The DevTox Germ Layer Reporter Platform: An Assay Adaptation of the Human Pluripotent Stem Cell Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. RUES2-GLR Pluripotent Stem Cell Culture Maintenance
2.2. TaqMan hPSC Scorecard Gene Expression Panel
2.3. Test Chemical Selection and Preparation
2.4. DevTox GLR Endoderm Differentiation Assay (DevTox GLR-Endo)
2.5. Image Acquisition and Analysis
2.6. ToxCast Pipeline Analysis
2.7. Assay Performance Evaluation
2.8. Comparative Analysis of Assay Predictive Performance
3. Results
3.1. Directed Endoderm Differentiation with the RUES2-GLR Cell Line
3.2. DevTox GLR-Endo Assay Workflow Optimization
3.3. Reference Chemical Testing
3.4. Chemical Training Set Evaluation
3.5. Comparative Analysis of the DevTox GLR-Endo Assay Predictive Performance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Acronyms and Abbreviations
| AC50 | activity concentration at 50% |
| ACC | active concentration at cutoff |
| bmad | baseline median absolute deviation |
| BRA | Brachyury |
| DevTox | developmental toxicity |
| Endo | endoderm |
| EPA | Environmental Protection Agency |
| ESC | embryonic stem cell |
| hPSC | human pluripotent stem cell |
| hPST | human pluripotent stem cell test |
| IC50 | inhibition concentration at 50% |
| mEST | mouse Embryonic Stem Cell Test |
| NAM | new approach method |
| OECD | Organization of Economic-Co-operation and Development |
| PSC | pluripotent stem cell |
| rCV | robust coefficient of variation |
| RUES2-GLR | Rockefeller University Embryonic Stem cell line 2–Germ Layer Reporter |
| rZ’ | robust Z-prime factor |
| SOX17 | SRY-box transcription factor 17 |
| SOX2 | SRY-box transcription factor 2 |
| tcpl | ToxCast Pipeline |
| TGF-β | transforming growth factor β |
| TSCA | Toxic Substance Control Act |
References
- Yoon, P.W.; Olney, R.S.; Khoury, M.J.; Sappenfield, W.M.; Chavez, G.F.; Taylor, D. Contribution of birth defects and genetic diseases to pediatric hospitalizations. A population-based study. Arch. Pediatr. Adolesc. Med. 1997, 151, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Hoyert, D.L.; Mathews, T.; Menacker, F.; Strobino, D.M.; Guyer, B. Annual summary of vital statistics: 2004. Pediatrics 2006, 117, 168–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stillerman, K.P.; Mattison, D.; Giudice, L.C.; Woodruff, T. Environmental exposures and adverse pregnancy outcomes: A review of the science. Reprod. Sci. 2008, 15, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Fantel, A.G. Culture of whole rodent embryos in teratogen screening. Teratog. Carcinog. Mutagen. 1982, 2, 231–242. [Google Scholar] [CrossRef]
- Webster, W.S.; Brown-Woodman, P.D.; Ritchie, H.E. A review of the contribution of whole embryo culture to the determination of hazard and risk in teratogenicity testing. Int. J. Dev. Biol. 2002, 41, 329–335. [Google Scholar]
- Caserta, D.; Grazianom, A.; Lo Monte, G.; Bordi, G.; Moscarini, M. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur. Rev. Med. Pharm. Sci. 2013, 17, 2198–2206. [Google Scholar]
- Birks, L.; Casas, M.; Garcia, A.M.; Alexander, J.; Barros, H.; Bergström, A.; Bonde, J.P.; Burdorf, A.; Costet, N.; Danileviciute, A.; et al. Occupational exposure to endocrine-disrupting chemicals and birth weight and length of gestation: A European meta-analysis. Environ. Health Perspect. 2016, 124, 1785–1793. [Google Scholar] [CrossRef]
- Grindler, N.M.; Vanderlinden, L.; Karthikraj, R.; Kannan, K.; Teal, S.; Polotsky, A.J.; Powell, T.L.; Yang, I.V.; Jansson, T. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women. Sci. Rep. 2018, 8, 6086. [Google Scholar] [CrossRef]
- Estevan, C.; Pamies, D.; Vilanova, E.; Sogorb, M.A. Chapter 9—OECD Guidelines for In Vivo Testing of Reproductive Toxicity. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 163–178. [Google Scholar]
- US Public Law 114-182 Frank R. Lautenberg Chemical Safety for the 21st Century Act. 2016.
