In Vitro Assessment of Pesticides Toxicity and Data Correlation with Pesticides Physicochemical Properties for Prediction of Toxicity in Gastrointestinal and Skin Contact Exposure
Abstract
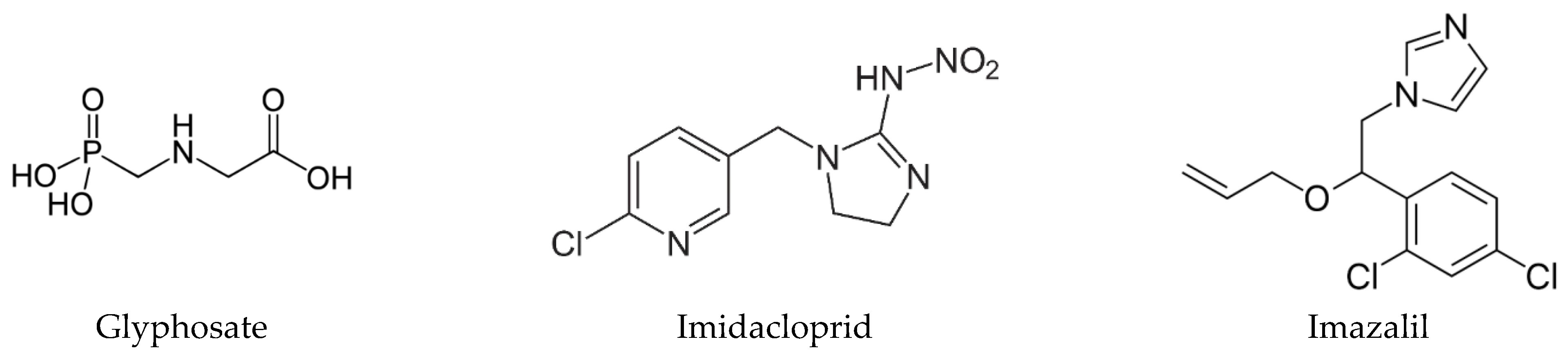
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cytotoxicity Evaluation
2.2.1. Cell Maintenance and Handling
2.2.2. Cell Viability/Cytotoxicity Assay
2.3. Evaluation of Cell Morphology
2.4. Data and Statistical Analysis
3. Results
Pesticide-Induced In Vitro Cytotoxicity
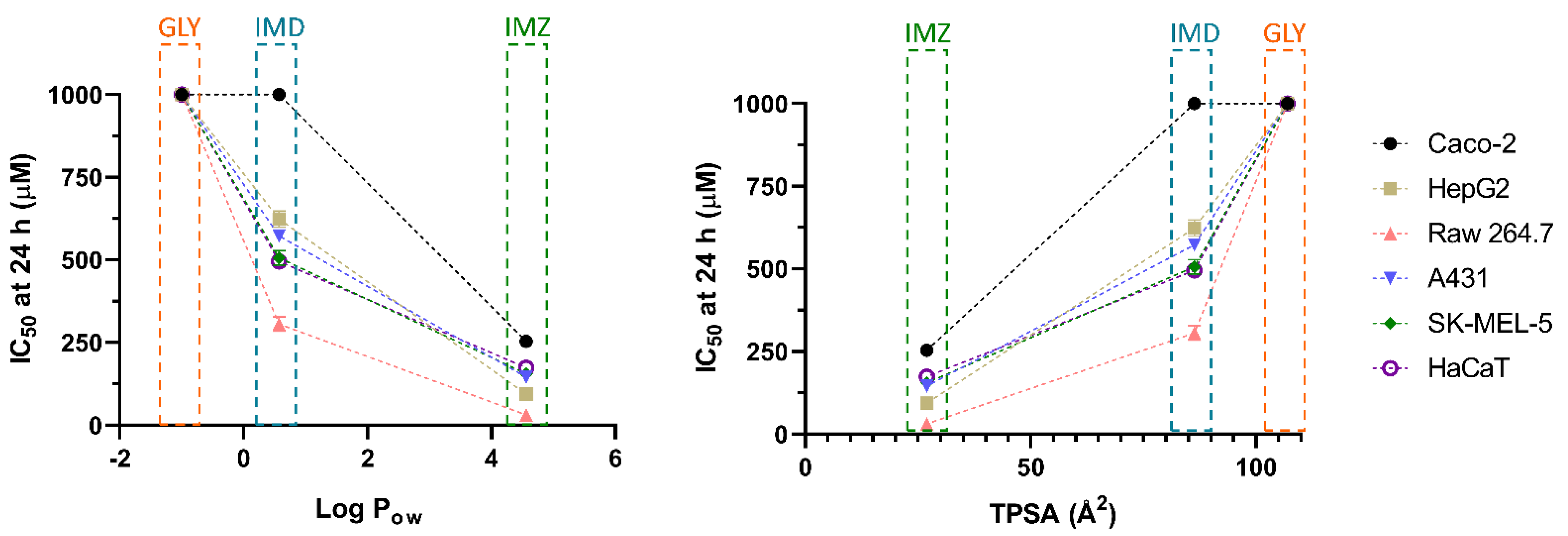
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martins-Gomes, C.; Silva, T.L.; Andreani, T.; Silva, A.M. Glyphosate vs. Glyphosate-Based Herbicides Exposure: A Review on Their Toxicity. J. Xenobiotics 2022, 12, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, N.N. The Role of Pesticides in Agricultural Crop Protection. Ann. N. Y. Acad. Sci. 1999, 894, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Tasheva, M. Pesticide Residues: Conazoles. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Caldas, E.D. Toxicological Aspects of Pesticides. In Sustainable Agrochemistry: A Compendium of Technologies; Vaz, S., Jr., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 275–305. [Google Scholar] [CrossRef]
- Casida, J.E. Pest toxicology: The primary mechanisms of pesticide action. Chem. Res. Toxicol. 2009, 22, 609–619. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, A.; Thorbek, P.; Chapman, P.; Grimm, V. Ecological models and pesticide risk assessment: Current modeling practice. Environ. Toxicol. Chem. 2010, 29, 1006–1012. [Google Scholar] [CrossRef]
- Dill, G.M.; Sammons, R.D.; Feng, P.C.C.; Kohn, F.; Kretzmer, K.; Mehrsheikh, A.; Bleeke, M.; Honegger, J.L.; Farmer, D.; Wright, D.; et al. Glyphosate: Discovery, Development, Applications, and Properties. In Glyphosate Resistance in Crops and Weeds; Wiley: New Jersey, NJ, USA, 2010; pp. 1–33. [Google Scholar] [CrossRef]
- Cuhra, M.; Bøhn, T.; Cuhra, P. Glyphosate: Too Much of a Good Thing? Front. Environ. Sci. 2016, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Tomizawa, M.; Casida, J.E. Molecular Recognition of Neonicotinoid Insecticides: The Determinants of Life or Death. Acc. Chem. Res. 2009, 42, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Schoville, S.; Peterson, N.; Lan, Q.; Groves, R.L. Characterizing Molecular Mechanisms of Imidacloprid Resistance in Select Populations of Leptinotarsa decemlineata in the Central Sands Region of Wisconsin. PLoS ONE 2016, 11, e0147844. [Google Scholar] [CrossRef] [PubMed]
