Growth of the Shiga Toxin-Producing Escherichia coli in Ground Beef" Foods 6, no. 8: 69. https://doi.org/10.3390/foods6080069





