Ambient Benzo[a]pyrene’s Effect on Kinetic Modulation of Amyloid Beta Peptide Aggregation: A Tentative Association between Ultrafine Particulate Matter and Alzheimer’s Disease
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.; Kim, W.-H.; Kim, Y.-Y.; Park, H.-Y. Air Pollution and Central Nervous System Disease: A Review of the Impact of Fine Particulate Matter on Neurological Disorders. Front. Public Health 2020, 8, 575330. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, S.; D’Angiulli, A. How air pollution alters brain development: The role of neuroinflammation. Transl. Neurosci. 2016, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.-C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Danti, S.; Carlesi, C.; Borin, G. Danger in the Air: Air Pollution and Cognitive Dysfunction. Am. J. Alzheimer’s Dis. Other Dementiasr. 2018, 33, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Heusinkveld, H.J.; Wahle, T.; Campbell, A.; Westerink, R.H.S.; Tran, L.; Johnston, H.; Stone, V.; Cassee, F.R.; Schins, R.P.F. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 2016, 56, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Mathis, C.A.; Wang, Y.; Klunk, W.E. Imaging beta-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr. Pharm. Des. 2004, 10, 1469–1492. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, F.; Zhang, S.; Xin, R.; Sun, Y. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. NPJ Park. Dis. 2021, 7, 70. [Google Scholar] [CrossRef]
- Cho, J.; Sohn, J.; Noh, J.; Jang, H.; Kim, W.; Cho, S.-K.; Seo, H.; Seo, G.; Lee, S.-K.; Noh, Y.; et al. Association between exposure to polycyclic aromatic hydrocarbons and brain cortical thinning: The Environmental Pollution-Induced Neurological EFfects (EPINEF) study. Sci. Total Environ. 2020, 737, 140097. [Google Scholar] [CrossRef]
