Alternative Methods for Skin-Sensitization Assessment
Abstract
:1. Introduction
- (i)
- In vitro methods, which are widely used in toxicology to assess topical toxicity, e.g., skin irritation/corrosion/sensitization, as well as systemic toxicity, e.g., genotoxicity and developmental and reproductive toxicology [4]. In these methods, the animal organism is replaced by a biological system consisting of cells or tissue models such as primary cultures, finite cell lines, continuous cell lines or reconstructed 3D tissues. In vitro systems are based on cell monolayer, coculture systems and three-dimensional (3D) tissues, taking into account the tissue microenvironment. There has been a lot of interest in recent years in the organs on chips (OoCs) which reflect the structure and physiological properties of tissues or organs.
- (ii)
- Ex vivo methods (isolated animal tissues and organs). In toxicology, ex vivo methods are mainly used to assess corrosion. Eye corrosion is evaluated by the methods adopted by the Organization for Economic Cooperation and Development (OECD) that use eyes obtained from slaughtered chickens (OECD TG 438) or cattle (OECD TG 437) [5,6]. In turn, skin corrosion is assessed by a rat skin transcutaneous electrical resistance (TER) test (OECD TG 430). This method uses skin fragments obtained from humanely euthanized rats [7]. Precision-cut tissue slices (PCS) are also used, both from rodents and from humans, e.g., from biopsy material [8].
- (iii)
- In chemico methods (chemical methods in which the properties of the substance under test are evaluated by reaction with appropriate material, without using human or animal cells). In chemico methods are used to evaluate reactive substances. In order to develop them, it is necessary to know about the interaction of the test substance with a biological substance. These methods are widely used in the skin-sensitization area.
- (iv)
- In silico methods: computer simulations and mathematical models such as Quantitative Structure–Activity Relationship (QSAR), Threshold of Toxicological Concern (TTC), etc. [9]. In silico methods are used to predict toxicity based on computational methods and are used in conjunction with other alternative methods to reduce in vivo testing. The particular advantage of in silico methods is the possibility of assessing the potential physico-chemical or biological properties of substances before they are synthesized [10,11,12].
2. Skin-Sensitization Mechanism and Standard Assessment
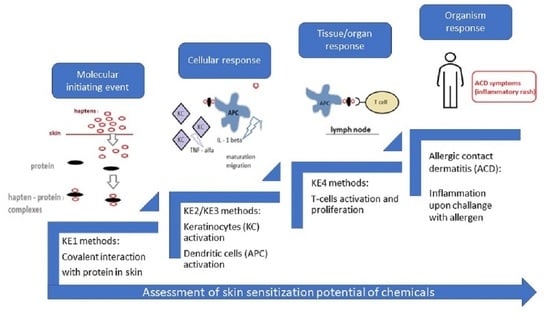
3. Skin-Sensitization Assessment—Alternative Methods
- KE1: the first key event, i.e., the molecular initiating event (MIE), is the covalent binding of electrophilic substances to nucleophilic centres in skin proteins;
- KE2: the second key event, i.e., the keratinocytes (KCs) activation;
- KE3: the third key event is the activation of dendritic cells (DCs);
- KE4: the fourth key event is Tcell proliferation.
- Unsatisfactory accuracy and predictability;
- Not all possible outcomes of interest are included in a single test;
- The need to consider different modes of action;
- In vitro tests represent only one or a few steps in complex in vivo processes;
- Not all classes of test substances are covered;
- Not all classes of severity of effects are covered;
- Positive test results are rare, and the number of false positives is too high;
- Lack of integrated data and evidence from different studies;
- Lack of integrated information on absorption, distribution, metabolism and excretion of test substances, which makes it impossible to extrapolate data from in vivo studies [4].
3.1. Key Event 1 (KE1)-Based Methods
| Assay | Detection Method | Type of Nucleophile | Dataset | Accuracy [%] | Specificity [%] | Sensitivity [%] | Source /Ref. |
|---|---|---|---|---|---|---|---|
| Compared to the LLNA | |||||||
| DPRA | HPLC-UV | synthetic peptide containing cysteine and lysine | 157 | 80 | 77 | 80 | [44] |
| ADRA | HPLC-UV | NAC, NAL | 136 | 76 | 79 | 76 | [44] |
| kDPRA | Fluorescence | cysteine peptide | 180 | 85 | 86 | 84 | [44] |
| ADRA-FL | HPLC-FL | NAC, NAL | 47 | - | - | - | [52] |
| COR1-C420 | LC-MS | COR1-C420 | 80 | 88.8 * | 89.5 * | 88.5 * | [46] |
| NMR | NMR spectroscopy | n-butylamine, 1-butanethiol | 8 | - | - | - | [53] |
| NMR-DCYA | NMR spectroscopy | DCYA | 17 | - | - | - | [54] |
| HTS-DCYA | Fluorescence detection | DCYA | 33 | 82 | 90 | 78 | [55] |
| HPLC/MS-MS method | HPLC/MS-MS | cysteine and lysine peptide | 18 | - | - | - | [58] |
| PPRA | HPLC/MS-MS | cysteine peptide | 15 | - | - | - | [63] |
| APIA | MALDI-TOF mass spectrometry | peptide-21 and peptide-20 | 3 | - | - | - | [66] |
| Spectro-DPRA | UV-VIS spectrophotometry/ fluorometry | cysteine and lysine peptide | 40 | 82.5 | 86.7 | 80 | [60] |
| Method using small, endogenous molecules | HPLC—PDA | cysteamine, gluthatione | 30 | 93 | 82 | 100 | [62] |
| EASA | absorbance and fluorescence | 4-nitrobenzenethiol (NTB) pyridoxylamine (PDA) | 92 | - | - | - | [69] |
| SH test | flow cytometry | cell surface thiols | 52 | - | - | - | [70] |
| Improved SH test | flow cytometry | cell surface thiols | 25 | 84 | 62.5 | 94.1 | [71] |
3.2. Key Event 2 (KE2)-Based Methods
| Method | Cell Line | Dataset | Accuracy [%] | Specificity [%] | Sensitivity [%] | Source /Ref. |
|---|---|---|---|---|---|---|
| Compared to the LLNA | ||||||
| ARE-Nrf2 Luciferase KeratinoSensTM Test | KeratinoSens™ transgenic cell line | 145 | 77 | 72 | 79 | [79] |
| The ARE-Nrf2 Luciferase LuSens test | LuSens transgenic cell line | 72 | 74 | 74 | 74 | [79] |
| AREc32 cell line assay | AREc32 | 102 | 83 | 86.6 | 81.4 | [89] |
| MDA-ARE assay | MDA-ARE reporter cell line | 22 | - | 100 | 92 | [86] |
| KeratinosensTM-resazurin assay | KeratinoSens™ transgenic cell line | 35 | - | - | - | [81] |
| Keratinosens – S9 assay | KeratinoSens™ transgenic cell line | 77 | - | - | - | [87] |
| Keratinosens assay with S9 and P450 assay | KeratinoSens™ transgenic cell line | 2 | - | - | - | [88] |
| Method | Marker Genes | Model | Dataset | Accuracy [%] | Specificity [%] | Sensitivity [%] | Source /Ref. |
|---|---|---|---|---|---|---|---|
| Compared to the LLNA | |||||||
| EpiSensA | ATF 3; GCLM DNAJB4 | 3D | 16 | 87.5–100 | 75–100 | 83.3–100 | [97] |
| Modified EpiSensA | ATF 3; GCLM DNAJB4; IL-8 | 3D | 72 | 90 | 78 | 94 | [98] |
| SENS-IS | REDOX group: 17 genes, SENS-IS group: 21 genes | 3D | 150 | 96.6 | 95.2 | 97.7 | [96] |
| HaCaT cell model assay | NQO1, AKR1C2, TXN, IL8, ALDH3A, HMOX1, MafF, GCLC, CYP1A1, MT1, MT2 | 2D | 58 | 84 | 92 | 81 | [90] |
| SenCeeTox | The expression of: eight Nrf2/ARE, one AhR/XRE, two Nrf1/MRE-controlled genes | 3D | 11 | - | - | - | [92] |
| HaCaT gene signature | HMOX1, STC2 ADM, SRD1, cFOS, FosL1, DNMT3b, RBM5, CDK12, ARD37 | 2D | 39 | 96.2 | 100 | 95 | [91] |
3.3. Key Event 3 (KE3)-Based Methods
- Human cell line activation test (h-CLAT);
- U937 cell line activation Test (U-SENS);
- Interleukin-8 Reporter Gene Assay (IL-8 Luc assay);
- Genomic Allergen Rapid Detection (GARD™) for assessment of skin sensitizers (GARD™skin) [118].
