Surface Water Processes Influencing Alterations in Pharmaceutical Chemical Composition following Wastewater Discharge into a Freshwater Estuary
Abstract
:1. Introduction
2. Materials and Methods
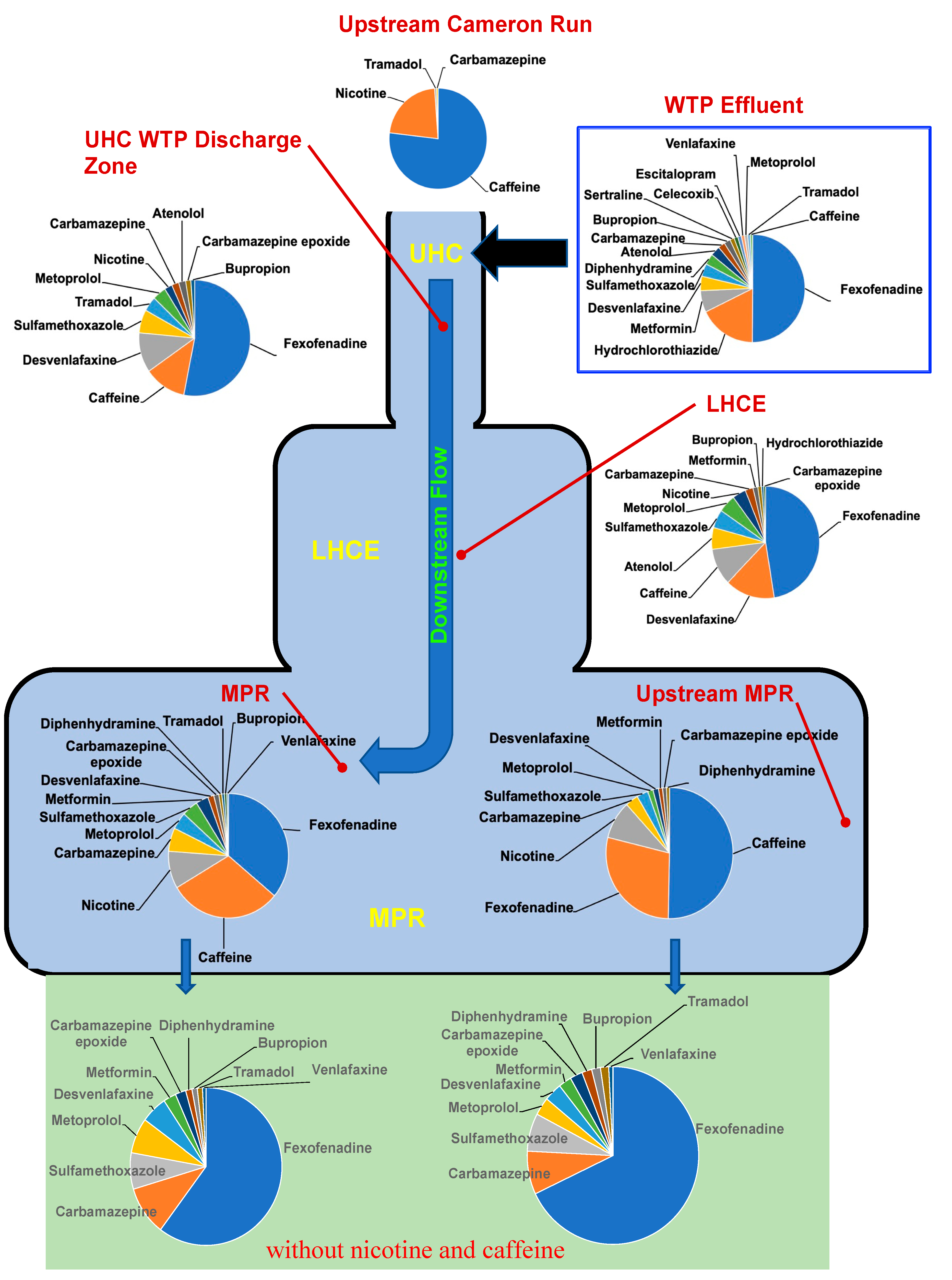
2.1. Study Area
2.2. Sampling
2.3. Materials
2.4. Sample Preparation
2.5. LC-MS/MS Analysis
2.6. Quality Assurance
2.7. Ancillary Measurements
3. Results and Discussion
PPCP Concentrations in Surface Water and Compositional Alteration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meador, J.P.; Yeh, A.; Young, G.; Gallagher, E.P. Contaminants of emerging concern in a large temperate estuary. Environ. Pollut. 2016, 213, 254–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Deo, R.P. Pharmaceuticals in the Surface Water of the USA: A Review. Curr. Environ. Health Rep. 2014, 1, 113–122. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- OECD. Pharmaceutical Residues in Freshwater: Hazards and Policy Responses; OECD Publishing: Paris, France, 2019. [Google Scholar]
- USEPA. Drinking Water Contaminant Candidate List 4—Final. Fed. Regist. 2016, 81, 81099–81114. [Google Scholar]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Persp. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Cela, R. Determination of drugs of abuse in water by solid-phase extraction, derivatisation and gas chromatography–ion trap-tandem mass spectrometry. J. Chrom. A 2010, 1217, 1748–1760. [Google Scholar] [CrossRef]
- Berset, J.-D.; Brenneisen, R.; Mathieu, C. Analysis of llicit and illicit drugs in waste, surface and lake water samples using large volume direct injection high performance liquid chromatography—Electrospray tandem mass spectrometry (HPLC–MS/MS). Chemosphere 2010, 81, 859–866. [Google Scholar] [CrossRef]
- Lee, S.S.; Paspalof, A.M.; Snow, D.D.; Richmond, E.K.; Rosi-Marshall, E.J.; Kelly, J.J. Occurrence and Potential Biological Effects of Amphetamine on Stream Communities. Environ. Sci. Technol. 2016, 50, 9727–9735. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.D.; Furlong, E.T.; Burkhardt, M.R.; Kolpin, D.; Anderson, L.G. Determination of pharmaceutical compounds in surface- and ground-water samples by solid-phase extraction and high-performance liquid chromatography–electrospray ionization mass spectrometry. J. Chrom. A 2004, 1041, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Blazer, V.S.; Gray, J.L.; Focazio, M.J.; Young, J.A.; Alvarez, D.A.; Iwanowicz, L.R.; Foreman, W.T.; Furlong, E.T.; Speiran, G.K.; et al. Chemical contaminants in water and sediment near fish nesting sites in the Potomac River basin: Determining potential exposures to smallmouth bass (Micropterus dolomieu). Sci. Total Environ. 2013, 443, 700–716. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Wat. Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Kaplan, S. Review: Pharmacological pollution in water. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1074–1116. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Ecotoxic pharmaceuticals, personal care products, and other emerging contaminants: A review of environmental, receptor-mediated, developmental, and epigenetic toxicity with discussion of proposed toxicity to humans. Crit. Rev. Environ. Sci. Technol. 2016, 46, 336–381. [Google Scholar] [CrossRef] [Green Version]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in treated wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Internat. 2020, 136, 105454. [Google Scholar] [CrossRef]