- Loser, D.; Grillberger, K.; Hinojosa, M.G.; Blum, J.; Haufe, Y.; Danker, T.; Johansson, Y.; Möller, C.; Nicke, A.; Bennekou, S.H.; et al. Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling. Arch. Toxicol. 2021, 95, 3695–3716. [Google Scholar] [CrossRef] [PubMed]
- Ghosoph, J.M.; Schmidt, L.S.; Margosan, D.A.; Smilanick, J.L. Imazalil resistance linked to a unique insertion sequence in the PdCYP51 promoter region of Penicillium digitatum. Postharvest Biol. Technol. 2007, 44, 9–18. [Google Scholar] [CrossRef]
- Sánchez-Torres, P. Molecular Mechanisms Underlying Fungicide Resistance in Citrus Postharvest Green Mold. J. Fungi 2021, 7, 783. [Google Scholar] [CrossRef]
- Mesnage, R.; Ferguson, S.; Mazzacuva, F.; Caldwell, A.; Halket, J.; Antoniou, M.N. Cytotoxicity mechanisms and composition of the glyphosate formulated herbicide RangerPro. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Tao, H.; Bao, Z.; Jin, C.; Miao, W.; Fu, Z.; Jin, Y. Toxic effects and mechanisms of three commonly used fungicides on the human colon adenocarcinoma cell line Caco-2. Environ. Pollut. 2020, 263, 114660. [Google Scholar] [CrossRef]
- Nedzvetsky, V.S.; Masiuk, D.M.; Gasso, V.Y.; Yermolenko, S.V.; Huslystyi, A.O.; Spirina, V.A. Low doses of imidacloprid induce disruption of intercellular adhesion and initiate proinflammatory changes in Caco-2 cells. Regul. Mech. Biosyst. 2021, 12, 430–437. [Google Scholar] [CrossRef]
- Kašuba, V.; Milić, M.; Rozgaj, R.; Kopjar, N.; Mladinić, M.; Žunec, S.; Vrdoljak, A.L.; Pavičić, I.; Čermak, A.M.M.; Pizent, A.; et al. Effects of low doses of glyphosate on DNA damage, cell proliferation and oxidative stress in the HepG2 cell line. Environ. Sci. Pollut. Res. 2017, 24, 19267–19281. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Andreani, T.; Kiill, C.P.; de Souza, A.L.; Fangueiro, J.F.; Fernandes, L.; Doktorovova, S.; Santos, D.L.; Garcia, M.L.; Gremiao, M.P.; Souto, E.B.; et al. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surf B Biointerfaces 2014, 123, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Coutinho, T.E.; Fangueiro, J.F.; Sanchez-Lopez, E.; Pashirova, T.N.; Andreani, T.; Souto, E.B. Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Sevior, D.K.; Pelkonen, O.; Ahokas, J.T. Hepatocytes: The powerhouse of biotransformation. Int. J. Biochem. Cell Biol. 2012, 44, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Konishi, T.; Han, G.; Campwala, K.H.; French, S.W.; Wan, Y.J. The role of hepatocyte RXR alpha in xenobiotic-sensing nuclear receptor-mediated pathways. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2002, 15, 89–96. [Google Scholar] [CrossRef]
- Vasiluk, L.; Pinto, L.J.; Moore, M.M. Oral bioavailability of glyphosate: Studies using two intestinal cell lines. Environ. Toxicol. Chem. 2005, 24, 153–160. [Google Scholar] [CrossRef]
- Louie, F.; Jacobs, N.F.B.; Yang, L.G.L.; Park, C.; Monnot, A.D.; Bandara, S.B. A comparative evaluation of dietary exposure to glyphosate resulting from recommended U.S. diets. Food Chem. Toxicol. 2021, 158, 112670. [Google Scholar] [CrossRef]
- Soares, D.; Silva, L.; Duarte, S.; Pena, A.; Pereira, A. Glyphosate Use, Toxicity and Occurrence in Food. Foods 2021, 10, 2785. [Google Scholar] [CrossRef] [PubMed]
- Gotti, R.; Fiori, J.; Bosi, S.; Dinelli, G. Field-amplified sample injection and sweeping micellar electrokinetic chromatography in analysis of glyphosate and aminomethylphosphonic acid in wheat. J. Chromatogr. A 2019, 1601, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Gillezeau, C.; van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Env. Health 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vass, A.; Korpics, E.; Dernovics, M. Follow-up of the fate of imazalil from post-harvest lemon surface treatment to a baking experiment. Food Addit. Contam. Part A 2015, 32, 1875–1884. [Google Scholar] [CrossRef]
- Kapoor, U.; Srivastava, M.K.; Srivastava, A.K.; Patel, D.K.; Garg, V.; Srivastava, L.P. Analysis of imidacloprid residues in fruits, vegetables, cereals, fruit juices, and baby foods, and daily intake estimation in and around Lucknow, India. Environ. Toxicol. Chem. 2013, 32, 723–727. [Google Scholar] [CrossRef]
- Klarich, K.L.; Pflug, N.C.; DeWald, E.M.; Hladik, M.L.; Kolpin, D.W.; Cwiertny, D.M.; LeFevre, G.H. Occurrence of Neonicotinoid Insecticides in Finished Drinking Water and Fate during Drinking Water Treatment. Environ. Sci. Technol. Lett. 2017, 4, 168–173. [Google Scholar] [CrossRef]
