Pharmacokinetics and the Dermal Absorption of Bromochlorophene, a Cosmetic Preservative Ingredient, in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Formulations
2.4. In Vitro Dermal Absorption
2.5. In Vivo Pharmacokinetics
2.6. LC–MS/MS Instruments and Conditions
2.7. Analytical Method Validation
2.8. Data Analysis
3. Results
3.1. Analytical Method Validation
3.2. In Vitro Dermal Absorption
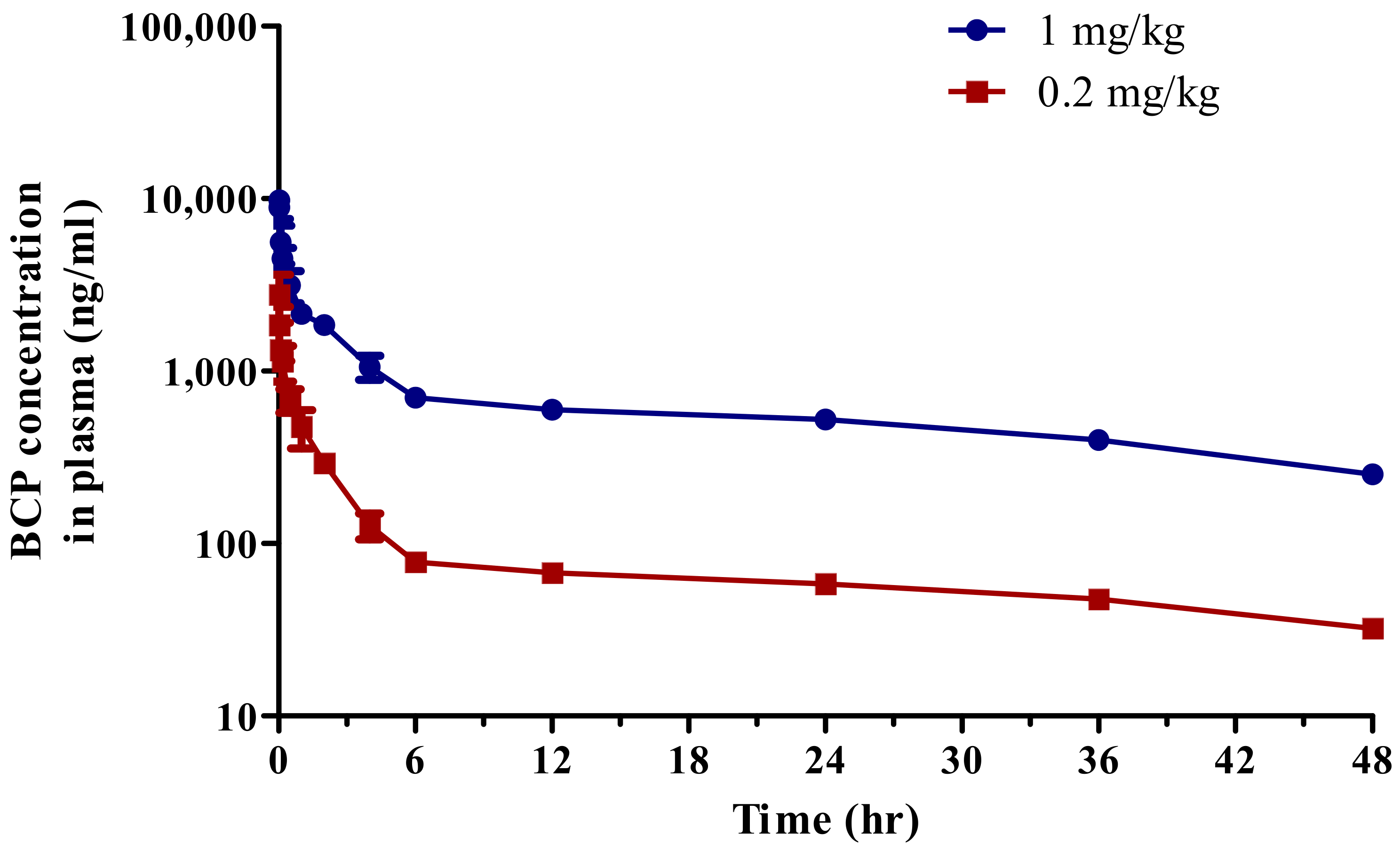