- National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy; The National Academies Press: Washington, DC, USA, 2007; 216p. [Google Scholar]
- National Research Council. Scientific Frontiers in Developmental Toxicology and Risk Assessment; The National Academies Press: Washington, DC, USA, 2000; 354p. [Google Scholar]
- Scialli, A.R.; Daston, G.; Chen, C.; Coder, P.S.; Euling, S.Y.; Foreman, J.; Hoberman, A.M.; Hui, J.; Knudsen, T.; Makris, S.L.; et al. Rethinking developmental toxicity testing: Evolution or revolution? Birth Defects Res. 2018, 110, 840–850. [Google Scholar] [CrossRef] [Green Version]
- U.S. EPA. Strategic Plan to Promote the Development and Implementation of Alternative Test Methods within the TSCA Program; EPA-740-R1-8004; U.S. EPA: Washington, DC, USA, 2018.
- U.S. EPA. Directive to Prioritize Efforts to Reduce Animal Testing; U.S. EPA: Washington, DC, USA, 2019.
- U.S. EPA. New Approach Methods Work Plan: Reducing Use of Animals in Chemical Testing; EPA 615B2001; U.S. Environmental Protection Agency: Washington, DC, USA, 2020.
- Richard, A.M.; Judson, R.S.; Houck, K.; Grulke, C.; Volarath, P.; Thillainadarajah, I.; Yang, C.; Rathman, J.F.; Martin, M.T.; Wambaugh, J.; et al. ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem. Res. Toxicol. 2016, 29, 1225–1251. [Google Scholar] [CrossRef] [Green Version]
- Judson, R.; Houck, K.; Martin, M.; Knudsen, T.; Thomas, R.S.; Sipes, N.; Shah, I.; Wambaugh, J.; Crofton, K. In vitro and modelling approaches to risk assessment from the U.S. Environmental Protection Agency ToxCast programme. Basic Clin. Pharmacol. Toxicol. 2014, 115, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dix, D.J.; Houck, K.A.; Martin, M.T.; Richard, A.M.; Setzer, R.W.; Kavlock, R.J. The ToxCast Program for Prioritizing Toxicity Testing of Environmental Chemicals. Toxicol. Sci. 2006, 95, 5–12. [Google Scholar] [CrossRef]
- Kavlock, R.; Chandler, K.; Houck, K.; Hunter, S.; Judson, R.; Kleinstreuer, N.; Knudsen, T.; Martin, M.; Padilla, S.; Reif, D.; et al. Update on EPA’s ToxCast program: Providing high throughput decision support tools for chemical risk management. Chem. Res. Toxicol. 2012, 25, 1287–1302. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [Green Version]
- Hong, E.-J.; Jeung, E.-B. Assessment of Developmental Toxicants using Human Embryonic Stem Cells. Toxicol. Res. 2013, 29, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siggia, E.D.; Warmflash, A. Chapter One—Modeling Mammalian Gastrulation with Embryonic Stem Cells. In Current Topics in Developmental Biology; Brivanlou, A.H., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–23. [Google Scholar]
- Sanz-Ezquerro, J.J.; Münsterberg, A.E.; Stricker, S. Editorial: Signaling Pathways in Embryonic Development. Front. Cell Dev. Biol. 2017, 5, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbazi, M.N. Mechanisms of human embryo development: From cell fate to tissue shape and back. Development 2020, 147, dev190629. [Google Scholar] [CrossRef]
- Zhu, M.; Zernicka-Goetz, M. Principles of Self-Organization of the Mammalian Embryo. Cell 2020, 183, 1467–1478. [Google Scholar] [CrossRef]
- Weitzer, W. Embryonic Stem Cell-Derived Embryoid Bodies: An in vitro Model of Eutherian Pregastrulation Development and Early Gastrulation. In Stem Cells; Wobus, A.M., Boheler, K.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 21–51. [Google Scholar]