- Gianelle, V.; Colombi, C.; Caserini, S.; Ozgen, S.; Galante, S.; Marongiu, A.; Lanzani, G. Benzo(a)pyrene air concentrations and emission inventory in Lombardy region, Italy. Atmos. Pollut. Res. 2013, 4, 257–266. [Google Scholar] [CrossRef]
- Schreiberová, M.; Vlasáková, L.; Vlček, O.; Šmejdířová, J.; Horálek, J.; Bieser, J. Benzo[a]pyrene in the Ambient Air in the Czech Republic: Emission Sources, Current and Long-Term Monitoring Analysis and Human Exposure. Atmosphere 2020, 11, 955. [Google Scholar] [CrossRef]
- Yu, Q.; Ding, X.; He, Q.; Yang, W.; Zhu, M.; Li, S.; Zhang, R.; Shen, R.; Zhang, Y.; Bi, X.; et al. Nationwide increase of polycyclic aromatic hydrocarbons in ultrafine particles during winter over China revealed by size-segregated measurements. Atmos. Chem. Phys. 2020, 20, 14581–14595. [Google Scholar] [CrossRef]
- Leikauf, G.D.; Kim, S.-H.; Jang, A.-S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, Y.; Jing, G.; Li, K.; Zhao, Y.; Sha, B.; Wang, Q.; Wu, D. Rapid and efficient crossing blood-brain barrier: Hydrophobic drug delivery system based on propionylated amylose helix nanoclusters. Biomaterials 2017, 113, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Wirnkor, V.A.; Ngozi, V.E.; Ajero, C.M.; Charity, L.K.; Ngozi, O.S.; Ebere, E.C.; Emeka, A.C. Biomonitoring of concentrations of polycyclic aromatic hydrocarbons in blood and urine of children at playgrounds within Owerri, Imo State, Nigeria. Environ. Anal. Health Toxicol. 2019, 34, e2019011. [Google Scholar] [CrossRef] [Green Version]