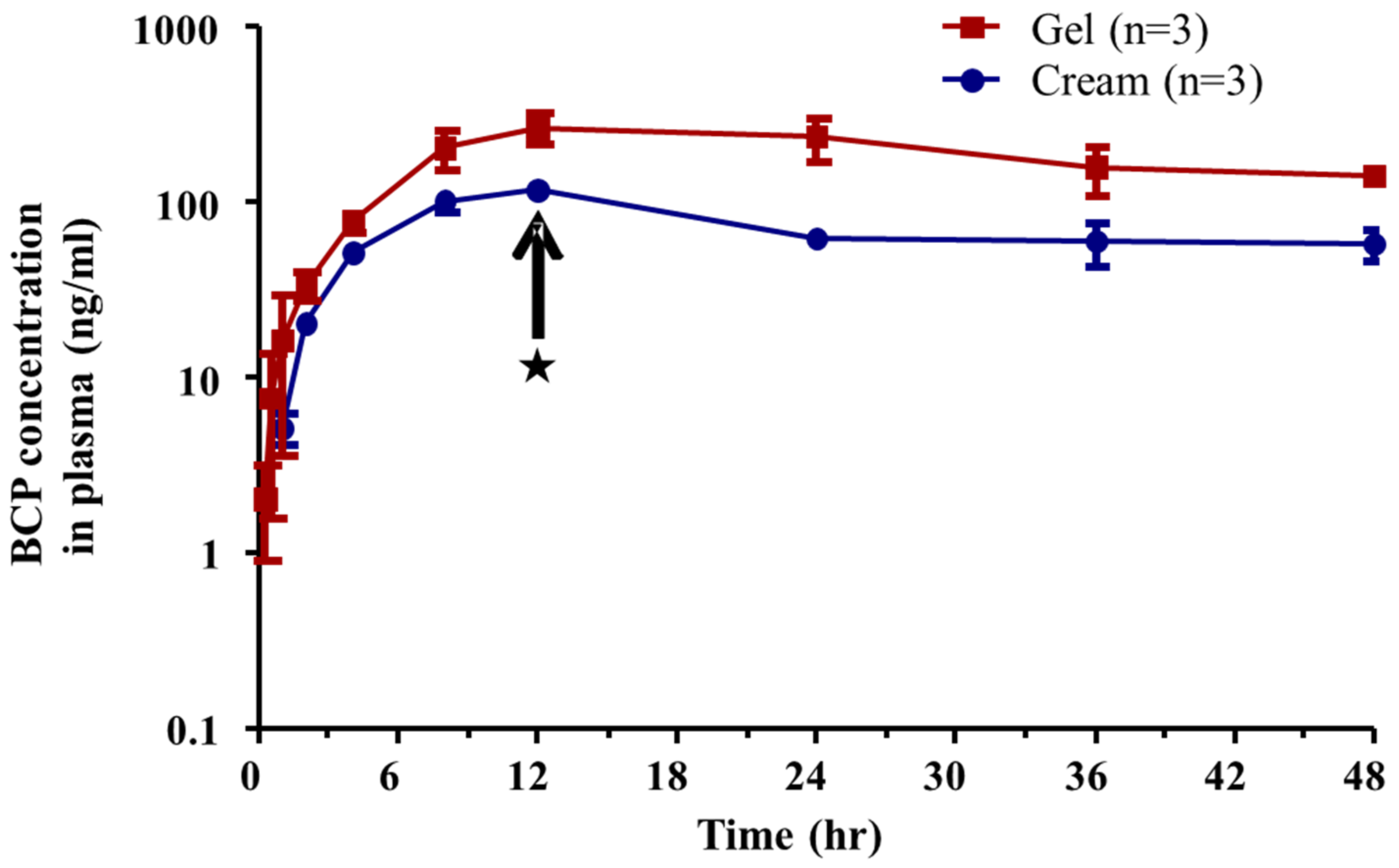
3.3. Pharmacokinetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russell, A.D. Mechanisms of bacterial resistance to non-antibiotics: Food additives and food and pharmaceutical preservatives. J. Appl. Bacteriol. 1991, 71, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety (MFDS). Regulation on Safety Standards for Cosmetics. Available online: https://www.law.go.kr (accessed on 12 April 2022).