| Method | Endpoint | Cell Line | Data Set | Accuracy [%] | Specificity [%] | Sensitivity [%] | Source /Ref. |
|---|---|---|---|---|---|---|---|
| Compared to the LLNA | |||||||
| h-CLAT | expression of CD86/CD54 | THP-1 | 142 | 85 | 66 | 93 | [118] |
| Original h-CLAT + LP h-CLAT | expression of CD86/CD54 | THP-1 | 132 | 88 | 70 | 95 | [122] |
| Animal-free h-CLAT | expression of CD86/CD54 | THP-1 | 10 | - | - | - | [123] |
| Serum free h-CLAT | expression of CD86/CD54 | THP-1 | 10 | - | - | - | [124] |
| U-SENS | expression of CD86 | U-937 | 166 | 86 | 65 | 91 | [118] |
| PBMDC assay | expression of CD86 | PBMDC | 12 | - | - | - | [127] |
| BMCDs assay | expression of MHC II/CD40/CD54/CD86 | BMCD | 20 | 75 | - | 69 | [129] |
| pDC assay | expression of CD86 | pDC | 45 | 91 | 86 | 96 | [126] |
| IL-Luc assay | IL-8 level | THP-G8 | 136 | 89 | 53 | 96 | [118] |
| GARDTMskin | gene expression196 transcripts | SenzaCell | 75 | 87.6 | 89.9 | 87.2 | [118] |
| GARD potency gene signature | gene expression–52 transcripts | SenzaCell | 18 | 78 | - | - | [136] |
| VitoSens | genes expression: CREM, CCR2 | CD34-DC | 73 | 89 | 97 | 82 | [137] |
| FSDC method | gene expression: Trxr1, Hmox1, Nqo1 and Cxcl10 —the p38 MAPK and JNK signalling pathways | FSDC | 18 | 94 | 100 | 92 | [139] |
| CD86 and IL-8 release assay | IL-8 level, expression of CD86 | THP-1 | 31 | 95.2 | - | 93.5 | [132] |
| U-937 activation test | IL-8 level and expression of CD86 | U-937 | 16 | - | - | - | [121] |
| moDC IL-8 assay | IL-8 level and expression of CD83 and DCD86 | moDC | 12 | - | - | - | [133] |
| THP-1 IL-8 | Il-8 level | THP-1 | 23 | - | - | - | [131] |
3.4. Key Event 4 (KE4)-Based Methods
3.5. Coculture Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peiser, M.; Tralau, T.; Heidler, J.; Api, A.M.; Arts, J.H.E.; Basketter, D.A.; English, J.; Diepgen, T.L.; Fuhlbrigge, R.C.; Gaspari, A.A.; et al. Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Cell. Mol. Life Sci. 2012, 69, 763–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thyssen, J.P.; Linneberg, A.; Menné, T.; Johansen, J.D. The epidemiology of contact allergy in the general population-prevalence and main findings. Contact Dermat. 2007, 57, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Clouet, E.; Kerdine-Römer, S.; Ferret, P.-J. Comparison and validation of an in vitro skin sensitization strategy using a data set of 33 chemical references. Toxicol. Vitr. 2017, 45, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Caloni, F.; De Angelis, I.; Hartung, T. Replacement of animal testing by integrated approaches to testing and assessment (IATA): A call for in vivitrosi. Arch. Toxicol. 2022, 96, 1935–1950. [Google Scholar] [CrossRef]
- OECD. Test No. 437: Bovine Corneal Opacity and Permeability Test Method for Identifying Ocular Corrosives and Severe Irritants [Internet]. 2009. Available online: https://www.oecd-ilibrary.org/content/publication/9789264076303-en (accessed on 20 June 2020).
- OECD. Test No. 438: Isolated Chicken Eye Test Method for Identifying (i) Chemicals Inducing Serious Eye Damage and (ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage [Internet]. 2018. Available online: https://www.oecd-ilibrary.org/content/publication/9789264203860-en (accessed on 8 September 2009).
- OECD. Test No. 430: In Vitro Skin Corrosion: Transcutaneous Electrical Resistance Test Method (TER) [Internet]. 2015. Available online: https://www.oecd-ilibrary.org/content/publication/9789264242739-en (accessed on 23 November 2004).
- Wick, P.; Chortarea, S.; Guenat, O.T.; Roesslein, M.; Stucki, J.D.; Hirn, S.; Fink, A.; Rothen-Rutishauser, B. In Vitro-ex vivo model systems for nanosafety assessment. Eur. J. Nanomed. 2015, 7, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Kandárová, H.; Letašiová, S. Alternative methods in toxicology: Pre-validated and validated methods. Interdiscip. Toxicol. 2011, 4, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Raies, A.B.; Bajic, V.B. In silico toxicology: Computational methods for the prediction of chemical toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 147–172. [Google Scholar] [CrossRef] [Green Version]
- Hemmerich, J.; Ecker, G.F. In silico toxicology: From structure–activity relationships towards deep learning and adverse outcome pathways. WIREs Comput. Mol. Sci. 2020, 10, e1475. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kim, M.K.; Kim, K.B.; Kim, H.S.; Lee, B.M. Quantitative structure-activity and quantitative structure-property relationship approaches as alternative skin sensitization risk assessment methods. J. Toxicol. Environ. Health A. 2019, 82, 447–472. [Google Scholar] [CrossRef]
- De Ávila, R.I.; Lindstedt, M.; Valadares, M.C. The 21st Century movement within the area of skin sensitization assessment: From the animal context towards current human-relevant in vitro solutions. Regul. Toxicol. Pharmacol. 2019, 108, 104445. [Google Scholar] [CrossRef]
- Hartung, T. From alternative methods to a new toxicology. Eur. J. Pharm. Biopharm. 2011, 77, 338–349. [Google Scholar] [CrossRef]
- Maertens, A.; Golden, E.; Luechtefeld, T.H.; Hoffmann, S.; Tsaioun, K.; Hartung, T. Probabilistic risk assessment-the keystone for the future of toxicology. ALTEX 2022, 39, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I. The activity of methacrylate esters in skin sensitisation test methods: A review. Regul. Toxicol. Pharmacol. 2019, 104, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W.; Api, A.M.; Aptula, A.O. Chemical applicability domain of the Local Lymph Node Assay (LLNA) for skin sensitisation potency. Part 2. The biological variability of the murine Local Lymph Node Assay (LLNA) for skin sensitisation. Regul. Toxicol. Pharmacol. 2016, 80, 255–259. [Google Scholar] [CrossRef]
- Martin, S.F.; Rustemeyer, T.; Thyssen, J.P. Recent advances in understanding and managing contact dermatitis. F1000Reserach 2018, 7, 810. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.E.A.M.; Dos Reis, V.M.S. Immunopathology of allergic contact dermatitis. An. Bras. De Dermatol. 2011, 86, 419–433. [Google Scholar] [CrossRef]
- Aptula, A.O.; Roberts, D.W.; Pease, C.K. Haptens, prohaptens and prehaptens, or electrophiles and proelectrophiles. Contact Dermat. 2007, 56, 54–56. [Google Scholar] [CrossRef]
- Urbisch, D.; Becker, M.; Honarvar, N.; Kolle, S.N.; Mehling, A.; Teubner, W.; Wareing, B.; Landsiedel, R. Assessment of Pre- and Pro-haptens Using Nonanimal Test Methods for Skin Sensitization. Chem. Res. Toxicol 2016, 29, 901–913. [Google Scholar] [CrossRef] [Green Version]
- Karlberg, A.-T.; Börje, A.; Duus Johansen, J.; Lidén, C.; Rastogi, S.; Roberts, D.; Uter, W.; White, I.R. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers-prehaptens and prohaptens. Contact Dermat. 2013, 69, 323–334. [Google Scholar] [CrossRef]
- Gądarowska, D.; Krakowian, D.; Daniel-Wójcik, A.; Mrzyk, I. Co-culture methods in the skin sensitization testing-review. In Advances in Biomedical Research-Cancer and Miscellaneous; Młynarczuk-Biały, I., Biały, Ł., Eds.; Wydawnictwo Naukowe Tygiel sp. z o.o.: Lublin-Warszawa, Poland, 2021; pp. 118–132. ISBN 978-83-67104-12-8. [Google Scholar]
- Kimber, I.; Basketter, D.A.; Gerberick, G.F.; Dearman, R.J. Allergic contact dermatitis. Int. Immunopharmacol. 2002, 2, 201–211. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 406: Skin Sensitisation [Internet]. 2022. Available online: https://www.oecd-ilibrary.org/content/publication/9789264070660-en (accessed on 30 June 2022).