- Genschow, E.; Spielmann, H.; Scholz, G.; Seiler, A.; Brown, N.; Piersma, A.; Brady, M.; Clemann, N.; Huuskonen, H.; Paillard, F.; et al. The ECVAM international validation study on in vitro embryotoxicity tests: Results of the definitive phase and evaluation of prediction models. European Centre for the Validation of Alternative Methods. Altern. Lab. Anim. 2002, 30, 151–176. [Google Scholar] [CrossRef]
- Paquette, J.A.; Kumpf, S.W.; Streck, R.D.; Thomson, J.J.; Chapin, R.E.; Stedman, D.B. Assessment of the Embryonic Stem Cell Test and application and use in the pharmaceutical industry. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2008, 83, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Buesen, R.; Genschow, E.; Slawik, B.; Visan, A.; Spielmann, H.; Luch, A.; Seiler, A. Embryonic stem cell test remastered: Comparison between the validated EST and the new molecular FACS-EST for assessing developmental toxicity in vitro. Toxicol. Sci. 2009, 108, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Barrier, M.; Jeffay, S.; Nichols, H.P.; Chandler, K.J.; Hoopes, M.R.; Slentz-Kesler, K.; Iii, E.S.H. Mouse embryonic stem cell adherent cell differentiation and cytotoxicity (ACDC) assay. Reprod. Toxicol. 2011, 31, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Chandler, K.J.; Barrier, M.; Jeffay, S.; Nichols, H.P.; Kleinstreuer, N.C.; Singh, A.V.; Reif, D.M.; Sipes, N.S.; Judson, R.S.; Dix, D.J.; et al. Evaluation of 309 environmental chemicals using a mouse embryonic stem cell adherent cell differentiation and cytotoxicity assay. PLoS ONE 2011, 6, e18540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Ando, S.; Yamashita, N.; Horie, N.; Saito, K. Evaluation of Novel High-Throughput Embryonic Stem Cell Tests with New Molecular Markers for Screening Embryotoxic Chemicals In Vitro. Toxicol. Sci. 2011, 124, 460–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Coz, F.; Suzuki, N.; Nagahori, H.; Omori, T.; Saito, K. Hand1-Luc embryonic stem cell test (Hand1-Luc EST): A novel rapid and highly reproducible in vitro test for embryotoxicity by measuring cytotoxicity and differentiation toxicity using engineered mouse ES cells. J. Toxicol. Sci. 2015, 40, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Uibel, F.; Mühleisen, A.; Köhle, C.; Weimer, M.; Stummann, T.C.; Bremer, S.; Schwarz, M. ReProGlo: A new stem cell-based reporter assay aimed to predict embryotoxic potential of drugs and chemicals. Reprod. Toxicol. 2010, 30, 103–112. [Google Scholar] [CrossRef]
- Van Dartel, D.A.M.; Pennings, J.L.A.; de la Fonteyne, L.J.J.; Brauers, K.J.J.; Claessen, S.; van Delft, J.H.; Kleinjans, J.C.S.; Piersma, A.H. Evaluation of Developmental Toxicant Identification Using Gene Expression Profiling in Embryonic Stem Cell Differentiation Cultures. Toxicol. Sci. 2010, 119, 126–134. [Google Scholar] [CrossRef]
- Van Dartel, D.A.; Piersma, A.H. The embryonic stem cell test combined with toxicogenomics as an alternative testing model for the assessment of developmental toxicity. Reprod. Toxicol. 2011, 32, 235–244. [Google Scholar] [CrossRef]
- Pennings, J.L.; Van Dartel, D.A.; Robinson, J.F.; Pronk, T.E.; Piersma, A.H. Gene set assembly for quantitative prediction of developmental toxicity in the embryonic stem cell test. Toxicology 2011, 284, 63–71. [Google Scholar] [CrossRef]