- Lim, D.S.; Roh, T.H.; Kim, M.K.; Kwon, Y.C.; Choi, S.M.; Kwack, S.J.; Kim, K.B.; Yoon, S.; Kim, H.S.; Lee, B.-M. Risk assessment of N-nitrosodiethylamine (NDEA) and N-nitrosodiethanolamine (NDELA) in cosmetics. J. Toxicol. Environ. Health A 2018, 81, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Lee, J.Y.; Kwack, S.J.; Shin, C.Y.; Jang, H.-J.; Kim, H.Y.; Kim, M.K.; Seo, D.-W.; Lee, B.-M.; Kim, K.-B. Risk Assessment of Triclosan, a Cosmetic Preservative. Toxicol. Res. 2019, 35, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Chevillotte, G.; Ficheux, A.S.; Morisset, T.; Roudot, A.C. Exposure method development for risk assessment to cosmetic products using a standard composition. Food Chem. Toxicol. 2014, 68, 108–116. [Google Scholar] [CrossRef]
- Hayes, A.W.; Wang, T.; Dixon, D.; Loomis, T.A. Loomis’s Essentials of Toxicology, 5th ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 17–31. [Google Scholar]
- KEMI. Triklosan Och Andra Konserveringsmedel i Kosmetiska Produkter–Rapport Fran ett Regeringsuppdrag. [In Swedish with English summary]. 2017. Available online: https://www.kemi.se/publikationer/rapporter/2017/rapport-3-17-triklosan-och-andra-konserveringsmedel-i-kosmetiska-produkter---rapport-fran-ett-regeringsuppdrag (accessed on 12 April 2022).
- Kenda, M.; Kuželički, N.K.; Iida, M.; Kojima, H.; Dolenc, M.S. riclocarban, Triclosan, Bromochlorophene, Chlorophene, and Climbazole Effects on Nuclear Receptors: An in Silico and in Vitro Study. Environ. Health Perspect. 2020, 128, 107005. [Google Scholar] [CrossRef]
- Won, H.; Jeong, D.H.; Shin, H.-S.; Lee, J.H.; Lee, J.P.; Yang, J.-Y.; Jung, K.; Jeong, J.; Oh, J.H. Toxicological Assessment of Bromochlorophene: Single and Repeated-Dose 28-Day Oral Toxicity, Genotoxicity, and Dermal Application in Sprague-Dawley Rats. Front. Pharmacol. 2021, 12, 690141. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety (SCCS). Basic Criteria for the In Vitro Assessment of Dermal Absorption of Cosmetic Ingredients. SCCS/1358/10. Available online: https://ec.europa.eu/health/system/files/2016-11/sccs_s_002_0.pdf (accessed on 12 April 2022).
- Gummer, C.L.; Hinz, R.S.; Maibach, H.I. The skin penetration cell: A design update. Int. J. Pharm. 1987, 40, 101–104. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Im, J.E.; Lee, J.D.; Kim, K.-B. Analytical method development and percutaneous absorption of propylidene phthalide, a cosmetic ingredient. J. Toxicol. Environ. Health A 2021, 84, 811–820. [Google Scholar]
- Frasch, H.F.; Dotson, G.S.; Barbero, A.M. In vitro human epidermal penetration of 1-bromopropane. J. Toxicol. Environ. Health A. 2011, 74, 1249–1260. [Google Scholar] [CrossRef]
- Jakasa, I.; Kezic, S. Evaluation of in-vivo animal and in-vitro models for prediction of dermal absorption in man. Hum. Exp. Toxicol. 2008, 27, 281–288. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Guideline for In Vitro Skin Absorption Method. Available online: http://www.nifds.go.kr/brd/m_15/view.do?seq=11430 (accessed on 12 April 2022).
- Organisation for Economic Cooperation and Development (OECD). Guideline for the Testing of Chemicals. Toxicokinetics. 417. Available online: http://www.oecd-ilibrary.org/environment/test-no-417-toxicokinetics_9789264070882-en (accessed on 12 April 2022).
- Organisation for Economic Cooperation and Development (OECD). Guideline for the Testing of Chemicals Skin Absorption: In Vitro Method. 427. Available online: http://www.oecd-ilibrary.org/environment/test-no-427-skin-absorption-in-vivo-method_9789264071063-en (accessed on 12 April 2022).
- Ministry of Food and Drug Safety (MFDS). Guideline on Bioanalytical Method Validation. Available online: http://www.nifds.go.kr/brd/m_15/view.do?seq=7018 (accessed on 12 April 2022).
- Huh, Y.; Lee, D.-H.; Choi, D.; Lim, K.-M. Effect of Cosmetics Use on the In Vitro Skin Absorption of a Biocide, 1,2-Benzisothiazolin-3-one. Toxics 2022, 10, 108. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, S.; Kim, K.B.; Seo, W.S.; Shin, J.C.; Choi, J.H.; Weon, K.Y.; Joo, S.H.; Jeong, S.W.; Shin, B.S. Determination of acrylamide and glycidamide in various biological matrices by liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic study. Talanta 2015, 131, 46–54. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Analytical Procedures and Methods Validation for Drugs and Biologics. ucm273423. Available online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm386366.pdf (accessed on 12 April 2022).
