T Cells Contribute to Pathological Responses in the Non-Targeted Rat Heart following Irradiation of the Kidneys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compliance with Design and Statistical Analysis Requirements
2.2. Experimental Animals
2.3. Generation of T Cell Knock down Rat on the WAG/RijCmcr Genetic Background
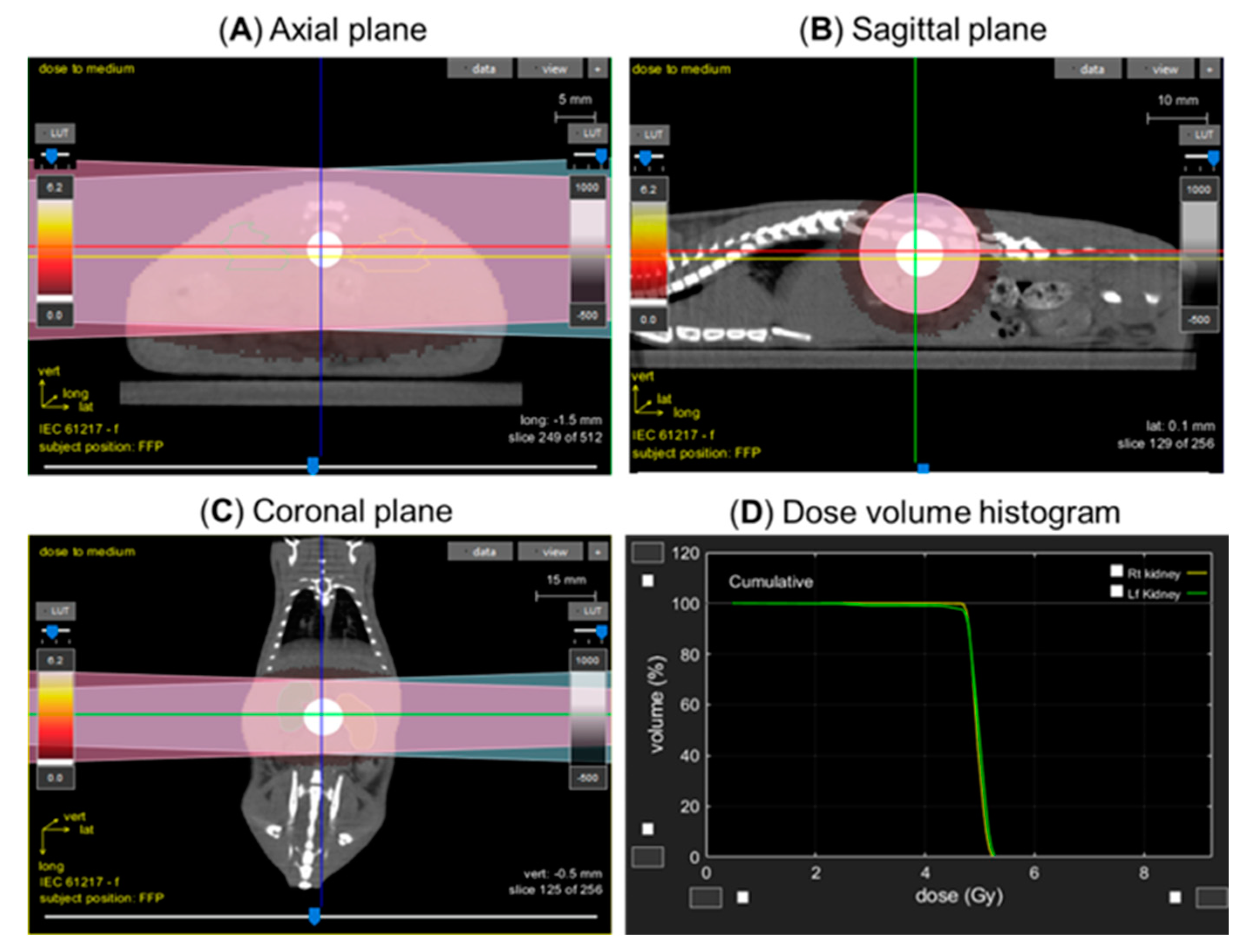
2.4. Lateral Irradiation
2.5. Irradiator and Dosimetry
2.6. Kidney Injury Measurement
2.7. Immune Cell Isolation and Flow Cytometry
2.8. Histology
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
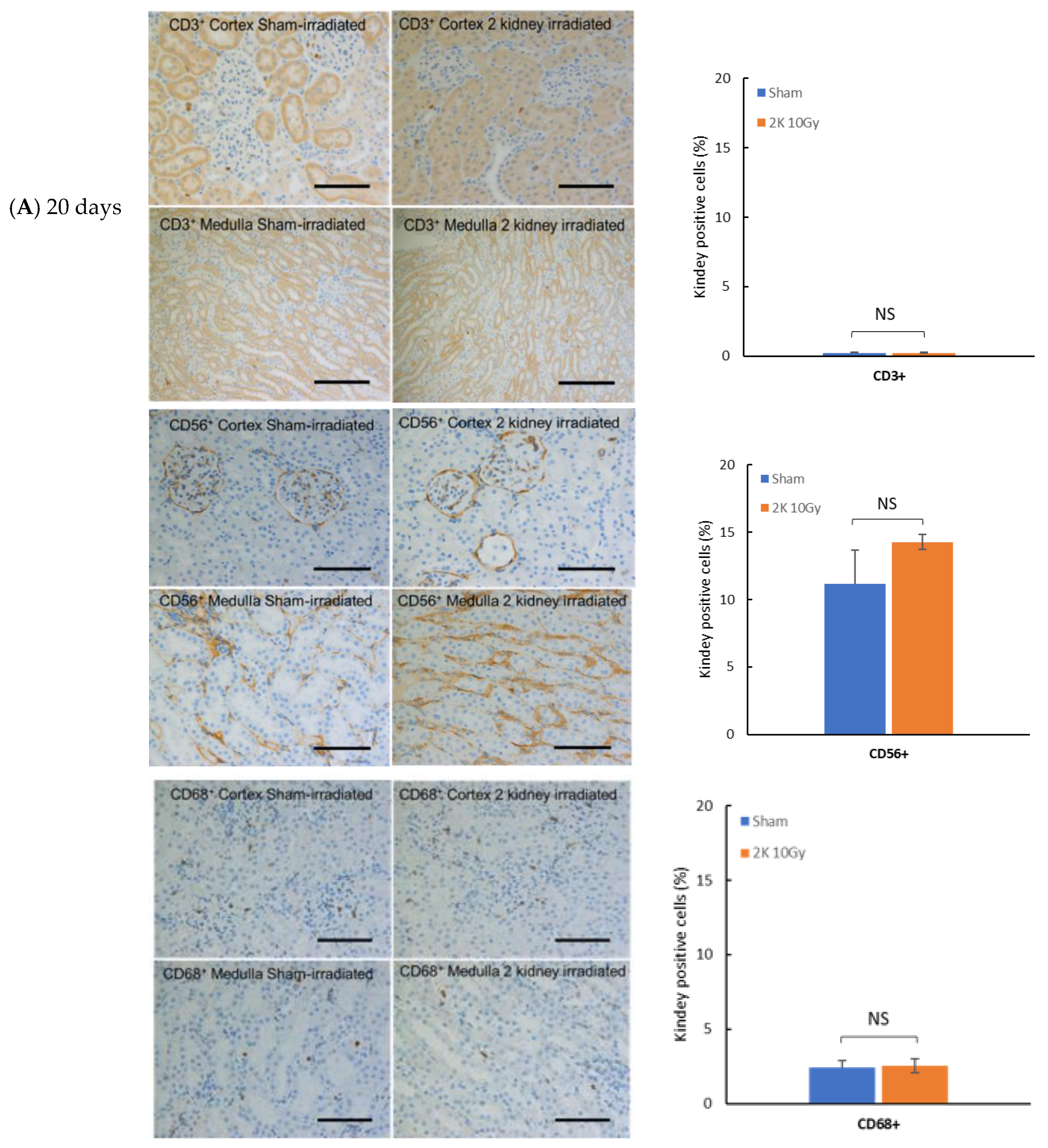
3.1. Immune Cell Infiltration in Kidney after Targeted Irradiation Is a Late Response
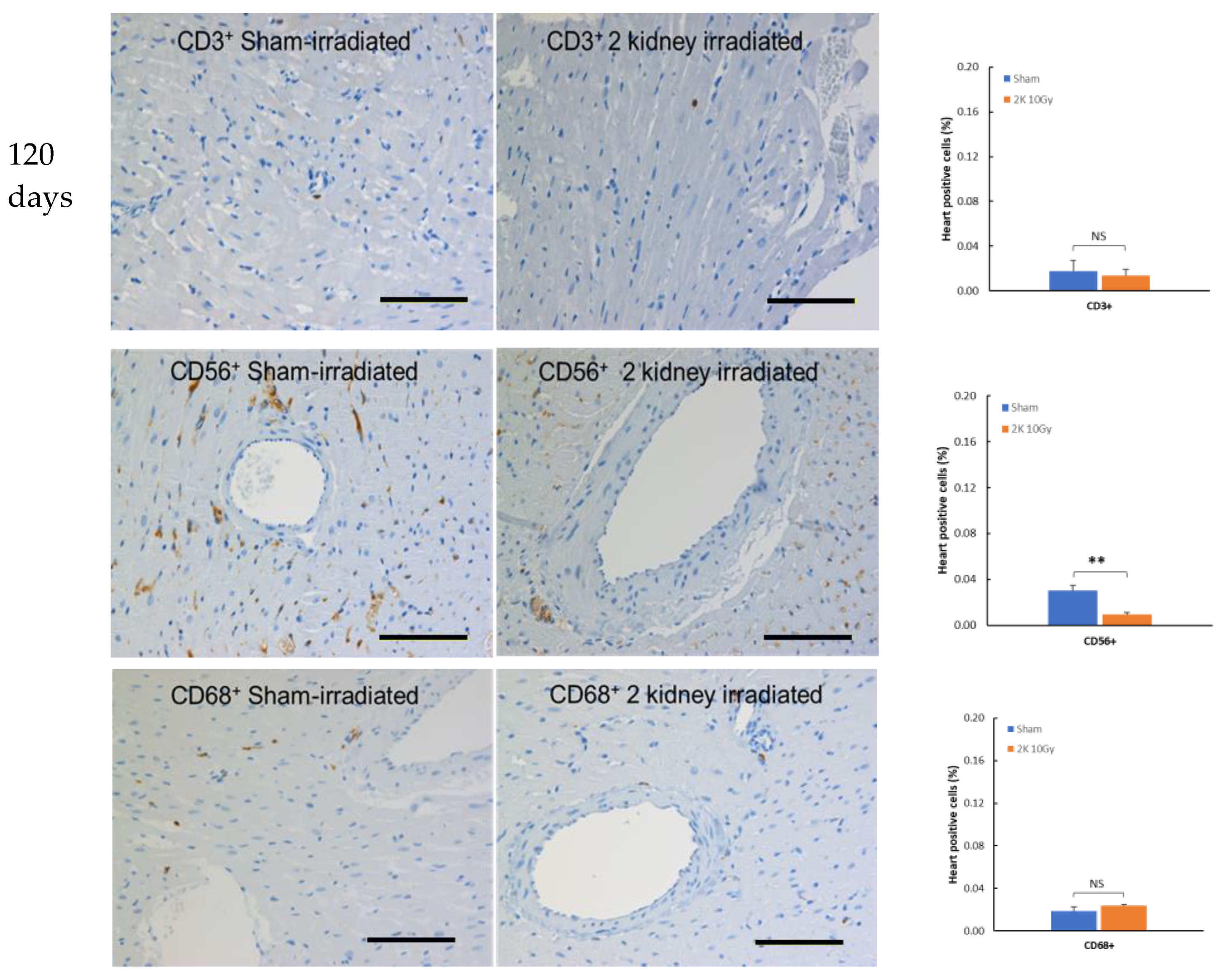
3.2. Immune Cells Do Not Infiltrate the Heart after Kidney Irradiation
3.3. Confirmation of the T Cell Knock down in the WAGCD247-/- Rat
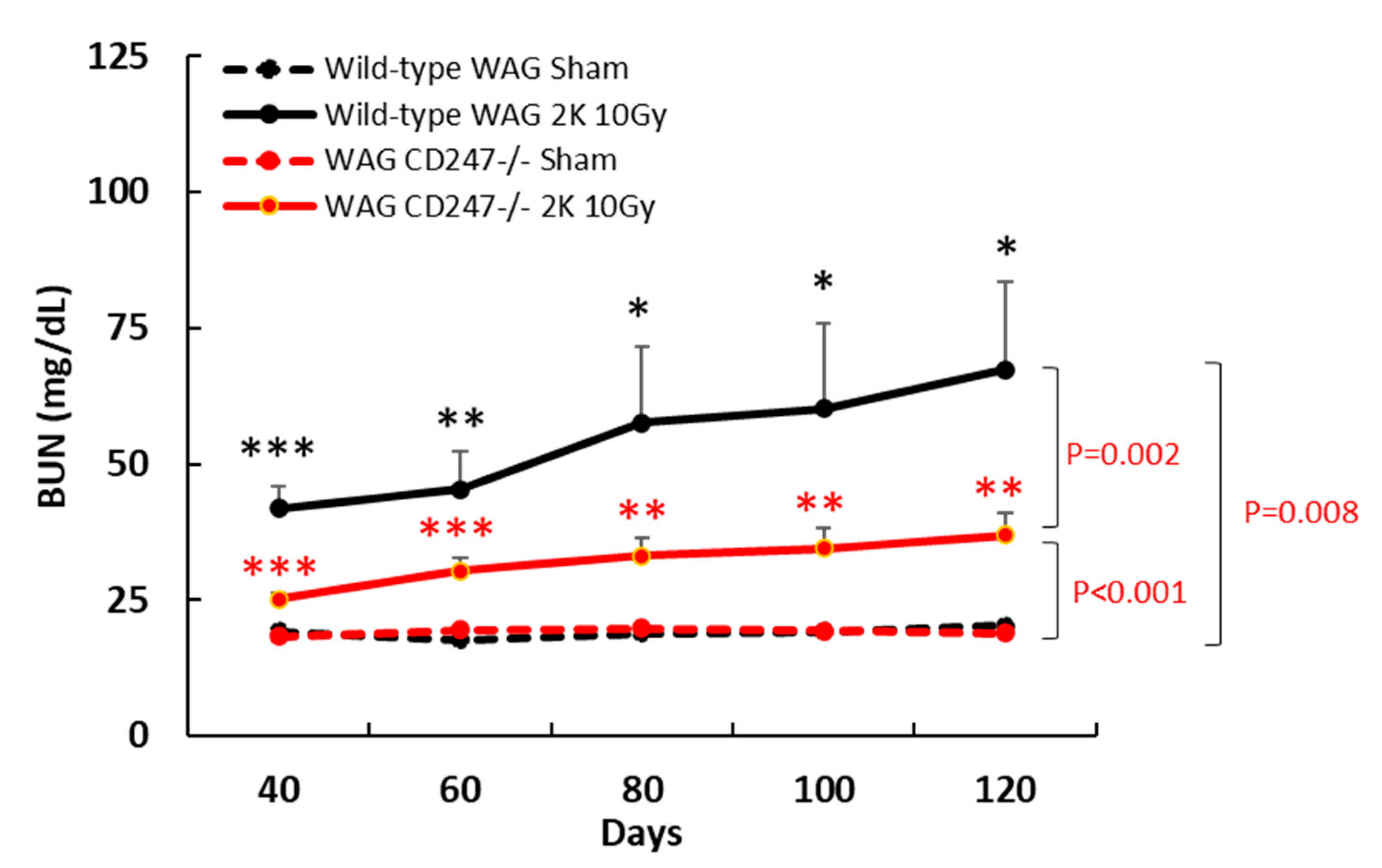
3.4. Genetic Knock down of T Cells Decreases Radiation Nephropathy in WAG Rats
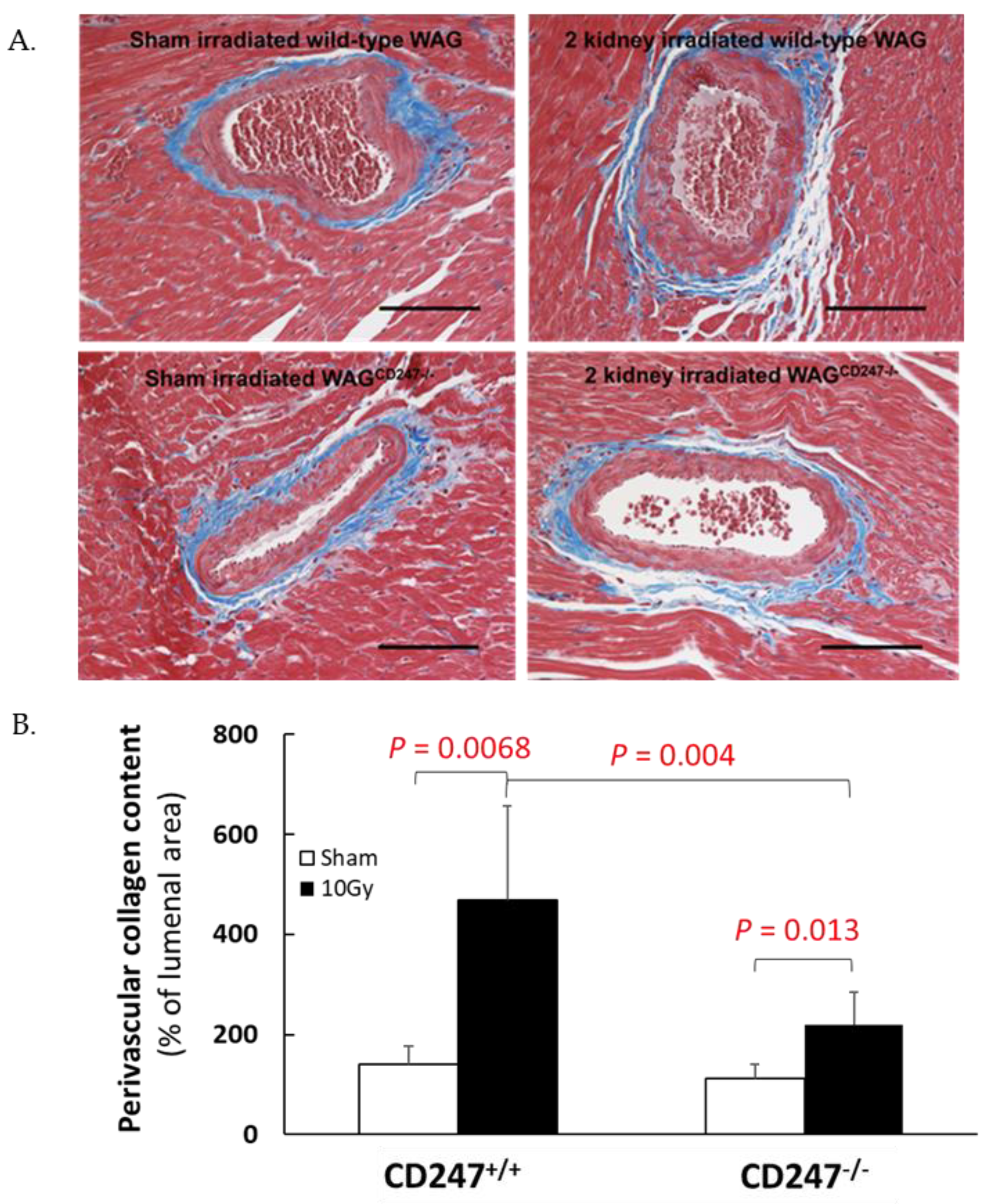
3.5. Genetic Knock down of T Cells Decreases Pathological Responses in the Non-Targeted Heart following Irradiation of the Kidneys in WAG Rats