- Chorfa, A.; Bétemps, D.; Morignat, E.; Lazizzera, C.; Hogeveen, K.; Andrieu, T.; Baron, T. Specific Pesticide-Dependent Increases in α-Synuclein Levels in Human Neuroblastoma (SH-SY5Y) and Melanoma (SK-MEL-2) Cell Lines. Toxicol. Sci. 2013, 133, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Heu, C.; Berquand, A.; Elie-Caille, C.; Nicod, L. Glyphosate-induced stiffening of HaCaT keratinocytes, a Peak Force Tapping study on living cells. J. Struct. Biol. 2012, 178, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kar, A.K.; Singh, D.; Verma, R.; Shraogi, N.; Zehra, A.; Gautam, K.; Anbumani, S.; Ghosh, D.; Patnaik, S. pH-responsive eco-friendly chitosan modified cenosphere/alginate composite hydrogel beads as carrier for controlled release of Imidacloprid towards sustainable pest control. Chem. Eng. J. 2022, 427, 131215. [Google Scholar] [CrossRef]
- de Jesus Santos, G.A.R.; Francisco Veiga Bizerra, P.; Araújo Miranda, C.; Mingatto, F.E. Effects of imidacloprid on viability and increase of reactive oxygen and nitrogen species in HepG2 cell line. Toxicol. Mech. Methods 2022, 32, 204–212. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings 1PII of original article: S0169-409X(96)00423-1. Adv. Drug Deliv. Rev. 1997, 23, 3–25, reprinted in Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the chemical beauty of drugs. Nat. Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujak, R.; Struck-Lewicka, W.; Kaliszan, M.; Kaliszan, R.; Markuszewski, M.J. Blood–brain barrier permeability mechanisms in view of quantitative structure–activity relationships (QSAR). J. Pharm. Biomed. Anal. 2015, 108, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Mekapati, S.B.; Kurup, A. QSAR and ADME. Bioorganic Med. Chem. 2004, 12, 3391–3400. [Google Scholar] [CrossRef]
- PubChem. National Center for Biotechnology Information. PubChem Compound Summary for CID 37175, Enilconazole. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Enilconazole (accessed on 6 June 2022).
- PubChem. National Center for Biotechnology Information. PubChem Compound Summary for CID 3496, Glyphosate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glyphosate (accessed on 6 June 2022).
- PubChem. National Center for Biotechnology Information. PubChem Compound Summary for CID 86287518, Imidacloprid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Imidacloprid (accessed on 6 June 2022).
- INCHEM; W.H.O. International Chemical Safety Cards. Availabe online: https://inchem.org/#/. Available online: https://inchem.org/#/ (accessed on 6 June 2022).