- Madison, K.C. Barrier function of the skin: “la raison d’etre” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Wiechers, J.W. The barrier function of the skin in relation to percutaneous absorption of drugs. Pharm. Weekbl. Sci. 1989, 11, 185–198. [Google Scholar] [CrossRef]
- Wertz, P.W.; Swartzendruber, D.C.; Madison, K.C.; Downing, D.T. Composition and morphology of epidermal cyst lipids. J. Investig. Dermatol. 1987, 89, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Doan, K.; Bronaugh, R.L.; Yourick, J.J. In vivo and in vitro skin absorption of lipophilic compounds, dibutyl phthalate, farnesol and geraniol in the hairless guinea pig. Food Chem. Toxicol. 2010, 48, 18–23. [Google Scholar] [CrossRef]
- Pozzo, A.D.; Pastori, N. Percutaneous absorption of parabens from cosmetic formulations. Int. J. Cosmet. Sci. 1996, 18, 57–66. [Google Scholar] [CrossRef]
- Hilton, J.; Woollen, B.H.; Scott, R.C.; Auton, T.R.; Trebilcock, K.L.; Wilks, M.F. Vehicle effects on in vitro percutaneous absorption through rat and human skin. Pharm. Res. 1994, 11, 1396–1400. [Google Scholar] [CrossRef]
- Chatelain, E.; Gabard, B.; Surber, C. Skin penetration and sun protection factor of five UV filters: Effect of the vehicle. Skin Pharmacol. Physiol. 2003, 16, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.C.; Ramsey, J.D. Comparison of the in vivo and in vitro percutaneous absorption of a lipophilic molecule (cypermethrin, a pyrethroid insecticide). J. Investig. Dermatol. 1987, 89, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.; Maibach, H.I. Effects of skin occlusion on percutaneous absorption: An overview. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 1–10. [Google Scholar] [CrossRef]
- Van Hal, D.A.; Jeremiasse, E.; Junginger, H.E.; Spies, F.; Bouwstra, J.A. Structure of fully hydrated human stratum corneum: A freeze-fracture electron microscopy study. J. Investig. Dermatol. 1996, 106, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, J.-E.; Kim, H.Y.; Lee, J.D.; Park, J.-J.; Kang, K.-S.; Kim, K.-B. Effect of Application Amounts on In Vitro Dermal Absorption Test Using Caffeine and Testosterone. Pharmaceutics 2021, 13, 641. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, Y.J.; Kim, K.-B. Analytical mehtod development of methyisothiazolinone, a preservative, in rat plasma using LC-MS/MS. J. Chromatogr. B 2018, 1100–1101, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Murp, M.R.; Hug, C.C., Jr.; McClain, D.A. Dose-independent pharmacokinetics of fentanyl. Anesthesiology. 1983, 59, 537–540. [Google Scholar] [CrossRef]
- Shin, J.H.; Choi, K.Y.; Kim, Y.C.; Lee, M.G. Dose-dependent pharmacokinetics of itraconazole after intravenous or oral administration to rats: Intestinal first-pass effect. Antimicrob. Agents Chemother. 2004, 48, 1756–1762. [Google Scholar] [CrossRef] [Green Version]
- Franz, T.J. Percutaneous absorption on the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Dermal Absorption. (Environmental Health Criteria, 235). Available online: https://apps.who.int/iris/handle/10665/43542 (accessed on 12 April 2022).
- Moody, R.P.; Nadeau, B.; Chu, I. In vivo and in vitro dermal absorption of benzo[a]pyrene in rat, guinea pig, human and tissue-cultured skin. J. Dermatol. Sci. 1995, 9, 48–58. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). Guideline for the Testing of Chemicals Skin Absorption: In Vitro Method. 428. Available online: http://www.oecd-ilibrary.org/environment/test-no-428-skin-absorption-in-vitro-method_9789264071087-en (accessed on 12 April 2022).