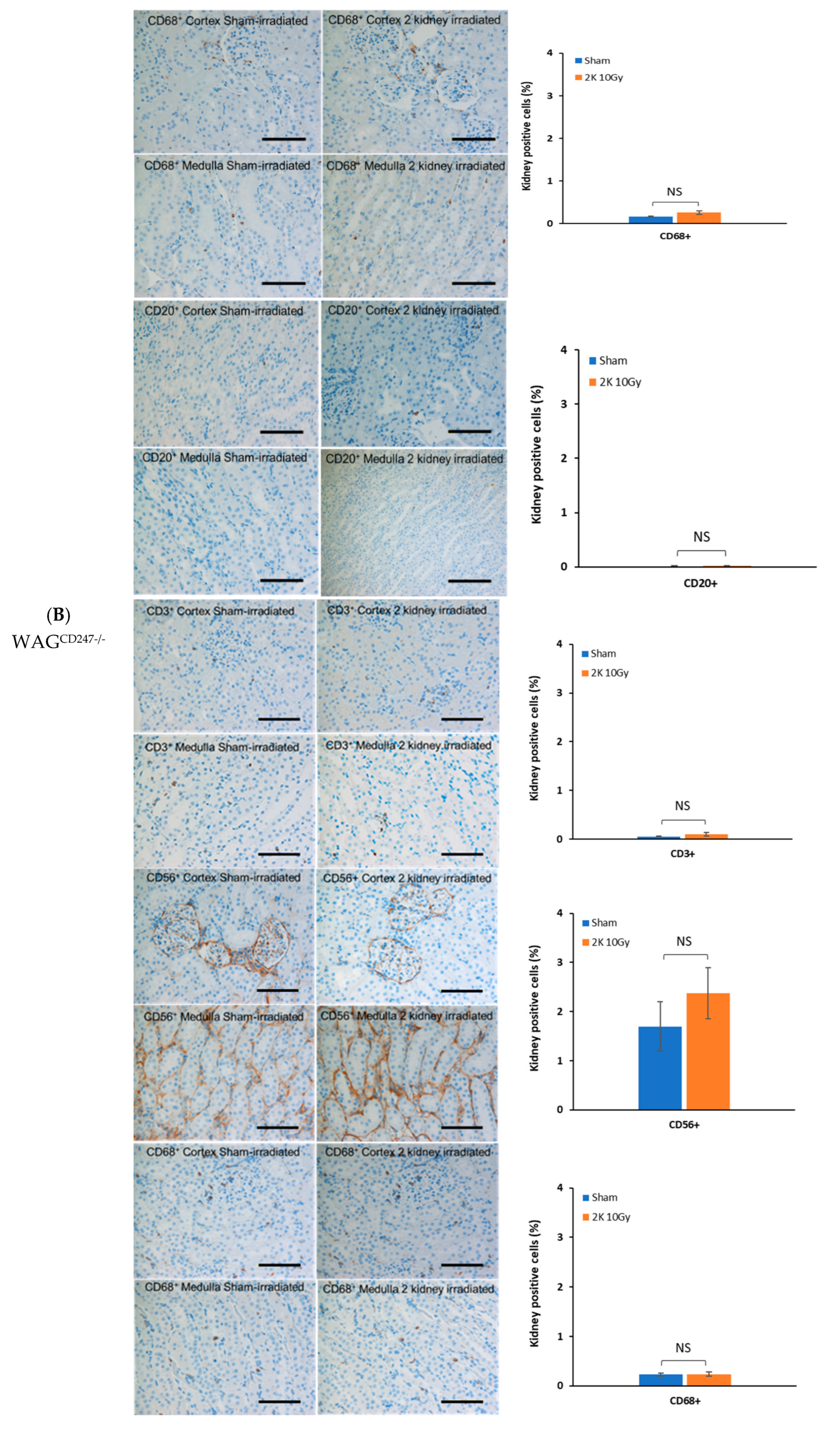
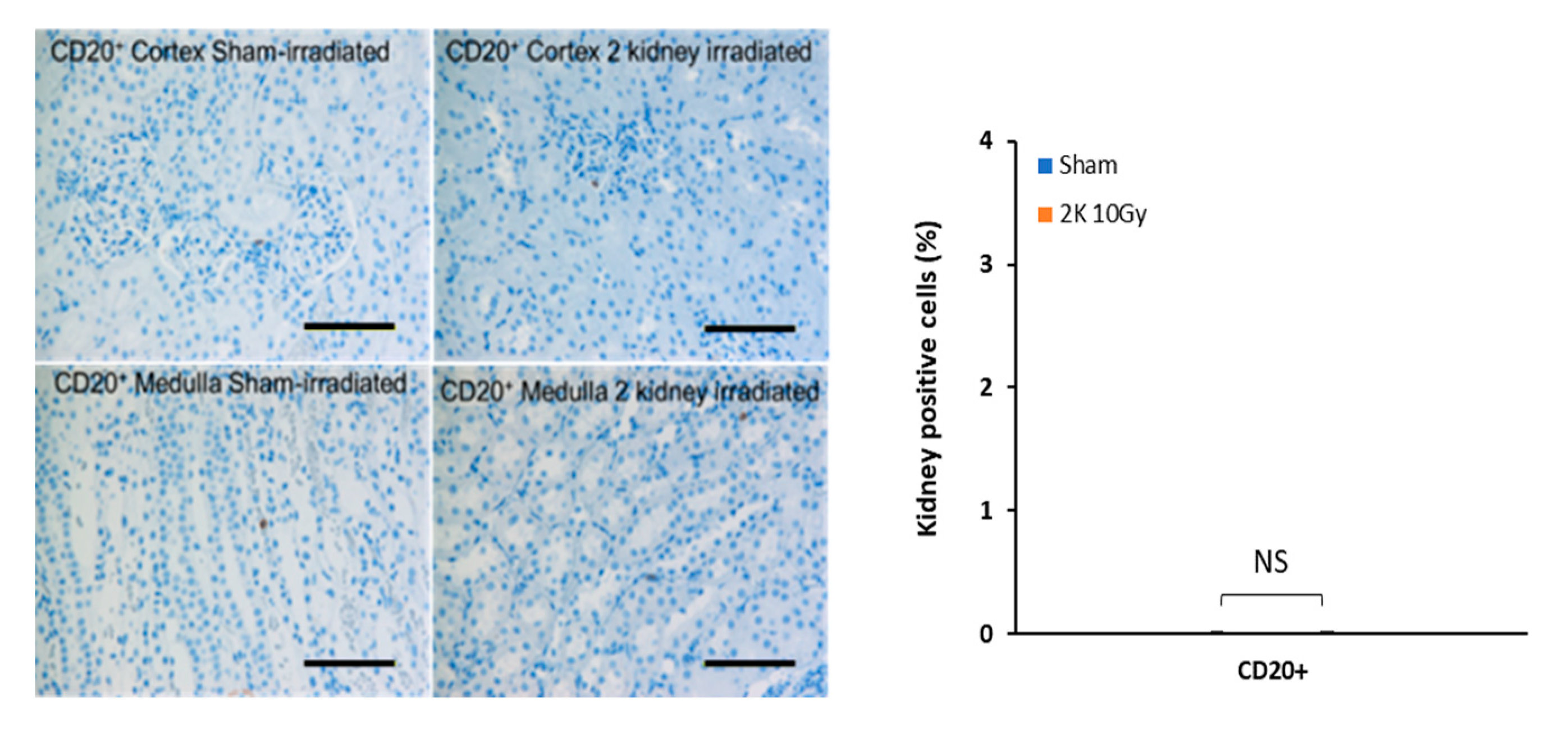
3.6. Early Radiation Nephropathy Is Not Associated with Engagement of Immune Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef] [PubMed]
- Lenarczyk, M.; Lam, V.; Jensen, E.; Fish, B.L.; Su, J.; Koprowski, S.; Komorowski, R.A.; Harmann, L.; Migrino, R.Q.; Li, X.A.; et al. Cardiac injury after 10 gy total body irradiation: Indirect role of effects on abdominal organs. Radiat Res 2013, 180, 247–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, W.F.; Sowa, M.B. Non-targeted effects induced by ionizing radiation: Mechanisms and potential impact on radiation induced health effects. Cancer Lett. 2015, 356, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenarczyk, M.; Laiakis, E.C.; Mattson, D.L.; Johnson, B.D.; Kronenberg, A.; North, P.E.; Komorowski, R.; Mäder, M.; Baker, J.E. Irradiation of the kidneys causes pathologic remodeling in the nontargeted heart: A role for the immune system. FASEB BioAdvances 2020, 2, 705–719. [Google Scholar] [CrossRef]