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and Its Relationship with Passive Drug Permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Amézqueta, S.; Subirats, X.; Fuguet, E.; Rosés, M.; Ràfols, C. Chapter 6-Octanol-Water Partition Constant. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–208. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A. Analysis of the glyphosate herbicide in water, soil and food using derivatising agents. Environ. Chem. Lett. 2017, 15, 85–100. [Google Scholar] [CrossRef]
- Prasanna, S.; Doerksen, R.J. Topological Polar Surface Area: A Useful Descriptor in 2D-QSAR. Curr. Med. Chem. 2009, 16, 21–41. [Google Scholar] [CrossRef]
- Fernandes, J.; Gattass, C.R. Topological Polar Surface Area Defines Substrate Transport by Multidrug Resistance Associated Protein 1 (MRP1/ABCC1). J. Med. Chem. 2009, 52, 1214–1218. [Google Scholar] [CrossRef]
- Cole, S.P. Targeting multidrug resistance protein 1 (MRP1, ABCC1): Past, present, and future. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.V.; Raub, T.J.; Blanco, M.-J. How hydrogen bonds impact P-glycoprotein transport and permeability. Bioorganic Med. Chem. Lett. 2012, 22, 6540–6548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; DeCrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorganic Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef] [PubMed]
- Nazir, I.; Shahzadi, I.; Jalil, A.; Bernkop-Schnürch, A. Hydrophobic H-bond pairing: A novel approach to improve membrane permeability. Int. J. Pharm. 2020, 573, 118863. [Google Scholar] [CrossRef]