- Niu, Q.; Zhang, H.; Li, X.; Li, M. Benzo[a]pyrene-induced neurobehavioral function and neurotransmitter alterations in coke oven workers. Occup. Environ. Med. 2009, 67, 444–448. [Google Scholar] [CrossRef]
- De, S.; Whiten, D.R.; Ruggeri, F.S.; Hughes, C.; Rodrigues, M.; Sideris, D.I.; Taylor, C.G.; Aprile, F.A.; Muyldermans, S.; Knowles, T.P.J.; et al. Soluble aggregates present in cerebrospinal fluid change in size and mechanism of toxicity during Alzheimer’s disease progression. Acta Neuropathol. Commun. 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- I Sideris, D.; Danial, J.S.H.; Emin, D.; Ruggeri, F.S.; Xia, Z.; Zhang, Y.P.; Lobanova, E.; Dakin, H.; De, S.; Miller, A.; et al. Soluble amyloid beta-containing aggregates are present throughout the brain at early stages of Alzheimer’s disease. Brain Commun. 2021, 3, fcab147. [Google Scholar] [CrossRef]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Walsh, D.M.; Selkoe, D.J. A beta oligomers—A decade of discovery. J. Neurochem. 2007, 101, 1172–1184. [Google Scholar] [CrossRef]
- Hölttä, M.; Hansson, O.; Andreasson, U.; Hertze, J.; Minthon, L.; Nägga, K.; Andreasen, N.; Zetterberg, H.; Blennow, K. Evaluating Amyloid-β Oligomers in Cerebrospinal Fluid as a Biomarker for Alzheimer’s Disease. PLoS ONE 2013, 8, e66381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, M.K.; Cappai, R.; Pham, C.L.L.; Ciccotosto, G.D. Membrane-bound tetramer and trimer Aβ oligomeric species correlate with toxicity towards cultured neurons. J. Neurochem. 2016, 136, 594–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Chen, W.; Wang, Y. Beta-Amyloid: The key peptide in the pathogenesis of Alzheimer’s disease. Front. Pharmacol. 2015, 6, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauk, A. Why is the amyloid beta peptide of Alzheimer’s disease neurotoxic? Dalton Trans. 2008, 10, 1273–1282. [Google Scholar] [CrossRef]