- OECD. Test No. 429: Skin Sensitisation [Internet]. 2010. Available online: https://www.oecd-ilibrary.org/content/publication/9789264071100-en (accessed on 23 July 2010).
- OECD. Test No. 442A: Skin Sensitization [Internet]. 2010. Available online: https://www.oecd-ilibrary.org/content/publication/9789264090972-en (accessed on 23 July 2010).
- OECD. Test No. 442B: Skin Sensitization [Internet]. 2018. Available online: https://www.oecd-ilibrary.org/content/publication/9789264090996-en (accessed on 27 October 2018).
- Gwaltney-Brant, S. Chapter 22-Immunotoxicity biomarkers. In Biomarkers in Toxicology; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 373–385. [Google Scholar] [CrossRef]
- Williams, W.C.; Copeland, C.; Boykin, E.; Quell, S.J.; Lehmann, D.M. Development and utilization of an ex vivo bromodeoxyuridine local lymph node assay protocol for assessing potential chemical sensitizers. J. Appl. Toxicol. 2015, 35, 29–40. [Google Scholar] [CrossRef] [PubMed]
- UE. Commission regulation (EU) 2016/1688 of 20 September 2016 amending Annex VII to Regulation (EC) No 1907/2006 of the European Parliament and of the council on the Registration. Off. J. Eur. Communities 2016, 255, 1–3. [Google Scholar]
- Casati, S.; Aschberger, K.; Barroso, J.; Casey, W.; Delgado, I.; Kim, T.S.; Kleinstreuer, N.; Kojima, H.; Lee, J.K.; Lowit, A.; et al. Standardisation of defined approaches for skin sensitisation testing to support regulatory use and international adoption: Position of the International Cooperation on Alternative Test Methods. Arch. Toxicol. 2018, 92, 611–617. [Google Scholar] [CrossRef] [Green Version]
- OECD. Guideline No. 497: Defined Approaches on Skin Sensitisation [Internet]. 2021. Available online: https://www.oecd-ilibrary.org/content/publication/b92879a4-en (accessed on 22 June 2021).
- Basketter, D.A.; Gerberick, G.F. Skin Sensitization Testing: The Ascendancy of Non-Animal Methods. Cosmetics 2022, 9, 38. Available online: https://www.mdpi.com/2079-9284/9/2/38 (accessed on 25 July 2022).
- Wilm, A.; Kühnl, J.; Kirchmair, J. Computational approaches for skin sensitization prediction. Crit. Rev. Toxicol. 2018, 48, 738–760. [Google Scholar] [CrossRef]
- Strickland, J.; Truax, J.; Corvaro, M.; Settivari, R.; Henriquez, J.; McFadden, J.; Gulledge, T.; Johnson, V.; Gehen, S.; Germolec, D.; et al. Application of Defined Approaches for Skin Sensitization to Agrochemical Products. Front. Toxicol. 2022, 4, 852856. [Google Scholar] [CrossRef]
- Reynolds, J.; MacKay, C.; Gilmour, N.; Miguel-Vilumbrales, D.; Maxwell, G. Probabilistic prediction of human skin sensitiser potency for use in next generation risk assessment. Comput. Toxicol. 2019, 9, 36–49. [Google Scholar] [CrossRef]
- Kolle, S.N.; Hill, E.; Raabe, H.; Landsiedel, R.; Curren, R. Regarding the references for reference chemicals of alternative methods. Toxicol. Vitr. 2019, 57, 48–53. [Google Scholar] [CrossRef]
- Kleinstreuer, N.C.; Hoffmann, S.; Alépée, N.; Allen, D.; Ashikaga, T.; Casey, W.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N.; et al. Non-animal methods to predict skin sensitization (II): An assessment of defined approaches. Crit. Rev. Toxicol. 2018, 48, 359–374. [Google Scholar] [CrossRef]
- Natsch, A.; Landsiedel, R.; Kolle, S.N. A triangular approach for the validation of new approach methods for skin sensitization. ALTEX 2021, 38, 669–677. [Google Scholar] [CrossRef]
- Gilmour, N.; Kimber, I.; Williams, J.; Maxwell, G. Skin sensitization: Uncertainties, challenges, and opportunities for improved risk assessment. Contact Dermat. 2019, 80, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, A.B.; Strickland, J.; Allen, D.; Casati, S.; Zuang, V.; Barroso, J.; Whelan, M.; Régimbald-Krnel, M.; Kojima, H.; Nishikawa, A.; et al. International regulatory requirements for skin sensitization testing. Regul. Toxicol. Pharmacol. 2018, 95, 52–65. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 442C: In Chemico Skin Sensitisation [Internet]. 2022. Available online: https://www.oecd-ilibrary.org/content/publication/9789264229709-en (accessed on 30 June 2022).