- Bingham, M.; Rankovic, Z. CHAPTER 18—Medicinal Chemistry Challenges in CNS Drug Discovery. In Drug Discovery for Psychiatric Disorders; The Royal Society of Chemistry: London, UK, 2012; pp. 465–509. [Google Scholar]
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F.; Pearlman, E. (Eds.) Chapter 6-General and ocular pharmacology. In The Eye, 4th ed.; W. B. Saunders Ltd.: Philadelphia, PA, USA, 2016; pp. 338–369.e1. [Google Scholar] [CrossRef]
- Mansouri, K.; Cariello, N.F.; Korotcov, A.; Tkachenko, V.; Grulke, C.M.; Sprankle, C.S.; Allen, D.; Casey, W.M.; Kleinstreuer, N.C.; Williams, A.J. Open-source QSAR models for pKa prediction using multiple machine learning approaches. J. Cheminformatics 2019, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Manallack, D.T. The pK(a) Distribution of Drugs: Application to Drug Discovery. Perspect Med. Chem. 2007, 1, 25–38. [Google Scholar]
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of physiological pH and chemical pKa under the umbrella of physiologically based pharmacokinetic modeling of drug absorption, distribution, metabolism, excretion, and toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1103–1124. [Google Scholar] [CrossRef]
- Xu, J.; Li, G.; Wang, Z.; Si, L.; He, S.; Cai, J.; Huang, J.; Donovan, M.D. The role of L-type amino acid transporters in the uptake of glyphosate across mammalian epithelial tissues. Chemosphere 2016, 145, 487–494. [Google Scholar] [CrossRef]
- Wang, L.-X.; Tao, S.; Zhang, Y.-C.; Pei, X.-G.; Gao, Y.; Song, X.-Y.; Yu, Z.-T.; Gao, C.-F. Overexpression of ATP-binding cassette transporter Mdr49-like confers resistance to imidacloprid in the field populations of brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2022, 78, 579–590. [Google Scholar] [CrossRef]