- Stewart, B.J.; Ferdinand, J.R.; Young, M.D.; Mitchell, T.J.; Loudon, K.W.; Riding, A.M.; Richoz, N.; Frazer, G.L.; Staniforth, J.U.; Vieira Braga, F.A. Spatiotemporal immune zonation of the human kidney. Science 2019, 365, 1461–1466. [Google Scholar] [CrossRef]
- Singh, N.; Avigan, Z.M.; Kliegel, J.A.; Shuch, B.M.; Montgomery, R.R.; Moeckel, G.W.; Cantley, L.G. Development of a 2-dimensional atlas of the human kidney with imaging mass cytometry. JCI Insight 2019, 4, e129477. [Google Scholar] [CrossRef] [Green Version]
- Rudemiller, N.; Lund, H.; Jacob, H.J.; Geurts, A.M.; Mattson, D.L. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 2014, 63, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.E.; Fish, B.L.; Su, J.; Haworth, S.T.; Strande, J.L.; Komorowski, R.A.; Migrino, R.Q.; Doppalapudi, A.; Harmann, L.; Allen Li, X. 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int. J. Radiat. Biol. 2009, 85, 1089–1100. [Google Scholar] [CrossRef]
- Abais-Battad, J.M.; Alsheikh, A.J.; Pan, X.; Fehrenbach, D.J.; Dasinger, J.H.; Lund, H.; Roberts, M.L.; Kriegel, A.J.; Cowley Jr, A.W.; Kidambi, S. Dietary effects on Dahl salt-sensitive hypertension, renal damage, and the T lymphocyte transcriptome. Hypertension 2019, 74, 854–863. [Google Scholar] [CrossRef]