| Compound | Matrix | Calibration Sample (ng/mL) | Linearity (r2) |
|---|---|---|---|
| Bromochlorophene | WASH | 1, 5, 10, 50, 100, 200 | 0.9994 |
| SC | 0.9996 | ||
| SKIN | 0.9994 | ||
| RF | 0.9993 | ||
| Plasma | 0.9995 |
| Compound | Conc. (ng/mL) | Intra-Day (%) | Inter-Day (%) | ||
|---|---|---|---|---|---|
| Accuracy | Precision | Accuracy | Precision | ||
| WASH | 1 | 91.70 | 2.93 | 99.30 | 4.78 |
| 3 | 100.56 | 4.46 | 97.50 | 4.23 | |
| 15 | 99.54 | 1.52 | 101.08 | 3.34 | |
| 150 | 101.77 | 2.04 | 100.68 | 2.43 | |
| S.C | 1 | 86.90 | 4.26 | 110.00 | 0.79 |
| 3 | 101.36 | 2.36 | 104.11 | 1.43 | |
| 15 | 100.63 | 4.33 | 98.30 | 0.58 | |
| 150 | 96.86 | 1.84 | 98.24 | 1.72 | |
| SKIN | 1 | 94.47 | 4.39 | 104.4 | 8.05 |
| 3 | 108.03 | 4.44 | 101.18 | 5.65 | |
| 15 | 103.62 | 1.29 | 106.05 | 0.96 | |
| 150 | 101.08 | 1.22 | 101.88 | 4.17 | |
| R.F | 1 | 102.4 | 10.66 | 93.17 | 4.29 |
| 3 | 95.94 | 4.60 | 102.87 | 8.59 | |
| 15 | 102.42 | 2.05 | 101.73 | 2.36 | |
| 150 | 104.75 | 2.47 | 96.79 | 3.63 | |
| Plasma | 1 | 107.70 | 4.93 | 104.5 | 8.04 |
| 3 | 100.68 | 6.35 | 107.61 | 4.31 | |
| 15 | 107.56 | 4.28 | 104.79 | 2.32 | |
| 150 | 105.04 | 5.11 | 102.18 | 3.22 | |
| Matrix | Formulation | |
|---|---|---|
| Gel (%) | Cream (%) | |
| WASH | 95.56 ± 10.86 | 101.88 ± 9.62 |
| S.C | 4.49 ± 1.52 | 0.89 ± 0.15 |
| SKIN | 7.42 ± 0.74 | 1.48 ± 0.93 |
| R.F | 0.0017 ± 0.74 | 0.0012 ± 0.001 |
| Total absorption (SKIN + RF) | 7.43 ± 0.74 | 1.48 ± 0.93 |
| Recovery | 109.12 ± 8.79 | 105.43 ± 11.07 |
| Parameter | Dose of Administration | |
|---|---|---|
| 0.2 mg/kg | 1 mg/kg | |
| T1/2 (h) | 34.48 ± 5.87 | 30.52 ± 1.83 |
| Cmax (ng/mL) | 4192.57 ± 1685.90 | 11,385.42 ± 1526.38 |
| AUCall (ng·h/mL) | 4190.78 ± 319.68 | 30,458.03 ± 366.90 |
| AUCinf (ng·h/mL) | 5855.42 ± 766.69 | 41,591.66 ± 939.34 |
| Vd (L/kg) | 1742.43 ± 71.97 | 1058.30 ± 50.17 |
| CL (mL/min/kg) | 34.58 ± 4.84 | 24.05 ± 0.54 |
| Parameter | Dose of Administration | |
|---|---|---|
| Gel | Cream | |
| T1/2 (h) | 38.54 ± 9.54 | 39.41 ± 11.70 |
| Tmax (h) | 12.00 ± 0.00 | 12.00 ± 0.00 |
| Cmax (ng/mL) | 259.77 ± 50.03 | 116.19 ± 10.38 |
| AUCall (ng·h/mL) | 8687.81 ± 1843.71 | 3309.16 ± 403.33 |
| AUCinf (ng·h/mL) | 16,356.04 ± 2518.43 | 6708.17 ± 2149.84 |
| F (%) | 12.20 ± 2.63 | 4.65 ± 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Kim, H.-Y.; Pham, Q.-L.; Lee, J.-D.; Kim, K.-B. Pharmacokinetics and the Dermal Absorption of Bromochlorophene, a Cosmetic Preservative Ingredient, in Rats. Toxics 2022, 10, 329. https://doi.org/10.3390/toxics10060329
Lee Y-J, Kim H-Y, Pham Q-L, Lee J-D, Kim K-B. Pharmacokinetics and the Dermal Absorption of Bromochlorophene, a Cosmetic Preservative Ingredient, in Rats. Toxics. 2022; 10(6):329. https://doi.org/10.3390/toxics10060329
Chicago/Turabian StyleLee, Yong-Jae, Hyang-Yeon Kim, Quynh-Lien Pham, Jung-Dae Lee, and Kyu-Bong Kim. 2022. "Pharmacokinetics and the Dermal Absorption of Bromochlorophene, a Cosmetic Preservative Ingredient, in Rats" Toxics 10, no. 6: 329. https://doi.org/10.3390/toxics10060329