- West, P.R.; Weir, A.M.; Smith, A.M.; Donley, E.L.; Cezar, G.G. Predicting human developmental toxicity of pharmaceuticals using human embryonic stem cells and metabolomics. Toxicol. Appl. Pharmacol. 2010, 247, 18–27. [Google Scholar] [CrossRef]
- Palmer, J.A.; Smith, A.M.; Egnash, L.A.; Conard, K.R.; West, P.R.; Burrier, R.E.; Donley, E.L.; Kirchner, F.R. Establishment and assessment of a new human embryonic stem cell-based biomarker assay for developmental toxicity screening. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2013, 98, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Marikawa, Y.; Tamashiro, D.A.A.; Fujita, T.C.; Alarcón, V.B. Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis 2009, 47, 93–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beccari, L.; Moris, N.; Girgin, M.; Turner, D.; Baillie-Johnson, P.; Cossy, A.-C.; Lutolf, M.P.; Duboule, D.; Arias, A.M. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 2018, 562, 272–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moris, N.; Anlas, K.; van den Brink, S.C.; Alemany, A.; Schröder, J.; Ghimire, S.; Balayo, T.; van Oudenaarden, A.; Arias, A.M. An in vitro model of early anteroposterior organization during human development. Nature 2020, 582, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Warkus, E.L.L.; Marikawa, Y. Exposure-Based Validation of an In Vitro Gastrulation Model for Developmental Toxicity Assays. Toxicol. Sci. 2017, 157, 235–245. [Google Scholar] [CrossRef]
- Xing, J.; Cao, Y.; Yu, Y.; Li, H.; Song, Z.; Yu, H. In Vitro Micropatterned Human Pluripotent Stem Cell Test (µP-hPST) for Morphometric-Based Teratogen Screening. Sci. Rep. 2017, 7, 8491. [Google Scholar] [CrossRef] [Green Version]
- Mantziou, V.; Baillie-Benson, P.; Jaklin, M.; Kustermann, S.; Arias, A.M.; Moris, N. In vitro teratogenicity testing using a 3D, embryo-like gastruloid system. Reprod. Toxicol. 2021, 105, 72–90. [Google Scholar] [CrossRef]
- Kameoka, S.; Babiarz, J.; Kolaja, K.; Chiao, E. A High-Throughput Screen for Teratogens Using Human Pluripotent Stem Cells. Toxicol. Sci. 2013, 137, 76–90. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chaturvedi, P.; Rankin, S.A.; Fish, M.B.; Wlizla, M.; Paraiso, K.D.; MacDonald, M.; Chen, X.; Weirauch, M.T.; Blitz, I.L.; et al. Sox17 and β-catenin co-occupy Wnt-responsive enhancers to govern the endoderm gene regulatory network. eLife 2020, 9, e58029. [Google Scholar] [CrossRef]
- Wells, J.M.; Melton, D.A. Vertebrate Endoderm Development. Annu. Rev. Cell Dev. Biol. 1999, 15, 393–410. [Google Scholar] [CrossRef]
- Kanai-Azuma, M.; Kanai, Y.; Gad, J.M.; Tajima, Y.; Taya, C.; Kurohmaru, M.; Sanai, Y.; Yonekawa, H.; Yazaki, K.; Tam, P.P.L.; et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 2002, 129, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Martyn, I.; Kanno, T.Y.; Ruzo, A.; Siggia, E.D.; Brivanlou, A.H. Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature 2018, 558, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Zurlinden, T.J.; Saili, K.S.; Rush, N.; Kothiya, P.; Judson, R.S.; Houck, K.A.; Hunter, E.S.; Baker, N.C.; Palmer, J.A.; Thomas, R.S.; et al. Profiling the ToxCast Library with a Pluripotent Human (H9) Stem Cell Line-Based Biomarker Assay for Developmental Toxicity. Toxicol. Sci. 2020, 174, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tsankov, A.M.; Akopian, V.; Pop, R.; Chetty, S.; Gifford, C.A.; Daheron, L.; Tsankova, N.M.; Meissner, A. A qPCR ScoreCard quantifies the differentiation potential of human pluripotent stem cells. Nat. Biotechnol. 2015, 33, 1182–1192. [Google Scholar] [CrossRef]