- Alsheikh, A.J.; Lund, H.; Dasinger, J.H.; Abais-Battad, J.M.; Fehrenbach, D.J.; Mattson, D.L. Renal nerves and leukocyte infiltration in the kidney during salt-sensitive hypertension. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2019, 317, R182–R189. [Google Scholar] [CrossRef]
- Ward, J.; Rehg, J. Rodent immunohistochemistry: Pitfalls and troubleshooting. Vet. Pathol. 2014, 51, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- McBride, W.H.; Chiang, C.S.; Olson, J.L.; Wang, C.C.; Hong, J.H.; Pajonk, F.; Dougherty, G.J.; Iwamoto, K.S.; Pervan, M.; Liao, Y.P. A sense of danger from radiation. Radiat Res 2004, 162, 1–19. [Google Scholar] [CrossRef]
- Purbey, P.K.; Scumpia, P.O.; Kim, P.J.; Tong, A.J.; Iwamoto, K.S.; McBride, W.H.; Smale, S.T. Defined Sensing Mechanisms and Signaling Pathways Contribute to the Global Inflammatory Gene Expression Output Elicited by Ionizing Radiation. Immunity 2017, 47, 421–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, S.M.; Benci, J.L.; Irianto, J.; Discher, D.E.; Minn, A.J.; Greenberg, R.A. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017, 548, 466. Available online: https://www.nature.com/articles/nature23470#supplementary-information (accessed on 2 August 2022). [CrossRef] [PubMed] [Green Version]
- Menendez, D.; Shatz, M.; Azzam, K.; Garantziotis, S.; Fessler, M.B.; Resnick, M.A. The Toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet 2011, 7, e1001360. [Google Scholar] [CrossRef] [Green Version]
- Montecino-Rodriguez, E.; Berent-Maoz, B.; Dorshkind, K. Causes, consequences, and reversal of immune system aging. J. Clin. Investig. 2013, 123, 958–965. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.E.; Moulder, J.E.; Hopewell, J.W. Radiation as a risk factor for cardiovascular disease. Antioxid Redox Signal 2011, 15, 1945–1956. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Moreira, S.; Moreno, S.G.; Ghinatti, G.; Lewandowski, D.; Hoffschir, F.; Ferri, F.; Gallouet, A.S.; Gay, D.; Motohashi, H.; Yamamoto, M.; et al. Low-Dose Irradiation Promotes Persistent Oxidative Stress and Decreases Self-Renewal in Hematopoietic Stem Cells. Cell Rep 2017, 20, 3199–3211. [Google Scholar] [CrossRef]
- Brush, J.M.; Kim, K.; Sayre, J.W.; McBride, W.H.; Iwamoto, K.S. Imaging of radiation effects on cellular 26S proteasome function in situ. Int. J. Radiat. Biol. 2009, 85, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Tsukimoto, M.; Homma, T.; Ohshima, Y.; Kojima, S. Involvement of purinergic signaling in cellular response to gamma radiation. Radiat Res 2010, 173, 298–309. [Google Scholar] [CrossRef]
- Schaue, D.; Iwamoto, K.S.; McBride, W.H. Immune Networks in the Context of Low Dose Ionizing Radiation. In Biomarkers of Radiation in the Environment. NATO Science for Peace and Security Series A: Chemistry and Biology; Wood, M.D., Mothersill, C.E., Tsakanova, G., Cresswell, T., Woloschak, G.E., Eds.; Springer: Dordrecht, The Netherlands, 2022; pp. 89–106. [Google Scholar]
- Segal, G.; Leblond, C. Reaction d’alarme produite par l’action des rayons X sur l’abdomen chez le rat. Compt Rend Soc De Biol 1938, 129, 279. [Google Scholar]
- Mole, R. Whole body irradiation—Radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Scharpfenecker, M.; Floot, B.; Russell, N.S.; Stewart, F.A. The TGF-β co-receptor endoglin regulates macrophage infiltration and cytokine production in the irradiated mouse kidney. Radiother. Oncol. 