- Yamamoto, Y.; Tahara, H.; Usami, R.; Kasahara, T.; Jimbo, Y.; Hioki, T.; Fujita, M. A novel in chemico method to detect skin sensitizers in highly diluted reaction conditions. J. Appl. Toxicol. 2015, 35, 1348–1360. [Google Scholar] [CrossRef]
- Natsch, A.; Gfeller, H. LC-MS–Based Characterization of the Peptide Reactivity of Chemicals to Improve the In Vitro Prediction of the Skin Sensitization Potential. Toxicol. Sci. 2008, 106, 464–478. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Yamamoto, Y.; Tahara, H.; Kasahara, T.; Jimbo, Y.; Hioki, T. Development of a prediction method for skin sensitization using novel cysteine and lysine derivatives. J. Pharmacol. Toxicol. Methods 2014, 70, 94–105. [Google Scholar] [CrossRef]
- Akimoto, M.; Yamamoto, Y.; Watanabe, S.; Yamaga, H.; Yoshida, K.; Wakabayashi, K.; Tahara, Y.; Horie, N.; Fujimoto, K.; Kusakari, K.; et al. Oxidation of a cysteine-derived nucleophilic reagent by dimethyl sulfoxide in the amino acid derivative reactivity assay. J. Appl. Toxicol. 2020, 40, 843–854. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujita, M.; Wanibuchi, S.; Katsuoka, Y.; Ono, A.; Kasahara, T. Expanding the applicability of the amino acid derivative reactivity assay: Determining a weight for preparation of test chemical solutions that yield a predictive capacity identical to the conventional method using molar concentration and demonstrating the capacity to detect sensitizers in liquid mixtures. J. Pharmacol. Toxicol. Methods 2019, 97, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Yamamoto, Y.; Wanibuchi, S.; Katsuoka, Y.; Kasahara, T. The underlying factors that explain why nucleophilic reagents rarely co-elute with test chemicals in the ADRA. J. Pharmacol. Toxicol. Methods 2019, 96, 95–105. [Google Scholar] [CrossRef]
- Fujita, M.; Yamamoto, Y.; Watanabe, S.; Sugawara, T.; Wakabayashi, K.; Tahara, Y.; Horie, N.; Fujimoto, K.; Kusakari, K.; Kurokawa, Y.; et al. Cause of and countermeasures for oxidation of the cysteine-derived reagent used in the amino acid derivative reactivity assay. J. Appl. Toxicol. 2018, 39, 191–208. [Google Scholar] [CrossRef]
- Fujita, M.; Yamamoto, Y.; Wanibuchi, S.; Katsuoka, Y.; Kasahara, T. A newly developed means of HPLC-fluorescence analysis for predicting the skin sensitization potential of multi-constituent substances using ADRA. Toxicol. Vitr. 2019, 59, 161–178. [Google Scholar] [CrossRef]
- Sanderson, P.N.; Simpson, W.; Cubberley, R.; Aleksic, M.; Gutsell, S.; Russell, P.J. Mechanistic understanding of molecular initiating events (MIEs) using NMR spectroscopy. Toxicol. Res. 2016, 5, 34–44. [Google Scholar] [CrossRef]
- Chittiboyina, A.G.; Avonto, C.; Rua, D.; Khan, I.A. Alternative Testing Methods for Skin Sensitization: NMR Spectroscopy for Probing the Reactivity and Classification of Potential Skin Sensitizers. Chem. Res. Toxicol. 2015, 28, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Avonto, C.; Chittiboyina, A.; Rua, D.; A Khan, I. A fluorescence high throughput screening method for the detection of reactive electrophiles as potential skin sensitizers. Toxicol. Appl. Pharmacol. 2015, 289, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Avonto, C.; Wang, Y.-H.; Chittiboyina, A.G.; Vukmanovic, S.; Khan, I.A. In chemico assessment of potential sensitizers: Stability and direct peptide reactivity of 24 fragrance ingredients. J. Appl. Toxicol. 2019, 39, 398–408. [Google Scholar] [CrossRef]
- Avonto, C.; Chittiboyina, A.G.; Sadrieh, N.; Vukmanovic, S.; Khan, I.A. In chemico skin sensitization risk assessment of botanical ingredients. J. Appl. Toxicol. 2018, 38, 1047–1053. [Google Scholar] [CrossRef]
- Zhang, F.; Erskine, T.; Klapacz, J.; Settivari, R.; Marty, S. A highly sensitive and selective high pressure liquid chromatography with tandem mass spectrometry (HPLC/MS-MS) method for the direct peptide reactivity assay (DPRA). J. Pharmacol. Toxicol. Methods 2018, 94, 1–15. [Google Scholar] [CrossRef]
- Jeong, Y.H.; An, S.; Shin, K.; Lee, T.R. Peptide reactivity assay using spectrophotometric method for high-throughput screening of skin sensitization potential of chemical haptens. Toxicol. Vitr. 2013, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-A.; Jeong, Y.H.; Kim, J.H.; Kim, S.; Cho, J.-C.; Heo, Y.; Suh, K.-D.; An, S.; Shin, K. Method for detecting the reactivity of chemicals towards peptides as an alternative test method for assessing skin sensitization potential. Toxicol. Lett. 2014, 225, 185–191. [Google Scholar] [CrossRef]
- Cho, S.-A.; An, S.; Park, J.-H. High-throughput screening (HTS)-based spectrophotometric direct peptide reactivity assay (Spectro-DPRA) to predict human skin sensitization potential. Toxicol. Lett. 2019, 314, 27–36. [Google Scholar] [CrossRef]
- Nepal, M.R.; Shakya, R.; Kang, M.J.; Jeong, T.C. A simple in chemico method for testing skin sensitizing potential of chemicals using small endogenous molecules. Toxicol. Lett. 2018, 289, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gerberick, G.F.; Troutman, A.J.; Foertsch, M.L.; Vassallo, D.J.; Quijano, M.; Dobson, L.M.R.; Goebel, C.; Lepoittevin, J.-P. Investigation of Peptide Reactivity of Pro-hapten Skin Sensitizers Using a Peroxidase-Peroxide Oxidation System. Toxicol. Sci. 2009, 112, 164–174. [Google Scholar] [CrossRef]
- Lalko, J.F.; Dearman, R.J.; Gerberick, G.F.; Troutman, J.A.; Api, A.M.; Kimber, I. Reactivity of chemical respiratory allergens in the Peroxidase Peptide Reactivity Assay. Toxicol. Vitr. 2013, 27, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Andersson, S.I.; Stenfeldt, A.-L.; Simonsson, C.; Bergström, J.; Ericson, M.B.; Jonsson, C.A.; Broo, K.S. Modification and Expulsion of Keratins by Human Epidermal Keratinocytes upon Hapten Exposure in Vitro. Chem. Res. Toxicol. 2011, 24, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Dietz, L.; Kinzebach, S.; Ohnesorge, S.; Franke, B.; Goette, I.; Koenig-Gressel, D.; Thierse, H.J. Proteomic allergen–peptide/protein interaction assay for the identification of human skin sensitizers. Toxicol. Vitr. 2013, 27, 1157–1162. [Google Scholar] [CrossRef]
- Chipinda, I.; Mbiya, W.; Adigun, R.A.; Morakinyo, M.K.; Law, B.F.; Simoyi, R.H.; Siegel, P.D. Pyridoxylamine reactivity kinetics as an amine based nucleophile for screening electrophilic dermal sensitizers. Toxicology 2014, 315, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Chipinda, I.; Ajibola, R.O.; Morakinyo, M.K.; Ruwona, T.B.; Simoyi, R.H.; Siegel, P.D. Rapid and simple kinetics screening assay for electrophilic dermal sensitizers using nitrobenzenethiol. Chem. Res. Toxicol. 2010, 23, 918–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, E.J.; Uhl, R.; Toman, B.; Elliott, J.T.; Strickland, J.; Truax, J.; Gordon, J. Development of a 96-Well Electrophilic Allergen Screening Assay for Skin Sensitization Using a Measurement Science Approach. Toxics 2022, 10, 257. [Google Scholar] [CrossRef]
- Suzuki, M.; Hirota, M.; Hagino, S.; Itagaki, H.; Aiba, S. Evaluation of changes of cell-surface thiols as a new biomarker for in vitro sensitization test. Toxicol. Vitr. 2009, 23, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Imai, N.; Takeyoshi, M.; Aizawa, S.; Tsurumaki, M.; Kurosawa, M.; Toyoda, A.; Sugiyama, M.; Kasahara, K.; Ogata, S.; Omori, T.; et al. Improved performance of the SH test as an in vitro skin sensitization test with a new predictive model and decision tree. J. Appl. Toxicol. 2022, 42, 1029–1043. [Google Scholar] [CrossRef]
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef]
- Helou, D.G.; Martin, S.F.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Römer, S. Nrf2 Involvement in Chemical-Induced Skin Innate Immunity. Front. Immunol. 2019, 10, 1004. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natsch, A. The Nrf2-Keap1-ARE Toxicity Pathway as a Cellular Sensor for Skin Sensitizers—Functional Relevance and a Hypothesis on Innate Reactions to Skin Sensitizers. Toxicol. Sci. 2009, 113, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Emter, R.; Ellis, G.; Natsch, A. Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers In Vitro. Toxicol. Appl. Pharmacol. 2010, 245, 281–290. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Kensler, T.W. The Role of Keap1 in Cellular Protective Responses. Chem. Res. Toxicol. 2005, 18, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [Green Version]
- OECD. Test No. 442D: In Vitro Skin Sensitisation [Internet]. 2022. Available online: https://www.oecd-ilibrary.org/content/publication/9789264229822-en (accessed on 30 June 2022).