- Belair, D.G.; Lu, G.; Waller, L.E.; Gustin, J.A.; Collins, N.D.; Kolaja, K. Thalidomide Inhibits Human iPSC Mesendoderm Differentiation by Modulating CRBN-dependent Degradation of SALL4. Sci. Rep. 2020, 10, 2864. [Google Scholar] [CrossRef] [Green Version]
- Collins, T.; Welsh, J.; Black, T.; Collins, E. A study of the teratogenic potential of caffeine given by oral intubation to rats. Regul. Toxicol. Pharmacol. 1981, 1, 355–378. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.Y.; Ku, S.-Y.; Kim, S.H.; Choi, Y.M.; Moon, S.Y. The Effect of Estrogen Compounds on Human Embryoid Bodies. Reprod. Sci. 2013, 20, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Xing, L.; Liu, R.; Jiang, J.; Wang, W.; Shang, L.; Wei, X.; Hao, W. Individual and combined developmental toxicity assessment of bisphenol A and genistein using the embryonic stem cell test in vitro. Food Chem. Toxicol. 2013, 60, 497–505. [Google Scholar] [CrossRef]
- Gill, S.; Kumara, V. Comparative Neurodevelopment Effects of Bisphenol A and Bisphenol F on Rat Fetal Neural Stem Cell Models. Cells 2021, 10, 793. [Google Scholar] [CrossRef]
- Inman, G.J.; Nicolás, F.J.; Callahan, J.F.; Harling, J.D.; Gaster, L.M.; Reith, A.D.; Laping, N.J.; Hill, C.S. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002, 62, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, G.A.D.; Fred, L. Python 3 Reference Manual. CreateSpace 2009, 10, 1593511. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Filer, D.L.; Kothiya, P.; Setzer, R.W.; Judson, R.S.; Martin, M.T. Tcpl: The ToxCast pipeline for high-throughput screening data. Bioinformatics 2016, 33, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Chung, T.D.Y.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Zhang, X.D. Illustration of SSMD, z Score, SSMD*, z* Score, and t Statistic for Hit Selection in RNAi High-Throughput Screens. J. Biomol. Screen. 2011, 16, 775–785. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Dean, A.; Walter, M.; Bao, Y.; Hu, Y.; Ruan, J.; Li, R. Cell density-dependent transcriptional activation of endocrine-related genes in human adipose tissue-derived stem cells. Exp. Cell Res. 2010, 316, 2087–2098. [Google Scholar] [CrossRef] [Green Version]
- Gage, B.K.; Webber, T.D.; Kieffer, T.J. Initial cell seeding density influences pancreatic endocrine development during in vitro differentiation of human embryonic stem cells. PLoS ONE 2013, 8, e82076. [Google Scholar] [CrossRef] [Green Version]
- Wilson, H.K.; Canfield, S.G.; Hjortness, M.K.; Palecek, S.P.; Shusta, E.V. Exploring the effects of cell seeding density on the differentiation of human pluripotent stem cells to brain microvascular endothelial cells. Fluids Barriers CNS 2015, 12, 13. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.J.; Turner, W.; Glaser, D.E.; McCloskey, K.E.; Filipp, F.V. Metabolic shift in density-dependent stem cell differentiation. Cell Commun. Signal. 2017, 15, 44. [Google Scholar] [CrossRef] [Green Version]