- Brunet, J.-L.; Maresca, M.; Fantini, J.; Belzunces, L.P. Human intestinal absorption of imidacloprid with Caco-2 cells as enterocyte model. Toxicol. Appl. Pharmacol. 2004, 194, 1–9. [Google Scholar] [CrossRef]
- Sergent, T.; Dupont, I.; Jassogne, C.; Ribonnet, L.; van der Heiden, E.; Scippo, M.-L.; Muller, M.; McAlister, D.; Pussemier, L.; Larondelle, Y.; et al. CYP1A1 induction and CYP3A4 inhibition by the fungicide imazalil in the human intestinal Caco-2 cells—Comparison with other conazole pesticides. Toxicol. Lett. 2009, 184, 159–168. [Google Scholar] [CrossRef]
- Moreau, A.; Le Vee, M.; Jouan, E.; Parmentier, Y.; Fardel, O. Drug transporter expression in human macrophages. Fundam. Clin. Pharmacol. 2011, 25, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Kaminski, W.E. ATP-binding cassette (ABC) transporters in atherosclerosis. Curr. Atheroscler. Rep. 2002, 4, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Mishra, V.; Mehra, N.K. Targeted drug delivery to macrophages. Expert Opin. Drug Deliv. 2013, 10, 353–367. [Google Scholar] [CrossRef] [PubMed]
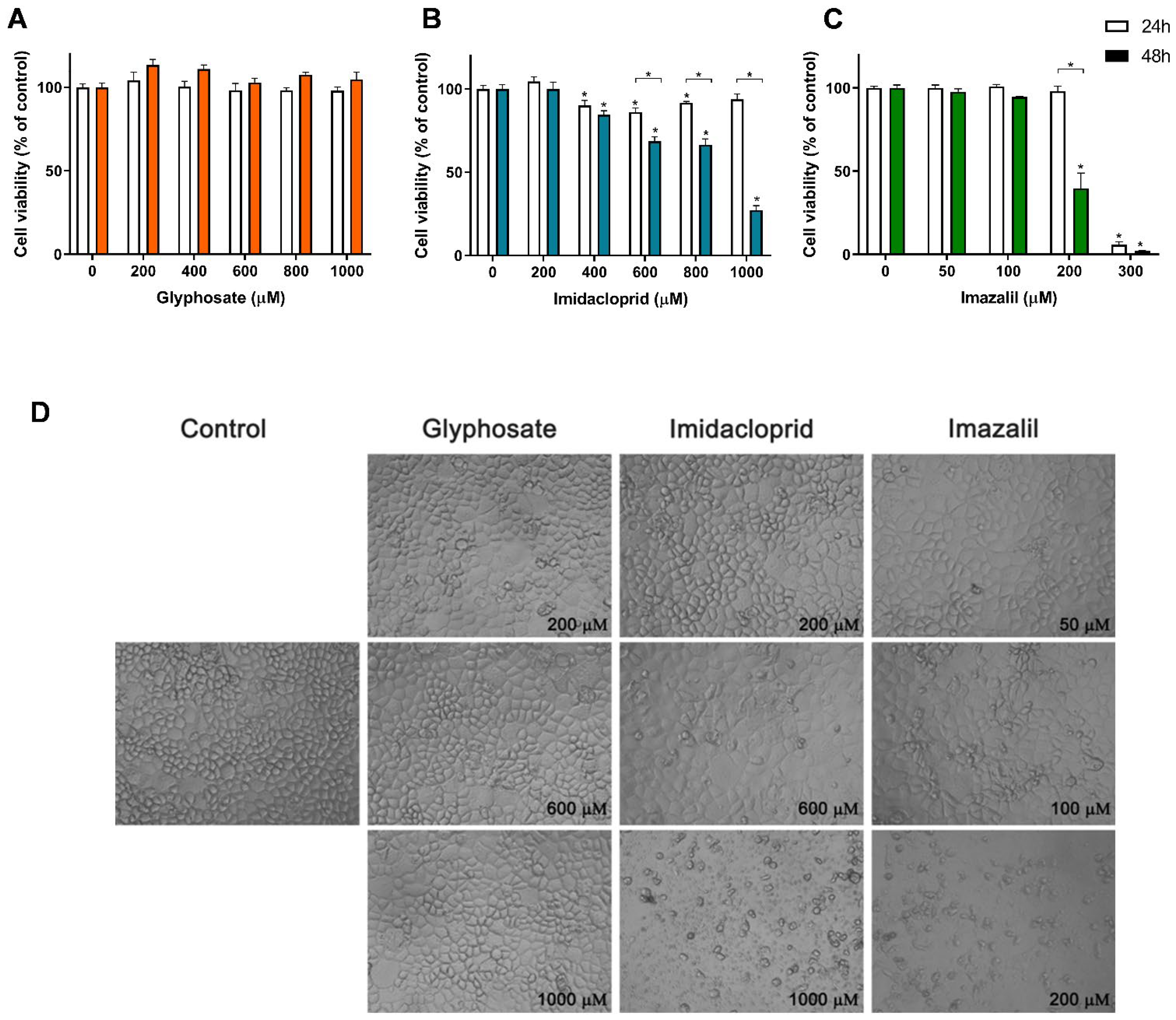
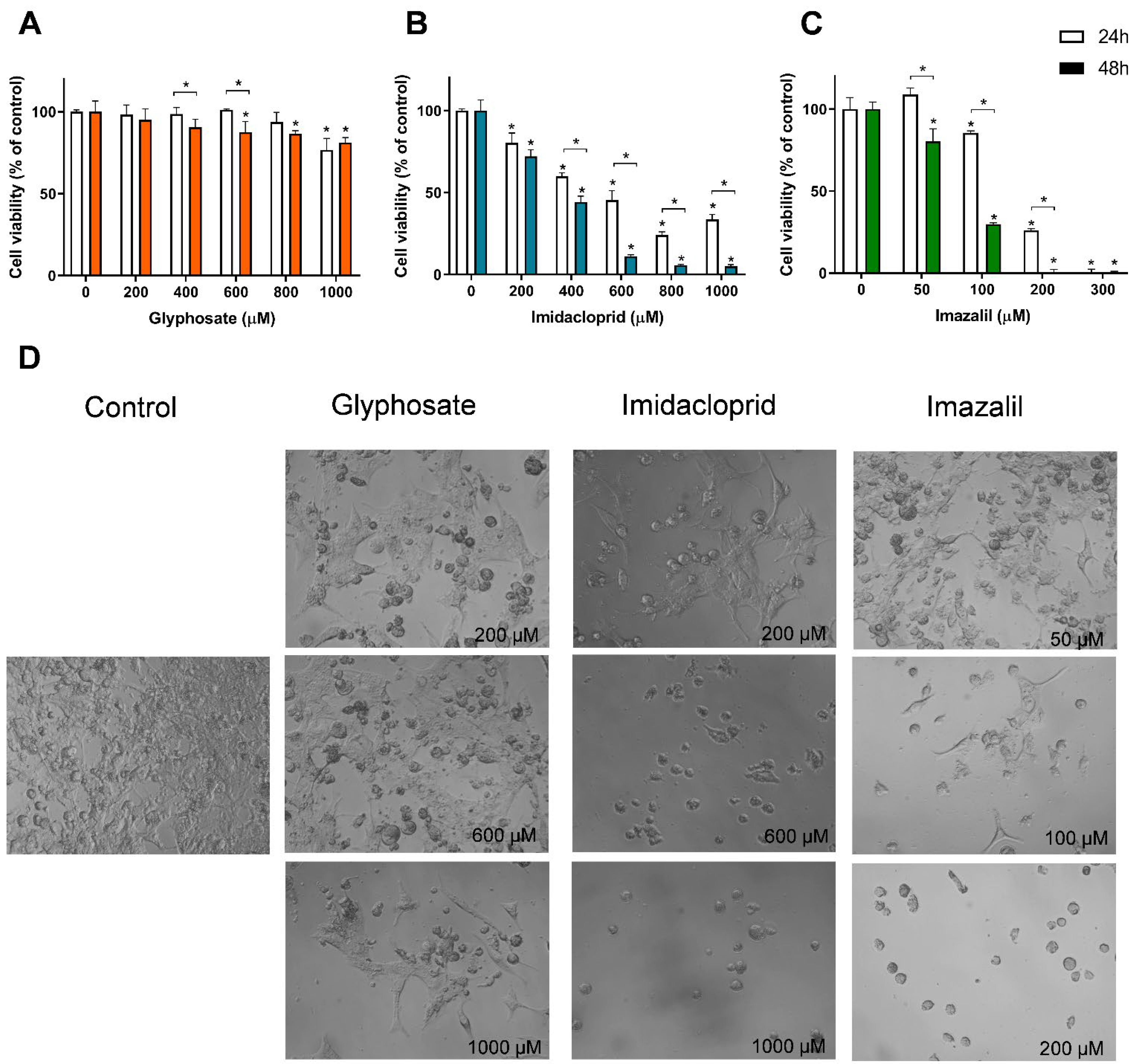

| Cell Line | Exposure Time | Glyphosate | Imidacloprid | Imazalil | |||
|---|---|---|---|---|---|---|---|
| A431 | 24 h | >1000 | n.s. | 573.2 ± 10.4 | * | 145.0 ± 1.7 | * |
| 48 h | >1000 | 389.3 ± 9.2 | 117.1 ± 6.5 | ||||
| HaCaT | 24 h | >1000 | n.s. | 495.3 ± 19.4 | n.s. | 173.6 ± 15.3 | * |