- Ramirez, T.; Mehling, A.; Kolle, S.N.; Wruck, C.J.; Teubner, W.; Eltze, T.; Aumann, A.; Urbisch, D.; van Ravenzwaay, B.; Landsiedel, R. LuSens: A keratinocyte based ARE reporter gene assay for use in integrated testing strategies for skin sensitization hazard identification. Toxicol. Vitr. 2014, 28, 1482–1497. [Google Scholar] [CrossRef] [Green Version]
- Emter, R.; Natsch, A. A fast Resazurin-based live viability assay is equivalent to the MTT-test in the KeratinoSens assay. Toxicol. Vitr. 2015, 29, 688–693. [Google Scholar] [CrossRef]
- Uibel, F.; Mühleisen, A.; Köhle, C.; Weimer, M.; Stummann, T.; Bremer, S.; Schwarz, M. ReProGlo: A new stem cell-based reporter assay aimed to predict embryotoxic potential of drugs and chemicals. Reprod. Toxicol. 2009, 30, 103–112. [Google Scholar] [CrossRef]
- Settivari, R.S.; Gehen, S.C.; Amado, R.A.; Visconti, N.R.; Boverhof, D.R.; Carney, E.W. Application of the KeratinoSensTM assay for assessing the skin sensitization potential of agrochemical active ingredients and formulations. Regul. Toxicol. Pharmacol. 2015, 72, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Andres, E.; Sá-Rocha, V.M.; Barrichello, C.; Haupt, T.; Ellis, G.; Natsch, A. The sensitivity of the KeratinoSensTM assay to evaluate plant extracts: A pilot study. Toxicol. Vitr. 2013, 27, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, T.; Miyazawa, M.; Saito, K.; Otsubo, Y.; Mizumachi, H.; Sakaguchi, H. Sensitivity of KeratinoSensTM and h-CLAT for detecting minute amounts of sensitizers to evaluate botanical extract. J. Toxicol. Sci. 2019, 44, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Mertl, E.; Riegel, E.; Glück, N.; Ettenberger-Bornberg, G.; Lin, G.; Auer, S.; Haller, M.; Wlodarczyk, A.; Steurer, C.; Kirchnawy, C.; et al. A dual luciferase assay for evaluation of skin sensitizing potential of medical devices. Mol. Biol. Rep. 2019, 46, 5089–5102. [Google Scholar] [CrossRef] [Green Version]
- Natsch, A.; Haupt, T. Utility of Rat Liver S9 Fractions to Study Skin-Sensitizing Prohaptens in a Modified KeratinoSens Assay. Toxicol. Sci. 2013, 135, 356–368. [Google Scholar] [CrossRef] [Green Version]
- Huth, L.; Moss, E.; Huth, S.; Skazik, C.; Karlberg, A.; Lepoittevin, J.; Baron, J.; Merk, H. 429 Prohapten-activation by human cutaneous cytochrome P450 isoenzymes—Identified with a modified KeratinoSens assay. J. Investig. Dermatol. 2017, 137, S265. [Google Scholar] [CrossRef] [Green Version]
- Natsch, A.; Emter, R. Skin Sensitizers Induce Antioxidant Response Element Dependent Genes: Application to the In Vitro Testing of the Sensitization Potential of Chemicals. Toxicol. Sci. 2007, 102, 110–119. [Google Scholar] [CrossRef] [Green Version]
- McKim, J.M.; Keller, D.J.; Gorski, J.R. A new in vitro method for identifying chemical sensitizers combining peptide binding with ARE/EpRE-mediated gene expression in human skin cells. Cutan. Ocul. Toxicol. 2010, 29, 171–192. [Google Scholar] [CrossRef]
- van der Veen, J.W.; Pronk, T.E.; van Loveren, H.; Ezendam, J. Applicability of a keratinocyte gene signature to predict skin sensitizing potential. Toxicol. Vitr. 2013, 27, 314–322. [Google Scholar] [CrossRef] [PubMed]
- McKim, J.M.; Keller, D.J.; Gorski, J.R. An in vitro method for detecting chemical sensitization using human reconstructed skin models and its applicability to cosmetic, pharmaceutical, and medical device safety testing. Cutan. Ocul. Toxicol. 2012, 31, 292–305. [Google Scholar] [CrossRef]
- Galbiati, V.; Gibbs, S.; Roggen, E.; Corsini, E. Development of an In Vitro Method to Estimate the Sensitization Induction Level of Contact Allergens. Curr. Protoc. Toxicol. 2018, 75, 20.15.1–20.15.20. [Google Scholar] [CrossRef]
- Cottrez, F.; Boitel, E.; Auriault, C.; Aeby, P.; Groux, H. Genes specifically modulated in sensitized skins allow the detection of sensitizers in a reconstructed human skin model. Development of the SENS-IS assay. Toxicol.Vitr. Int. J. Publ. Assoc. BIBRA 2015, 29, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Uruno, A.; Motohashi, H. The Keap1–Nrf2 system as an in vivo sensor for electrophiles. Nitric. Oxide 2011, 25, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Cottrez, F.; Boitel, E.; Ourlin, J.-C.; Peiffer, J.-L.; Fabre, I.; Henaoui, I.-S.; Mari, B.; Vallauri, A.; Paquet, A.; Barbry, P.; et al. SENS-IS, a 3D reconstituted epidermis based model for quantifying chemical sensitization potency: Reproducibility and predictivity results from an inter-laboratory study. Toxicol. Vitr. 2016, 32, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nukada, Y.; Takenouchi, O.; Miyazawa, M.; Sakaguchi, H.; Nishiyama, N. Development of a new in vitro skin sensitization assay (Epidermal Sensitization Assay; EpiSensA) using reconstructed human epidermis. Toxicol. Vitr. 2013, 27, 2213–2224. [Google Scholar] [CrossRef]
- Saito, K.; Takenouchi, O.; Nukada, Y.; Miyazawa, M.; Sakaguchi, H. An in vitro skin sensitization assay termed EpiSensA for broad sets of chemicals including lipophilic chemicals and pre/pro-haptens. Toxicol. Vitr. 2017, 40, 11–25. [Google Scholar] [CrossRef]