- Daston, G.P.; Beyer, B.K.; Carney, E.W.; Chapin, R.E.; Friedman, J.M.; Piersma, A.H.; Rogers, J.M.; Scialli, A.R. Exposure-Based Validation List for Developmental Toxicity Screening Assays. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2014, 101, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Panzica-Kelly, J.M.; Brannen, K.C.; Ma, Y.; Zhang, C.X.; Flint, O.P.; Lehman-McKeeman, L.D.; Augustine-Rauch, K.A. Establishment of a molecular embryonic stem cell developmental toxicity assay. Toxicol. Sci. 2013, 131, 447–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine-Rauch, K.; Zhang, C.X.; Panzica-Kelly, J.M. A Developmental Toxicology Assay Platform for Screening Teratogenic Liability of Pharmaceutical Compounds. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2016, 107, 4–20. [Google Scholar] [CrossRef]
- Wise, L.D. Numeric Estimates of Teratogenic Severity from Embryo-Fetal Developmental Toxicity Studies. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2016, 107, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, B. Environmental Factors in Birth Defects: What We Need to Know. Environ. Health Perspect. 2009, 117, A440–A447. [Google Scholar] [CrossRef] [Green Version]
- Südbeck, P.; Scherer, G. Two Independent Nuclear Localization Signals Are Present in the DNA-binding High-mobility Group Domains of SRY and SOX9. J. Biol. Chem. 1997, 272, 27848–27852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagga, B.; Edwards, M.; Pagin, M.; Wagstaff, K.M.; Aragão, D.; Roman, N.; Nanson, J.D.; Raidal, S.R.; Dominado, N.; Stewart, M.; et al. Structural basis for nuclear import selectivity of pioneer transcription factor SOX2. Nat. Commun. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Chapin, R.; Augustine-Rauch, K.; Beyer, B.; Daston, G.; Finnell, R.; Flynn, T.; Hunter, S.; Mirkes, P.; O’Shea, K.S.; Piersma, A. State of the art in developmental toxicity screening methods and a way forward: A meeting report addressing embryonic stem cells, whole embryo culture, and zebrafish. Birth Defects Res. B Dev. Reprod. Toxicol. 2008, 83, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Luz, A.L.; Tokar, E.J. Pluripotent Stem Cells in Developmental Toxicity Testing: A Review of Methodological Advances. Toxicol. Sci. 2018, 165, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Piersma, A.H.; Hessel, E.V.; Staal, Y.C. Retinoic acid in developmental toxicology: Teratogen, morphogen and biomarker. Reprod. Toxicol. 2017, 72, 53–61. [Google Scholar] [CrossRef]
- Mirkes, P.E. Cyclophosphamide teratogenesis: A review. Teratog. Carcinog. Mutagen. 1985, 5, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Ozolinsš, T.R. Cyclophosphamide and the Teratology society: An awkward marriage. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.; Banton, M.; Klapacz, J.; Shen, H. A toxicological review of the ethylene glycol series: Commonalities and differences in toxicity and modes of action. Toxicol. Lett. 2017, 278, 66–83. [Google Scholar] [CrossRef]
- Ritchie, H.E.; Moore, N.P.; Webster, W.S. Editor’s Highlight: Ethylene Glycol Teratogenicity: A Role for Embryonic Acidosis? Toxicol. Sci. 2017, 161, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Piersma, A.; Bosgra, S.; van Duursen, M.; Hermsen, S.; Jonker, L.; Kroese, E.; van der Linden, S.; Man, H.; Roelofs, M.; Schulpen, S.; et al. Evaluation of an alternative in vitro test battery for detecting reproductive toxicants. Reprod. Toxicol. 2013, 38, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Mao, Y.; Gurr, J.A.; Hickok, N.J. Retinoic acid regulates ornithine decarboxylase gene expression at the transcriptional level. Biochem. J. 1993, 295, 641–644. [Google Scholar] [CrossRef] [Green Version]