- Wu, M.-h.; Xie, D.-g.; Xu, G.; Sun, R.; Xia, X.-y.; Liu, W.-l.; Tang, L. Benzophenone-type UV filters in surface waters: An assessment of profiles and ecological risks in Shanghai, China. Ecotoxicol. Environ. Saf. 2017, 141, 235–241. [Google Scholar] [CrossRef]
- Lang, D.J. Water Quality of the three Major Tributaries to the Chesapeake Bay, the Susquehanna, Potomac, and James Rivers, January 1979–April 1981. In Water-Resources Investigations Report; U.S. Geological Survey: Towson, MD, USA, 2016. [Google Scholar]
- Jones, R.C. Recovery of a Tidal Freshwater Embayment from Eutrophication: A Multidecadal Study. Estuaries Coasts 2020, 43, 1318–1334. [Google Scholar] [CrossRef] [Green Version]
- Bavumiragira, J.P.; Ge, J.N.; Yin, H. Fate and transport of pharmaceuticals in water systems: A processes review. Sci. Total Environ. 2022, 823, 153635. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, X.; Xu, B.; Peng, D.; Guo, X. Sorption of pharmaceuticals and personal care products on soil and soil components: Influencing factors and mechanisms. Sci. Total Environ. 2021, 753, 141891. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, K.; Benoit, P.; Mamy, L.; Patureau, D. Transformation of PPCPs in the environment: Review of knowledge and classification of pathways according to parent molecule structures. Crit. Rev. Environ. Sci. Technol. 2022, 53, 47–69. [Google Scholar] [CrossRef]
- Ator, S.W.; Denver, J.M.; Krantz, D.E.; Newell, W.L.; Martucci, S.K. A Surficial Hydrogeologic Framework for the Mid-Atlantic Coastal Plain; Professional Paper; U.S. Geological Survey: Towson, MD, USA, 2016. [Google Scholar]
- Jones, R.C.; Kelso, D.P.; Schaeffer, E. Spatial and seasonal patterns in water quality in an embayment-mainstem reach of the tidal freshwater Potomac River, USA: A multiyear study. Environ. Monit. Assess. 2008, 147, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Dulaurent, S.; El Balkhi, S.; Poncelet, L.; Gaulier, J.M.; Marquet, P.; Saint-Marcoux, F. QuEChERS sample preparation prior to LC-MS/MS determination of opiates, amphetamines, and cocaine metabolites in whole blood. Anal. Bioanal. Chem. 2016, 408, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.B.R.; Guilherme, J.R.; Caldas, S.S.; Martins, M.L.; Zanella, R.; Primel, E.G. Evaluation of the QuEChERS method for the extraction of pharmaceuticals and personal care products from drinking-water treatment sludge with determination by UPLC-ESI-MS/MS. Chemosphere 2014, 107, 74–82. [Google Scholar] [CrossRef]
- Foster, G.D.; Leahigh, A. Sediment–Water Distribution and Benthic Boundary Layer Fluxes of Pharmaceuticals and Personal Care Products near Wastewater Discharge into a Tidal River Shoal. ACS EST Water 2021, 1, 1447–1455. [Google Scholar] [CrossRef]
- Baena-Nogueras, R.M.; González-Mazo, E.; Lara-Martín, P.A. Degradation kinetics of pharmaceuticals and personal care products in surface waters: Photolysis vs biodegradation. Sci. Total Environ. 2017, 590–591, 643–654. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Wu, L. Sorption and degradation of pharmaceuticals and personal care products (PPCPs) in soils. Environ. Sci. Pollut. Res. Int. 2013, 20, 4261–4267. [Google Scholar] [CrossRef]
- Li, Z.; Chang, P.H.; Jiang, W.T.; Jean, J.S.; Hong, H.; Liao, L. Removal of diphenhydramine from water by swelling clay minerals. J. Colloid. Interface Sci. 2011, 360, 227–232. [Google Scholar] [CrossRef]