- Mizumachi, H.; Sakuma, M.; Ikezumi, M.; Saito, K.; Takeyoshi, M.; Imai, N.; Okutomi, H.; Umetsu, A.; Motohashi, H.; Watanabe, M.; et al. Transferability and within- and between-laboratory reproducibilities of EpiSensA for predicting skin sensitization potential in vitro: A ring study in three laboratories. J. Appl. Toxicol. 2018, 38, 1233–1243. [Google Scholar] [CrossRef]
- Cumberbatch, M.; Dearman, R.J.; Antonopoulos, C.; Groves, R.W.; Kimber, I. Interleukin (IL)-18 induces Langerhans cell migration by a tumour necrosis factor-alpha- and IL-1beta-dependent mechanism. Immunology 2001, 102, 323–330. [Google Scholar] [CrossRef]
- Naik, S.M.; Cannon, G.; Burbach, G.J.; Singh, S.R.; Swerlick, R.A.; Ansel, J.C.; Caughman, S.W.; Wilcox, J.N. Human Keratinocytes Constitutively Express Interleukin-18 and Secrete Biologically Active Interleukin-18 After Treatment with Pro-Inflammatory Mediators and Dinitrochlorobenzene. J. Investig. Dermatol. 1999, 113, 766–772. [Google Scholar] [CrossRef] [Green Version]
- Corsini, E.; Mitjans, M.; Galbiati, V.; Lucchi, L.; Galli, C.; Marinovich, M. Use of IL-18 production in a human keratinocyte cell line to discriminate contact sensitizers from irritants and low molecular weight respiratory allergens. Toxicol. Vitr. 2009, 23, 789–796. [Google Scholar] [CrossRef]
- Corsini, E.; Galbiati, V.; Mitjans, M.; Galli, C.; Marinovich, M. NCTC 2544 and IL-18 production: A tool for the identification of contact allergens. Toxicol. Vitr. 2013, 27, 1127–1134. [Google Scholar] [CrossRef]
- Van der Veen, J.W.; Rorije, E.; Emter, R.; Natsch, A.; van Loveren, H.; Ezendam, J. Evaluating the performance of integrated approaches for hazard identification of skin sensitizing chemicals. Regul. Toxicol. Pharmacol. 2014, 69, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, V.; Corsini, E. The NCTC 2544 IL-18 Assay for the In Vitro Identification of Contact Allergens. Curr. Protoc. Toxicol. 2012, 54, 20.8.1–20.8.18. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.; Corsini, E.; Spiekstra, S.W.; Galbiati, V.; Fuchs, H.W.; Degeorge, G.; Troese, M.; Hayden, P.; Deng, W.; Roggen, E. An epidermal equivalent assay for identification and ranking potency of contact sensitizers. Toxicol. Appl. Pharmacol. 2013, 272, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Quan, H.; Jung, D.; Ravi, G.; Cho, A.; Kang, M.J.; Kim, E.; Che, J.-H.; Lee, E.-S.; Jeong, T.C.; et al. Intra- and inter-laboratory reproducibility and predictivity of the HaCaSens assay: A skin sensitization test using human keratinocytes, HaCaT. Toxicol. Vitr. 2018, 46, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.; Kim, M.O.; Kim, Y.; Han, H.; Yun, J.-H.; Kim, J.; Huang, Y.; Choi, Y.; Cho, C.-H.; Kang, B.-C.; et al. Optimization and validation of a method to identify skin sensitization hazards using IL-1 α and IL-6 secretion from HaCaT. Toxicol. Vitr. 2019, 61, 104589. [Google Scholar] [CrossRef]
- Och, F.M.M.V.; Loveren, H.V.; Wolfswinkel, J.C.V.; Machielsen, A.J.C.; Vandebriel, R.J. Assessment of potency of allergenic activity of low molecular weight compounds based on IL-1α and IL-18 production by a murine and human keratinocyte cell line. Toxicology 2005, 210, 95–109. [Google Scholar] [CrossRef]
- Son, D.; Na, Y.; Cho, W.-S.; Lee, B.-H.; Heo, Y.; Park, J.-H.; Seok, S.H. Differentiation of skin sensitizers from irritant chemicals by interleukin-1α and macrophage inflammatory protein-2 in murine keratinocytes. Toxicol. Lett. 2013, 216, 65–71. [Google Scholar] [CrossRef]
- dos Santos, G.G.; Spiekstra, S.W.; Sampat-Sardjoepersad, S.C.; Reinders, J.; Scheper, R.J.; Gibbs, S. A potential in vitro epidermal equivalent assay to determine sensitizer potency. Toxicol. Vitr. 2011, 25, 347–357. [Google Scholar] [CrossRef]
- Andres, E.; Barry, M.; Hundt, A.; Dini, C.; Corsini, E.; Gibbs, S.; Roggen, E.L.; Ferret, P. Preliminary performance data of the RHE/IL-18 assay performed on SkinEthicTM RHE for the identification of contact sensitizers. Int. J. Cosmet. Sci. 2017, 39, 121–132. [Google Scholar] [CrossRef]
- Gibbs, S.; Kosten, I.; Veldhuizen, R.; Spiekstra, S.; Corsini, E.; Roggen, E.; Rustemeyer, T.; Feilzer, A.J.; Kleverlaan, C.J. Assessment of metal sensitizer potency with the reconstructed human epidermis IL-18 assay. Toxicology 2018, 393, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Che, J.-H.; Lim, K.-M.; Chun, Y.-J.; Heo, Y.; Seok, S.H. Discrimination of skin sensitizers from non-sensitizers by interleukin-1α and interleukin-6 production on cultured human keratinocytes. J. Appl. Toxicol. 2016, 36, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Stössel, H.; Hefel, L.; Imamura, S.; Fritsch, P.; Sepp, N.T.; Schuler, G.; Romani, N. Human Cutaneous Dendritic Cells Migrate Through Dermal Lymphatic Vessels in a Skin Organ Culture Model. J. Investig. Dermatol. 1996, 106, 1293–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gober, M.D.; Gaspari, A.A. Allergic Contact Dermatitis. Dermatol. Immun. 2008, 10, 1–26. [Google Scholar] [CrossRef]
- Stępnik, M.; Arkusz, J. Molecular events associated with dendritic cells activation by contact sensitizers. Int. J. Occup. Med. Environ. Health 2003, 16, 191–199. [Google Scholar] [PubMed]
- OECD. Test No. 442E: In Vitro Skin Sensitisation [Internet]. 2022. Available online: https://www.oecd-ilibrary.org/content/publication/9789264264359-en (accessed on 30 June 2022).