2012, 105, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Bonow, R.O.; Mann, D.L.; Tomaselli, G.F.; Bhatt, D.; Solomon, S.D.; Braunwald, E. Braunwald’s Heart Disease-E-Book: A Textbook of Cardiovascular Medicine; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Zhao, J.; Pei, L. Cardiac endocrinology: Heart-derived hormones in physiology and disease. Basic Transl. Sci. 2020, 5, 949–960. [Google Scholar]
- Mulrooney, D.A.; Yeazel, M.W.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; Robison, L.L. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009, 339, b4606. [Google Scholar] [CrossRef] [Green Version]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Huff, J.; Cucinotta, F.A. Risk of degenerative tissue or other health effects from radiation exposure. Human Health and performance risks of space exploration missions National Aeronautics and Space Administration, NASA SP-2009-3405. In Human Health and Performance Risks of Space Exploration Missions; WHO: Houston, TX, USA, 2009; pp. 213–235. [Google Scholar]
- Wang, Z.; Jia, Z.; Zhou, Z.; Zhao, X.; Wang, F.; Zhang, X.; Tse, G.; Li, G.; Liu, Y.; Liu, T. Long-Term Cardiac Damage Associated With Abdominal Irradiation in Mice. Front. Pharmacol. 2022, 13, 850735. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenarczyk, M.; Alsheikh, A.J.; Cohen, E.P.; Schaue, D.; Kronenberg, A.; Geurts, A.; Klawikowski, S.; Mattson, D.; Baker, J.E. T Cells Contribute to Pathological Responses in the Non-Targeted Rat Heart following Irradiation of the Kidneys. Toxics 2022, 10, 797. https://doi.org/10.3390/toxics10120797
Lenarczyk M, Alsheikh AJ, Cohen EP, Schaue D, Kronenberg A, Geurts A, Klawikowski S, Mattson D, Baker JE. T Cells Contribute to Pathological Responses in the Non-Targeted Rat Heart following Irradiation of the Kidneys. Toxics. 2022; 10(12):797. https://doi.org/10.3390/toxics10120797
Chicago/Turabian StyleLenarczyk, Marek, Ammar J. Alsheikh, Eric P. Cohen, Dörthe Schaue, Amy Kronenberg, Aron Geurts, Slade Klawikowski, David Mattson, and John E. Baker. 2022. "T Cells Contribute to Pathological Responses in the Non-Targeted Rat Heart following Irradiation of the Kidneys" Toxics 10, no. 12: 797. https://doi.org/10.3390/toxics10120797