- Kepp, K.P. Bioinorganic Chemistry of Alzheimer’s Disease. Chem. Rev. 2012, 112, 5193–5239. [Google Scholar] [CrossRef] [Green Version]
- Kaumbekova, S.; Torkmahalleh, M.A.; Sakaguchi, N.; Umezawa, M.; Shah, D. Effect of ambient polycyclic aromatic hydrocarbons and nicotine on the structure of Aβ42 protein. Front. Environ. Sci. Eng. 2022, 17, 15. [Google Scholar] [CrossRef]
- Wallin, C.; Sholts, S.B.; Österlund, N.; Luo, J.; Jarvet, J.; Roos, P.M.; Ilag, L.; Gräslund, A.; Wärmländer, S.K. Alzheimer’s disease and cigarette smoke components: Effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-β peptide aggregation. Sci. Rep. 2017, 7, 14423. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Wu, M.; Wang, C.; Wang, Y.; Zuo, Z. Chronic exposure to low benzo[a]pyrene level causes neurodegenerative disease-like syndromes in zebrafish (Danio rerio). Aquat. Toxicol. 2015, 167, 200–208. [Google Scholar] [CrossRef]
- Gao, D.; Wang, C.; Xi, Z.; Zhou, Y.; Wang, Y.; Zuo, Z. Early-Life Benzo[a]Pyrene Exposure Causes Neurodegenerative Syndromes in Adult Zebrafish (Danio rerio) and the Mechanism Involved. Toxicol. Sci. 2017, 157, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhao, Y.; Qi, Y.; Gao, Y.; Tu, D.; Wang, Y.; Gao, H.-M.; Zhou, H. Benzo(a)pyrene exposure induced neuronal loss, plaque deposition, and cognitive decline in APP/PS1 mice. J. Neuroinflammation 2020, 17, 258. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Pall, S.; Smith, J.C.; Hess, B.; Lindahl, E. Gromacs: High performance molecular simulations through multi-level parallelism from laprtops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Gerben, S.; Lemkul, J.; Brown, A.M.; Bevan, D. Comparing atomistic molecular mechanics force fields for a difficult target: A case study on the Alzheimer’s amyloid β-peptide. J. Biomol. Struct. Dyn. 2013, 32, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.; Fraaije, J.G. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Brown, A.M.; Bevan, D.R. Molecular Dynamics Simulations of Amyloid β -Peptide (1-42): Tetramer Formation and Membrane Interactions. Biophys. J. 2016, 111, 937–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Östersund, N.; Kulkarni, Y.S.; Misiaszek, A.D.; Wallin, C.; Krüger, D.M.; Liao, Q.; Mashayekhy Rad, F.; Jarvet, J.; Strodel, B.; Wärmländer, S.K.; et al. Amyloid-β Peptide Interactions with Amphiphilic Surfactants: Electrostatic and Hydrophobic Effects. ACS Chem. Neurosci. 2018, 9, 1680–1692. [Google Scholar] [CrossRef]
- Berhanu, W.M.; Hansmann, U.H.E. Structure and Dynamics of Amyloid-β Segmental Polymorphisms. PLoS ONE 2012, 7, e41479. [Google Scholar] [CrossRef] [Green Version]
- Jokar, S.; Erfani, M.; Bavi, O.; Khazaei, S.; Sharifzadeh, M.; Hajiramezanali, M.; Beiki, D.; Shamloo, A. Design of peptide-based inhibitor agent against amyloid-β aggregation: Molecular docking, synthesis and in vitro evaluation. Bioorganic Chem. 2020, 102, 104050. [Google Scholar] [CrossRef]
- Grasso, G.; Lionello, C.; Stojceski, F. Highlighting the effect of amyloid beta assemblies on the mechanical properties and conformational stability of cell membrane. J. Mol. Graph. Model. 2020, 100, 107670. [Google Scholar] [CrossRef]