- Stein, K.; Ramil, M.; Fink, G.; Sander, M.; Ternes, T.A. Analysis and Sorption of Psychoactive Drugs onto Sediment. Environ. Sci. Technol. 2008, 42, 6415–6423. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, X.; Song, W.; Pan, Y.; Lambropoulou, D.; Zhong, Y.; Du, Y.; Nie, J.; Yang, X. Photochemical oxidation of PPCPs using a combination of solar irradiation and free available chlorine. Sci. Total Environ. 2019, 682, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Seid, M.G.; Lee, C.; Cho, K.; Hong, S.W. Degradation of ranitidine and changes in N-nitrosodimethylamine formation potential by advanced oxidation processes: Role of oxidant speciation and water matrix. Wat. Res. 2021, 203, 117495. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Williams, R.; Walsh, J.J.; Gilmer, J.F. The aqueous stability of bupropion. J. Pharm. Biomed. Anal. 2010, 53, 376–381. [Google Scholar] [CrossRef]
- Jones, R.C.; De Mutsert, K.; Jonas, R.; Huff, T.B. An Ecological Study of Hungting Creek 2015. Final Report to Alexandria Renew Enterprises. Alexandria, Virginia, 4 December 2016. Available online: http://mars.gmu.edu/handle/1920/10634?show=full (accessed on 12 November 2022).
- Pan, B.; Ning, P.; Xing, B. Part V—Sorption of pharmaceuticals and personal care products. Environ. Sci. Pollut. Res. 2009, 16, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, V.; Meffe, R.; Herrera, S.; Arranz, E.; de Bustamante, I. Sorption/desorption of non-hydrophobic and ionisable pharmaceutical and personal care products from reclaimed water onto/from a natural sediment. Sci. Total Environ. 2014, 472, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Takemoto, K.; Tamura, I.; Shin-oka, N.; Nakano, T.; Nishida, M.; Honda, Y.; Moriguchi, S.; Nakamura, Y. Contribution of inorganic and organic components to sorption of neutral and ionizable pharmaceuticals by sediment/soil. Environ. Sci. Pollut. Res. 2018, 25, 7250–7261. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Claybourne, J.; Shi, E.; Strand, S.; Schultz, M.; Snider, M. Biodegradation of venlafaxine (LB289). FASEB J. 2014, 28, LB289. [Google Scholar] [CrossRef]
- EPA, U. Estimation Programs Interface Suite™ for Microsoft® Windows; United States Environmental Protection Agency: Washington, DC, USA, 2018. [Google Scholar]



| PPCP | Eigen Rank a | Sorption Dominant | log Dow (7.4) b | Reaction Dominant | Half-life (h) | Reference/Other Comment |
|---|---|---|---|---|---|---|
| Sertraline | 1 | X | 3.14 | |||
| Diphenhydramine | 2 | X | 2.34 | |||
| Escitalopram | 4 | X | 1.27 | |||
| Desvenlafaxine | 7 | X | 0.89 | |||
| Fexofenadine | 10 | X | 2.43 | |||
| Venlafaxine | 3 | X (Bio) c | 15 | [43] | ||
| Nicotine | 5 | upstream source | ||||
| Caffeine | 6 | upstream source | ||||
| Hydrochlorothiazide | 7 | X (P) d | 0.43 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foster, G.; Leahigh, A.; Huff, T. Surface Water Processes Influencing Alterations in Pharmaceutical Chemical Composition following Wastewater Discharge into a Freshwater Estuary. Toxics 2022, 10, 702. https://doi.org/10.3390/toxics10110702
Foster G, Leahigh A, Huff T. Surface Water Processes Influencing Alterations in Pharmaceutical Chemical Composition following Wastewater Discharge into a Freshwater Estuary. Toxics. 2022; 10(11):702. https://doi.org/10.3390/toxics10110702
Chicago/Turabian StyleFoster, Gregory, Arion Leahigh, and Thomas Huff. 2022. "Surface Water Processes Influencing Alterations in Pharmaceutical Chemical Composition following Wastewater Discharge into a Freshwater Estuary" Toxics 10, no. 11: 702. https://doi.org/10.3390/toxics10110702