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Ashikaga, T.; Yoshida, Y.; Hirota, M.; Yoneyama, K.; Itagaki, H.; Sakaguchi, H.; Miyazawa, M.; Ito, Y.; Suzuki, H.; Toyoda, H. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT): I. Optimization of the h-CLAT protocol. Toxicol. Vitr. 2006, 20, 767–773. [Google Scholar] [CrossRef]
- Python, F.; Goebel, C.; Aeby, P. Assessment of the U937 cell line for the detection of contact allergens. Toxicol. Appl. Pharmacol. 2007, 220, 113–124. [Google Scholar] [CrossRef]
- Narita, K.; Ishii, Y.; Vo, P.T.H.; Nakagawa, F.; Ogata, S.; Yamashita, K.; Kojima, H.; Itagaki, H. Improvement of human cell line activation test (h-CLAT) using short-time exposure methods for prevention of false-negative results. J. Toxicol. Sci. 2018, 43, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.; Roscoe, L.; Longmore, C.; Bailey, F.; Sim, B.; Treasure, C. Adaptation of the human Cell Line Activation Test (h-CLAT) to animal-product-free conditions. ALTEX 2018, 35, 477–488. [Google Scholar] [CrossRef]
- Marigliani, B.; Silva, J.; Balottin, L.; Silva, K.; Baptista, L.; de Campos, C.B.L.; Augusto, E.D.F.P. Adaptation of a skin sensitization assay to a chemically defined culture. Toxicol. Vitr. 2018, 57, 145–153. [Google Scholar] [CrossRef]
- Dos Santos, G.G.; Reinders, J.; Ouwehand, K.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Progress on the development of human in vitro dendritic cell based assays for assessment of the sensitizing potential of a compound. Toxicol. Appl. Pharmacol. 2009, 236, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Ayehunie, S.; Snell, M.; Child, M.; Klausner, M. A plasmacytoid dendritic cell (CD123+/CD11c−) based assay system to predict contact allergenicity of chemicals. Toxicology 2009, 264, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, H.; Spieker, J.; Gerlach, S.; Engels, U.; Pape, W.; Kolbe, L.; Schmucker, R.; Wenck, H.; Diembeck, W.; Wittern, K.-P.; et al. In Vitro detection of contact allergens: Development of an optimized protocol using human peripheral blood monocyte-derived dendritic cells. Toxicol. Vitr. 2011, 25, 315–323. [Google Scholar] [CrossRef]
- Pépin, E.; Goutet, M.; Ban, M. Murine bone marrow-derived dendritic cells as a potential in vitro model for predictive identification of chemical sensitizers. Toxicol. Lett. 2007, 175, 89–101. [Google Scholar] [CrossRef]
- Battais, F.; Huppert, C.; Langonné, I.; Muller, S.; Sponne, I. In Vitro detection of chemical allergens: An optimized assay using mouse bone marrow-derived dendritic cells. Contact Dermat. 2017, 77, 311–322. [Google Scholar] [CrossRef]
- Takahashi, T.; Kimura, Y.; Saito, R.; Nakajima, Y.; Ohmiya, Y.; Yamasaki, K.; Aiba, S. An In Vitro Test to Screen Skin Sensitizers Using a Stable THP-1–Derived IL-8 Reporter Cell Line, THP-G8. Toxicol. Sci. 2011, 124, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Nukada, Y.; Miyazawa, M.; Kosaka, N.; Ito, Y.; Sakaguchi, H.; Nishiyama, N. Production of IL-8 in THP-1 cells following contact allergen stimulation via mitogen-activated protein kinase activation or tumor necrosis factor-alpha production. J. Toxicol. Sci. 2008, 33, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Parise, C.B.; Sá-Rocha, V.M.; Moraes, J.Z. Skin sensitizer identification by IL-8 secretion and CD86 expression on THP-1 cells. Toxicol.Vitr. 2015, 30, 318–324. [Google Scholar] [CrossRef]
- Toebak, M.J.; Pohlmann, P.R.; Sampat-Sardjoepersad, S.C.; von Blomberg, B.M.E.; Bruynzeel, D.P.; Scheper, R.J.; Rustemeyer, T.; Gibbs, S. CXCL8 secretion by dendritic cells predicts contact allergens from irritants. Toxicol. Vitr. 2006, 20, 117–124. [Google Scholar] [CrossRef]
- Johansson, H.; Lindstedt, M.; Albrekt, A.-S.; Borrebaeck, C.A. A genomic biomarker signature can predict skin sensitizers using a cell-based in vitro alternative to animal tests. BMC Genom. 2011, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Gradin, R.; Johansson, A.; Adriaens, E.; Edwards, A.; Zuckerstätter, V.; Jerre, A.; Burleson, F.; Gehrke, H.; Roggen, E.L. Validation of the GARDTMskin Assay for Assessment of Chemical Skin Sensitizers: Ring Trial Results of Predictive Performance and Reproducibility. Toxicol. Sci. 2019, 170, 374–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeller, K.S.; Forreryd, A.; Lindberg, T.; Gradin, R.; Chawade, A.; Lindstedt, M. The GARD platform for potency assessment of skin sensitizing chemicals. ALTEX 2017, 34, 539–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooyberghs, J.; Schoeters, E.; Lambrechts, N.; Nelissen, I.; Witters, H.; Schoeters, G.; Heuvel, R.V.D. A cell-based in vitro alternative to identify skin sensitizers by gene expression. Toxicol. Appl. Pharmacol. 2008, 231, 103–111. [Google Scholar] [CrossRef]
- Lambrechts, N.; Vanheel, H.; Nelissen, I.; Witters, H.; Van Den Heuvel, R.; Van Tendeloo, V.; Schoeters, G.; Hooyberghs, J. Assessment of Chemical Skin-Sensitizing Potency by an In Vitro Assay Based on Human Dendritic Cells. Toxicol. Sci. 2010, 116, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.; Rosa, S.; Martins, J.; Silva, A.; Gonçalo, M.; Lopes, M.C.; Cruz, M.T. Development of an in Vitro Dendritic Cell-Based Test for Skin Sensitizer Identification. Chem. Res. Toxicol. 2013, 26, 368–378. [Google Scholar] [CrossRef]
- Nagahata, T.; Tsujino, Y.; Takayama, E.; Hikasa, H.; Satoh, A. Evaluation of skin sensitization based on interleukin-2 promoter activation in Jurkat cells. Biomed. Rep. 2022, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Schmucker, S.S.; Esser, P.R.; Traska, V.; Weber, V.; Dietz, L.; Thierse, H.-J.; Pennino, D.; Cavani, A.; Martin, S.F. Human T cell priming assay (hTCPA) for the identification of contact allergens based on naive T cells and DC-IFN-γ and TNF-α readout. Toxicol. Vitr. 2013, 27, 1180–1185. [Google Scholar] [CrossRef]
- Vocanson, M.; Achachi, A.; Mutez, V.; Tailhardat, M.; Le Varlet, B.; Rozières, A.; Fournier, P.; Nicolas, J.-F. Human T Cell Priming Assay: Depletion of Peripheral Blood Lymphocytes in CD25+ Cells Improves the In Vitro Detection of Weak Allergen-Specific T Cells. EXS 2014, 104, 89–100. [Google Scholar] [CrossRef]
- Claesson, M.H.; Dissing, S.; Tscherning, T.; Geisler, C. T-cell activation. V. Anti-major histocompatibility complex class I antibody-induced activation and clonal abortion in Jurkat T-leukaemic cells. Immunology 1993, 78, 444–448. [Google Scholar]
- Hou, F.; Xing, C.; Li, B.; Cheng, J.; Chen, W. Performance of a novel In Vitro assay for skin sensitization based on activation of T lymphocytes. ALTEX 2020, 37, 451–468. [Google Scholar] [CrossRef]
- Thélu, A.; Catoire, S.; Kerdine-Römer, S. Immune-competent in vitro co-culture models as an approach for skin sensitisation assessment. Toxicol. Vitr. 2020, 62, 104691. [Google Scholar] [CrossRef] [PubMed]