- Saili, K.S.; Antonijevic, T.; Zurlinden, T.J.; Shah, I.; Deisenroth, C.; Knudsen, T.B. Molecular characterization of a toxicological tipping point during human stem cell differentiation. Reprod. Toxicol. 2020, 91, 1–13. [Google Scholar] [CrossRef]
- Sewell, F.; Doe, J.; Gellatly, N.; Ragan, I.; Burden, N. Steps towards the international regulatory acceptance of non-animal methodology in safety assessment. Regul. Toxicol. Pharmacol. 2017, 89, 50–56. [Google Scholar] [CrossRef]


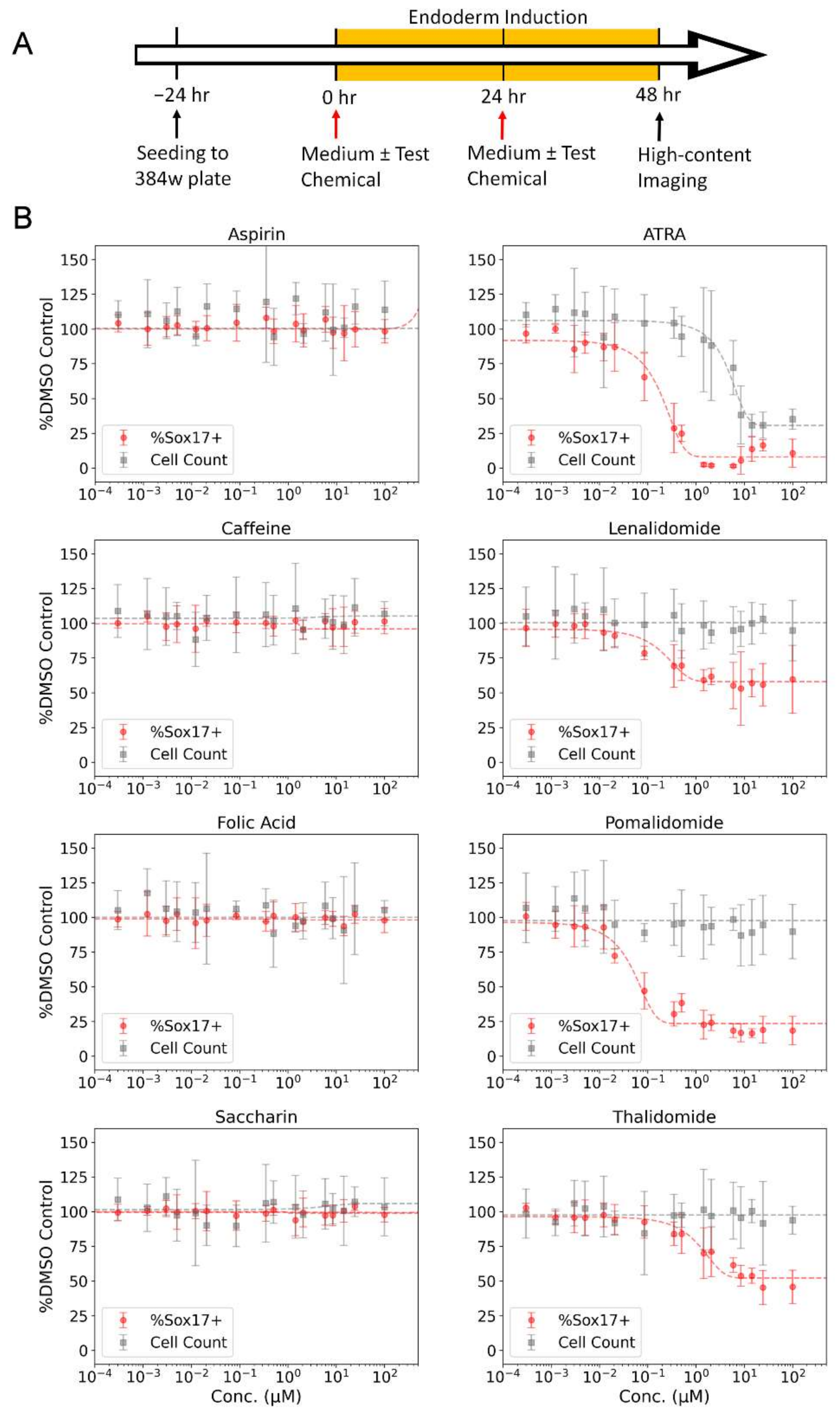


| Chemical | CASRN | DTXSID | Developmental Toxicant Classification | FDA Pregnancy Category | IC50 (µM) | % Max Inhibition |
|---|---|---|---|---|---|---|
| All-Trans Retinoic Acid | 302-79-4 | DTXSID7021239 | Positive | X | 0.16 | 93 |
| Lenalidomide | 191732-72-6 | DTXSID8046664 | Positive | X | 0.13 | 44 |
| Pomalidomide | 19171-19-8 | DTXSID40893458 | Positive | X | 0.05 | 81 |
| Thalidomide | 50-35-1 | DTXSID9022524 | Positive | X | 1.71 | 58 |
| Aspirin | 50-78-2 | DTXSID5020108 | Negative | C | - | - |
| Caffeine | 58-08-2 | DTXSID0020232 | Negative | B | - | - |
| Folic Acid | 59-30-3 | DTXSID0022519 | Negative | A | - | - |
| Saccharin | 81-07-2 | DTXSID5021251 | Negative | A | - | - |
| Assay Endpoint ID (AEID) | Assay Endpoint Name | Interpretation |
|---|---|---|
| 3093 | CCTE_Deisenroth_DEVTOX_RUES2-GLR_Endo_Sox17_up | SOX17 positive |
| 3094 | CCTE_Deisenroth_DEVTOX_RUES2-GLR_Endo_Sox17_dn | SOX17 negative |
| 3095 | CCTE_Deisenroth_DEVTOX_RUES2-GLR_Endo_Sox2_up | SOX2 positive |
| 3096 | CCTE_Deisenroth_DEVTOX_RUES2-GLR_Endo_Bra_up | BRA positive |
| 3097 | CCTE_Deisenroth_DEVTOX_RUES2-GLR_Endo_ CellCount_up | Cell count positive |
| 3098 | CCTE_Deisenroth_DEVTOX_RUES2-GLR_Endo_ CellCount_dn | Cell count negative |
| CASRN | Chemical Name | DT Class. | hPST | DevTox-GLR Endo | ||||
|---|---|---|---|---|---|---|---|---|
| TC50 (µM) | IC50 (µM) | Call | CC50 (µM) | AC50 (µM) | Call | |||
| 50-76-0 | Actinomycin D | Pos | <0.01 | <0.01 | TP | 4.8 × 10−4 | 3.7 × 10−5 | TP |
| 25316-40-9 | Doxorubicin | Pos | <0.3 | 0.3 | TP | 3.3 × 10−3 | 1.9 × 10−3 | TP |
| 302-79-4 | All-Trans-Retinoic Acid | Pos | 33 | 0.07 | TP | 5.53 | 0.10 | TP |
| 4759-48-2 | 13-cis Retinoic Acid | Pos | 140 | 0.2 | TP | 10.24 | 0.16 | TP |
| 59-14-3 | 5-Bromo-2’-deoxyuridine | Pos | 3.4 | 0.2 | TP | 9.37 | 0.55 | TP |
| 284461-73-0 | Sorafenib | Pos | <2 | <2 | TP | 0.61 | 0.62 | TP |
| 341031-54-7 | Sunitinib | Pos | 3.6 | 2.8 | TP | 1.26 | 0.63 | TP |
| 56-53-1 | Diethylstilbestrol | Pos | 4.4 | 4.4 | TP | 0.71 | 0.64 | TP |
| 302962-49-8 | Dasatinib | Pos | <1 | <1 | TP | 0.05 | 0.72 | TP |
| 50-35-1 | Thalidomide | Pos | >200 | 0.5 | TP | >200 | 1.64 | TP |
| 146939-27-7 | Ziprasidone | Pos | 5.1 | 2.8 | TP | 2.42 | 3.57 | TP |
| 443913-73-3 | Vandetanib | Pos | 4.4 | 3.5 | TP | 4.32 | 4.90 | TP |
| 184475-35-2 | Gefitinib | Pos | 10 | 10 | TP | 6.60 | 5.75 | TP |
| 51-21-8 | 5-Fluorouracil | Pos | 0.7 | 0.6 | TP | 5.72 | 8.48 | TP |
| 87051-43-2 | Ritanserin | Pos | 27 | 7.3 | TP | 9.47 | 9.98 | TP |
| 220127-57-1 | Imatinib | Pos | 22 | 11 | TP | 13.41 | 13.45 | TP |