- Paul, A.; Samantray, S.; Anteghini, M.; Khaled, M.; Strodel, B. Thermodynamics and kinetics of the amyloid-β peptide revealed by Markov state models based on MD data in agreement with experiment. Chem. Sci. 2021, 12, 6652–6669. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, N.T.; Derreumaux, P.; Vu, V.V.; Nam, P.C.; Ngo, S.T. C-Terminal Plays as the Possible Nucleation of the Self-Aggregation of the S-Shape Aβ. ACS Omega 2019, 4, 11066–11073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.L.; Krupa, P.; Hai, N.M.; Linh, H.Q.; Li, M.S. Structure and Physicochemical Properties of the Aβ42 Tetramer: Multiscale Molecular Dynamics Simulations. J. Phys. Chem. B 2019, 123, 7253–7269. [Google Scholar] [CrossRef]
- Fatafta, H.; Khaled, M.; Owen, M.C.; Sayyed-Ahmad, A.; Strodel, B. Amyloid-β peptide dimers undergo a random coil to β-sheet transition in the aqueous phase but not at the neuronal membrane. Proc. Natl. Acad. Sci. USA 2021, 118, e2106210118. [Google Scholar] [CrossRef]
- Aitken, J.F.; Loomes, K.M.; Konarkowska, B.; Cooper, G.J.S. Suppression by polycyclic compounds of the conversion of human amylin into insoluble amyloid. Biochem. J. 2003, 374 Pt 3, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Tang, Q.; Xiong, Y.; Qing, G.; Sun, T. Protein/Peptide Aggregation and Amyloidosis on Biointerfaces. Materials 2016, 9, 740. [Google Scholar] [CrossRef]
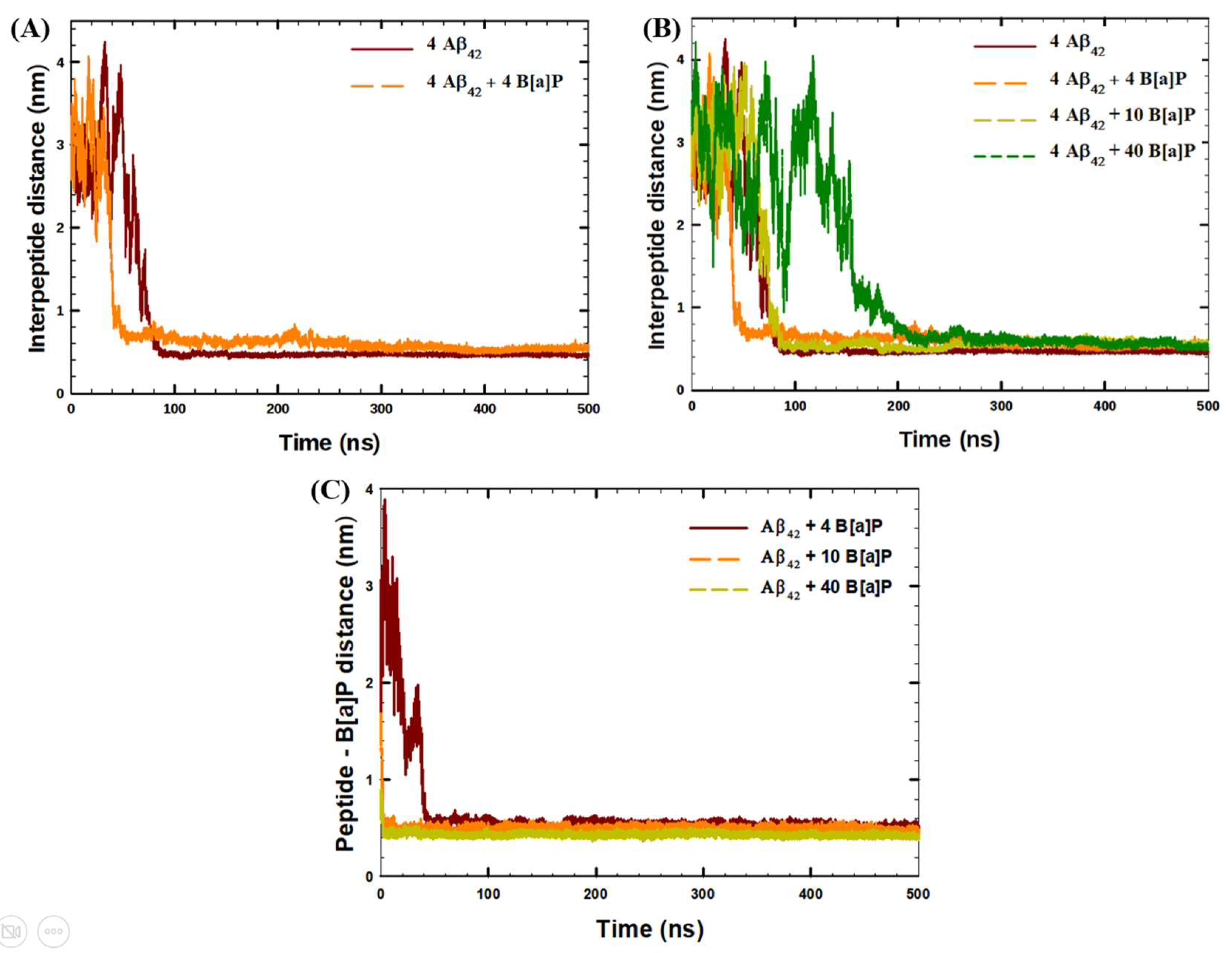
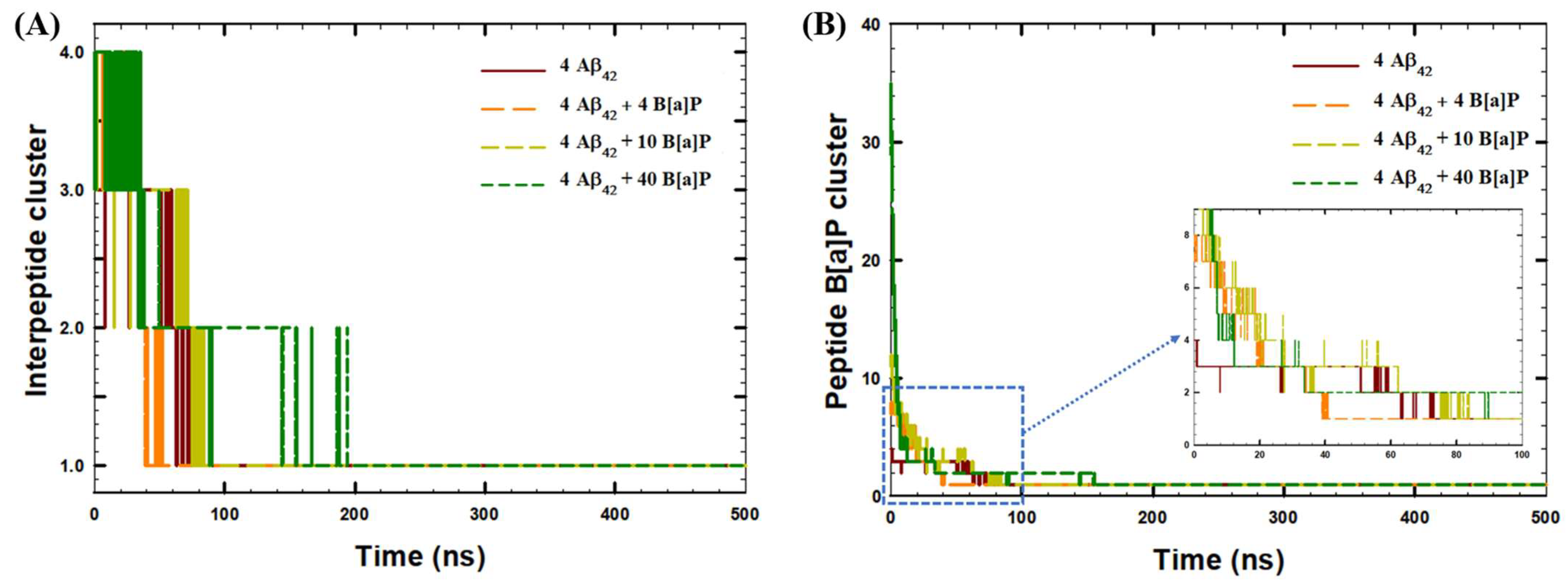
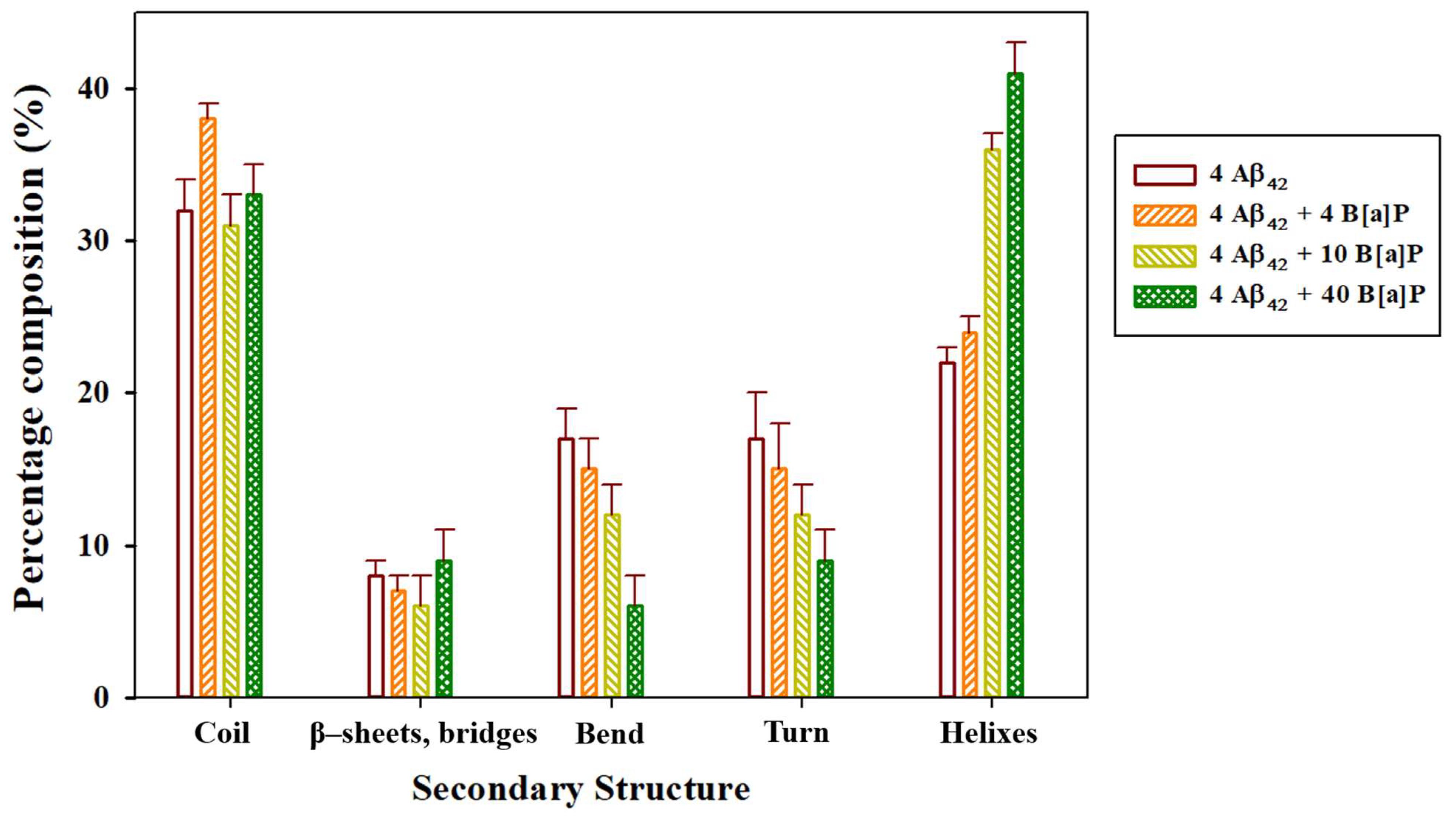
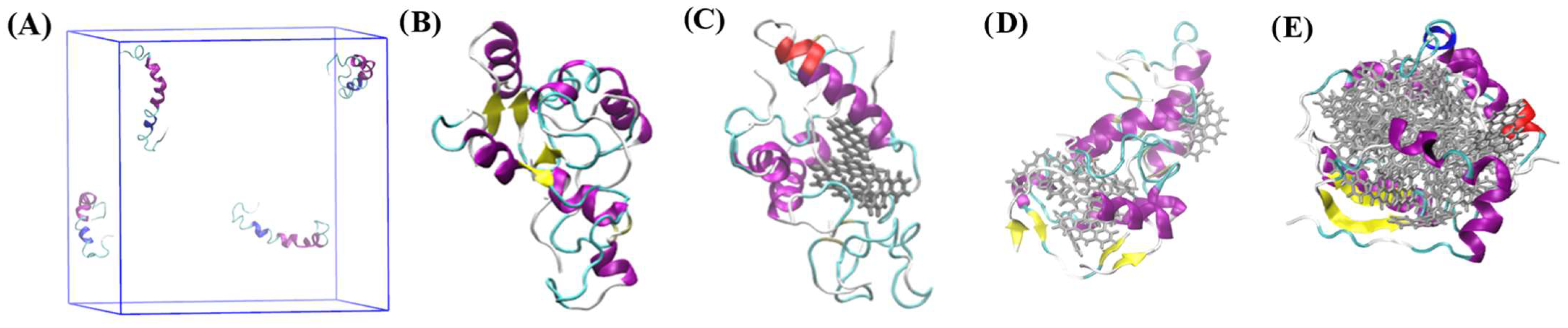


| System | Aβ42 | B[a]P | B[a]P Concentration | H2O | Na+ | Cl− |
|---|---|---|---|---|---|---|
| 4 Aβ42 | 4 | 0 | 0 mM | 42,296 | 128 | 120 |
| 4 Aβ42 + 4 B[a]P | 4 | 4 | 5.00 mM | 42,270 | 128 | 120 |
| 4 Aβ42 + 10 B[a]P | 4 | 10 | 12.5 mM | 42,179 | 128 | 120 |
| 4 Aβ42 + 40 B[a]P | 4 | 40 | 50.0 mM | 41,798 | 128 | 120 |
| SASA0 ns (nm2) | SASA500 ns (nm2) | SASAmin (nm2) | H-Bonds (Last 30 ns) | |