| 48 h | >1000 | 482.3 ± 12.9 | 120.2 ± 6.2 | ||||
| SK-MEL-5 | 24 h | >1000 | n.s. | 506.6 ± 22.07 | * | 155.7 ± 9.0 | * |
| 48 h | >1000 | 318.8 ± 11.5 | 76.7 ± 1.8 | ||||
| Caco-2 | 24 h | >1000 | n.s. | >1000 | * | 253.5 ± 3.37 | * |
| 48 h | >1000 | 832.1 ± 29.6 | 186.5 ± 2.27 | ||||
| HepG2 | 24 h | >1000 | n.s. | 623.8 ± 24.3 | n.s. | 93.7 ± 2.2 | * |
| 48 h | >1000 | 620.2 ± 10.6 | 47.1 ± 0.5 | ||||
| RAW 264.7 | 24 h | >1000 | n.s. | 305.9 ± 22.4 | n.s. | 31.3 ± 2.7 | * |
| 48 h | >1000 | 306.6 ± 22.1 | 7.21 ± 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.M.; Martins-Gomes, C.; Silva, T.L.; Coutinho, T.E.; Souto, E.B.; Andreani, T. In Vitro Assessment of Pesticides Toxicity and Data Correlation with Pesticides Physicochemical Properties for Prediction of Toxicity in Gastrointestinal and Skin Contact Exposure. Toxics 2022, 10, 378. https://doi.org/10.3390/toxics10070378
Silva AM, Martins-Gomes C, Silva TL, Coutinho TE, Souto EB, Andreani T. In Vitro Assessment of Pesticides Toxicity and Data Correlation with Pesticides Physicochemical Properties for Prediction of Toxicity in Gastrointestinal and Skin Contact Exposure. Toxics. 2022; 10(7):378. https://doi.org/10.3390/toxics10070378
Chicago/Turabian StyleSilva, Amélia M., Carlos Martins-Gomes, Tânia L. Silva, Tiago E. Coutinho, Eliana B. Souto, and Tatiana Andreani. 2022. "In Vitro Assessment of Pesticides Toxicity and Data Correlation with Pesticides Physicochemical Properties for Prediction of Toxicity in Gastrointestinal and Skin Contact Exposure" Toxics 10, no. 7: 378. https://doi.org/10.3390/toxics10070378