- Balszuweit, F.; Menacher, G.; Bloemeke, B.; Schmidt, A.; Worek, F.; Thiermann, H.; Steinritz, D. Development of a co-culture of keratinocytes and immune cells for in vitro investigation of cutaneous sulfur mustard toxicity. Chem. Biol. Interact. 2014, 223, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hennen, J.; Blömeke, B. Keratinocytes improve prediction of sensitization potential and potency of chemicals with THP-1 cells. ALTEX 2017, 34, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frombach, J.; Sonnenburg, A.; Krapohl, B.-D.; Zuberbier, T.; Peiser, M.; Stahlmann, R.; Schreiner, M. Lymphocyte surface markers and cytokines are suitable for detection and potency assessment of skin-sensitizing chemicals in an in vitro model of allergic contact dermatitis: The LCSA-ly. Arch. Toxicol. 2018, 92, 1495–1505. [Google Scholar] [CrossRef]
- Lee, S.; Greenstein, T.; Shi, L.; Maguire, T.; Schloss, R.; Yarmush, M. Tri-culture system for pro-hapten sensitizer identification and potency classification. Technology (Singap. World Sci.) 2018, 6, 67–74. [Google Scholar] [CrossRef]
- Karri, V.; Lidén, C.; Fyhrquist, N.; Högberg, J.; Karlsson, H.L. Impact of mono-culture vs. Co-culture of keratinocytes and monocytes on cytokine responses induced by important skin sensitizers. J. Immunotoxicol. 2021, 18, 74–84. [Google Scholar] [CrossRef]
- Eskes, C.; Hennen, J.; Schellenberger, M.T.; Hoffmann, S.; Frey, S.; Goldinger-Oggier, D.; Peter, N.; van Vliet, E.; Blömeke, B. The HaCaT/THP-1 Cocultured Activation Test (COCAT) for skin sensitization: A study of intra-lab reproducibility and predictivity. ALTEX 2019, 36, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Galbiati, V.; Maddalon, A.; Iulini, M.; Marinovich, M.; Corsini, E. Human keratinocytes and monocytes co-culture cell system: An important contribution for the study of moderate and weak sensitizers. Toxicol. Vitr. 2020, 68, 104929. [Google Scholar] [CrossRef]
- Meloni, M.; De Servi, B.; Le Varlet, B. New approach for chemical sensitizing potential assessment using THP-1 and NCTC 2544 co-culture. ALTEX 2010, 27, 90–91. [Google Scholar]
- Hennen, J.; Aeby, P.; Goebel, C.; Schettgen, T.; Oberli, A.; Kalmes, M.; Blömeke, B. Cross talk between keratinocytes and dendritic cells: Impact on the prediction of sensitization. Toxicol. Sci. 2011, 123, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.P.; Ma, P.C.; Liu, W.-D.; Zhou, W.-Q.; Tao, Y.; Zhang, M.-L.; Li, L.-J.; Chen, Z.-Y. Evaluation of the skin sensitization potential of chemicals in THP-1/keratinocyte co-cultures. Immunopharmacol. Immunotoxicol. 2012, 34, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Tsukumo, H.; Fukuda, J.; Iijima, K.; Itagaki, H. Co-Culture of THP-1 Cells and Normal Human Epidermal Keratinocytes (NHEK) for Modified Human Cell Line Activation Test (h-CLAT). Appl. Sci. 2022, 12, 6207. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiner, M.; Peiser, M.; Briechle, D.; Stahlmann, R.; Zuberbier, T.; Wanner, R. A loose-fit coculture of activated keratinocytes and dendritic cell-related cells for prediction of sensitizing potential. Allergy 2007, 62, 1419–1428. [Google Scholar] [CrossRef]
- Koppes, S.A.; Engebretsen, K.A.; Agner, T.; Angelova-Fischer, I.; Berents, T.; Brandner, J.; Brans, R.; Clausen, M.-L.; Hummler, E.; Jakasa, I.; et al. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermat. 2017, 77, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtani, T.; Mizuashi, M.; Nakagawa, S.; Sasaki, Y.; Fujimura, T.; Okuyama, R.; Aiba, S. TGF-beta1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation. Immunology 2009, 126, 485–499. [Google Scholar] [CrossRef]
- Miyazawa, M.; Ito, Y.; Yoshida, Y.; Sakaguchi, H.; Suzuki, H. Phenotypic alterations and cytokine production in THP-1 cells in response to allergens. Toxicol In Vitro 2007, 21, 428–437. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Ashikaga, T.; Miyazawa, M.; Yoshida, Y.; Ito, Y.; Yoneyama, K.; Hirota, M.; Itagaki, H.; Toyoda, H.; Suzuki, H. Development of an in Vitro skin sensitization test using human cell lines; human Cell Line Activation Test (h-CLAT). II. An inter-laboratory study of the h-CLAT. Toxicol. Vitr. 2006, 20, 774–784. [Google Scholar] [CrossRef]
- Schellenberger, M.T.; Bock, U.; Hennen, J.; Groeber-Becker, F.; Walles, H.; Blömeke, B. A coculture system composed of THP-1 cells and 3D reconstructed human epidermis to assess activation of dendritic cells by sensitizing chemicals after topical exposure. Toxicol. Vitr. 2019, 57, 62–66. [Google Scholar] [CrossRef]
- Bock, S.; Said, A.; Müller, G.; Schäfer-Korting, M.; Zoschke, C.; Weindl, G. Characterization of reconstructed human skin containing Langerhans cells to monitor molecular events in skin sensitization. Toxicol. Vitr. 2018, 46, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- OECD. Guidance Document on the Reporting of Defined Approaches and Individual Information Sources to Be Used within Integrated Approaches to Testing and Assessment (IATA) for Skin Sensitisation [Internet]. 2017. Available online: https://www.oecd-ilibrary.org/content/publication/9789264279285-en (accessed on 11 July 2017).


| Type of DA | Types of Covered KE | Methods | Prediction |
|---|---|---|---|
| 2o3 DA | KE1, KE2, KE3 | DPRA, KeratinoSensTM, h-CLAT | Hazard |
| ITSv1 | KE1, KE3 | DPRA, h-CLAT, DEREK Nexus v6.1.0 | Hazard, potency |
| ITSv2 | KE1, KE3 | DPRA, h-CLAT, OECD QSAR Toolbox v4.5 | Hazard, potency |
| Method | Cytokine | Test System | Dataset | Accuracy [%] | Specificity [%] | Sensitivity [%] | Source/ Ref. |
|---|---|---|---|---|---|---|---|
| Compared to the LLNA | |||||||
| NCTC 2544 IL-18 assay | IL-18 | 2D/ NCTC 2544 | 33 | 97 | 94.1 | 100 | [105] |
| HaCaT IL-18 assay | IL-18 | 2D/HaCaT | 41 * | 57.2 | 91.7 | 22.2 | [104] |
| IL-18 EE potency assay | IL-18 | 3D/ RhE | 27 | 95 | - | - | [106] |
| HaCaSens | IL-1α, IL-6 | 2D/HaCaT | 20 | 83.3 | 87.5 | 81.8 | [107] |
| Optimized HaCaSens | IL-1α IL-6 | 2D/HaCaT | 22 | 81.8 | 80 | 83.3 | [108] |
| HaCaT/HEL30 assay | IL-1α IL-18 | 2D/ HEL-30/HaCaT | 4 | - | - | - | [109] |
| HEL-30 IL-1α, MIP-2 | IL-1α MIP-2 | 2D/HEL-30 | 22 | 86 | - | - | [110] |
| IL-1α EE potency assay | IL-1α | 3D/RhE | 16 | - | - | - | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gądarowska, D.; Kalka, J.; Daniel-Wójcik, A.; Mrzyk, I. Alternative Methods for Skin-Sensitization Assessment. Toxics 2022, 10, 740. https://doi.org/10.3390/toxics10120740
Gądarowska D, Kalka J, Daniel-Wójcik A, Mrzyk I. Alternative Methods for Skin-Sensitization Assessment. Toxics. 2022; 10(12):740. https://doi.org/10.3390/toxics10120740
Chicago/Turabian StyleGądarowska, Dominika, Joanna Kalka, Anna Daniel-Wójcik, and Inga Mrzyk. 2022. "Alternative Methods for Skin-Sensitization Assessment" Toxics 10, no. 12: 740. https://doi.org/10.3390/toxics10120740