|---|---|---|---|---|
| 4 Aβ42 | 171.6 | 94.60 | 87.30 | 115 ± 5 |
| 4 Aβ42 + 4 B[a]P | 169.0 | 102.7 | 95.10 | 119 ± 5 |
| 4 Aβ42 + 10 B[a]P | 169.6 | 110.0 | 101.5 | 113 ± 5 |
| 4 Aβ42 + 40 B[a]P | 166.5 | 139.2 | 132.7 | 109 ± 5 |
| System | Peptide–Peptide (kJ/mol) | Peptide–B[a]P (kJ/mol) | ||||
|---|---|---|---|---|---|---|
| Coul–SR | LJ–SR | Coul–LR | LJ–LR | Coul–SR | LJ–SR | |
| 4 Aβ42 | −44,048 ± 26 | −3712 ± 11 | 27,804 ± 8 | −38.5 ± 3.4 | - | - |
| 4 Aβ42 + 4 B[a]P | −44,333 ± 10 | −3577 ± 7 | 28,052 ± 12 | −44.1 ± 1.9 | −49.2 ± 2.2 | −423 ± 2 |
| 4 Aβ42 + 10 B[a]P | −43,963 ± 40 | −3536 ± 9 | 28,042 ± 7 | −7.9 ± 3 | −60.6 ± 2.8 | −762 ± 16 |
| 4 Aβ42 + 40 B[a]P | −43,945 ± 22 | −3016 ± 7 | 28,043 ± 6 | 9.8 ± 3 | −191.7 ± 3 | −2020 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaumbekova, S.; Torkmahalleh, M.A.; Shah, D. Ambient Benzo[a]pyrene’s Effect on Kinetic Modulation of Amyloid Beta Peptide Aggregation: A Tentative Association between Ultrafine Particulate Matter and Alzheimer’s Disease. Toxics 2022, 10, 786. https://doi.org/10.3390/toxics10120786
Kaumbekova S, Torkmahalleh MA, Shah D. Ambient Benzo[a]pyrene’s Effect on Kinetic Modulation of Amyloid Beta Peptide Aggregation: A Tentative Association between Ultrafine Particulate Matter and Alzheimer’s Disease. Toxics. 2022; 10(12):786. https://doi.org/10.3390/toxics10120786
Chicago/Turabian StyleKaumbekova, Samal, Mehdi Amouei Torkmahalleh, and Dhawal Shah. 2022. "Ambient Benzo[a]pyrene’s Effect on Kinetic Modulation of Amyloid Beta Peptide Aggregation: A Tentative Association between Ultrafine Particulate Matter and Alzheimer’s Disease" Toxics 10, no. 12: 786. https://doi.org/10.3390/toxics10120786