| 329-89-5 | 6-Aminonicotinamide | Pos | 8 | 7 | TP | 23.85 | 16.61 | TP |
| 866405-64-3 | Dorsomorphin | Pos | 2.4 | 0.3 | TP | 16.7 | 17.1 | TP |
| 21535-47-7 | Mianserin | Pos | 60 | 3.4 | TP | 33.56 | 34.85 | TP |
| 298-46-4 | Carbamazepine | Pos | 0.19 | 0.08 | TP | 136.8 | 102.7 | TP |
| 99-66-1 | Valproic Acid | Pos | 3.3 | 2.6 | TP | >200 | 123.9 | TP |
| 59277-89-3 | Acyclovir | Neg | >200 | >200 | TN | >200 | >200 | TN |
| 50-78-2 | Aspirin | Neg | >200 | >200 | TN | >200 | >200 | TN |
| 58-08-2 | Caffeine | Neg | >400 | >400 | TN | >200 | >200 | TN |
| 59-30-3 | Folic Acid | Neg | 84 | 39 | TN | >200 | >200 | TN |
| 59-67-6 | Niacin | Neg | >200 | >200 | TN | >200 | >200 | TN |
| 113-98-4 | Penicillin G | Neg | >400 | >400 | TN | >200 | >200 | TN |
| 81-07-2 | Saccharin | Neg | >400 | >400 | TN | >200 | >200 | TN |
| 189188-57-6 | Tegaserod Maleate | Neg | 4.5 | 4.6 | FP | 5.06 | 5.32 | FP |
| 668985-31-7 | Esomeprazole | Neg | 101 | 32 | TN | 38.5 | 33.8 | FP |
| 74050-98-9 | Ketanserin | Neg | >200 | >200 | TN | 57.5 | 36.6 | FP |
| 154-23-4 | Cianidanol | Neg | 200 | 97 | TN | 193.6 | 92.9 | FP |
| 41372-08-1 | Methyldopa | Neg | 462 | 272 | TN | >200 | 113.1 | FP |
| 15687-27-1 | Ibuprofen | Neg | >200 | >200 | TN | 79.4 | 161.8 | FP |
| Sensitivity | 100.0% | 100.0% | ||||||
| Specificity | 92.3% | 53.8% | ||||||
| Balanced Accuracy | 96.2% | 76.9% | ||||||
| CASRN | Chemical Name | DT Class. | quickPredict | DevTox-GLR Endo | ||||
|---|---|---|---|---|---|---|---|---|
| CV (µM) | TI (µM) | Call | CC (µM) | ACC (µM) | Call | |||
| 302-79-4 | All-Trans-Retinoic Acid | Pos | NA | 0.003 | TP | 3.84 | 0.04 | TP |
| 50-35-1 | Thalidomide | Pos | NA | 1.27 | TP | NA | 0.25 | TP |
| 69-74-9 | Cytarabine | Pos | 0.083 | 0.054 | TP | 0.05 | 0.46 | TP |
| 56-53-1 | Diethylstilbestrol | Pos | NA | NA | FN | 0.60 | 0.54 | TP |
| 75330-75-5 | Lovastatin | Pos | NA | 5.1 | TP | 0.71 | 0.93 | TP |
| 51-21-8 | 5-Fluorouracil | Pos | 1.45 | 2.02 | TP | 20.34 | 7.07 | TP |
| 53-86-1 | Indomethacin | Pos | 44.1 | 72.7 | TP | 8.70 | 9.38 | TP |
| 19774-82-4 | Amiodarone | Pos | NA | 5.1 | TP | 11.14 | 14.22 | TP |
| 147-24-0 | Diphenhydramine | Pos | 3.76 | 0.588 | TP | 14.34 | 16.78 | TP |
| 55-98-1 | Busulfan | Pos | 4.91 | 2.31 | TP | 5.79 | 25.26 | TP |
| 80-05-7 | Bisphenol A | Pos | 39.4 | NA | FN | 21.12 | 31.75 | TP |
| 13292-46-1 | Rifampicin | Pos | NA | 2.46 | TP | 18.11 | 37.85 | TP |
| 298-46-4 | Carbamazepine | Pos | NA | 2.29 | TP | 57.78 | 64.02 | TP |
| 4376-20-9 | MEHP | Pos | NA | 167 | TP | 61.55 | 76.33 | TP |
| 81-81-2 | Warfarin | Pos | NA | NA | FN | 197.75 | 93.33 | TP |
| 99-66-1 | Valproic Acid | Pos | 271 | 155 | TP | NA | 177.33 | TP |
| 57-41-0 | 5,5-Diphenylhydantoin | Pos | NA | NA | FN | 191.82 | 201.56 | TP |
| 57-55-6 | 1,2-Propylene glycol | Neg | 246,664 | 327,552 | TN | NA | NA | TN |
| 103-90-2 | Acetaminophen | Neg | NA * | NA | TN | NA | NA | TN |
| 79-06-1 | Acrylamide | Neg | NA | NA | TN | NA | NA | TN |
| 50-78-2 | Aspirin | Neg | NA * | NA | TN | NA | NA | TN |
| 58-08-2 | Caffeine | Neg | NA | NA | TN | NA | NA | TN |
| 464-49-3 | D-Camphor | Neg | NA | NA | TN | NA | NA | TN |
| 131-11-3 | Dimethyl Phthalate | Neg | NA | NA | TN | NA | NA | TN |
| 59-30-3 | Folic Acid | Neg | NA | NA | TN | NA | NA | TN |
| 54-85-3 | Isoniazid | Neg | NA * | NA | TN | NA | NA | TN |
| 81-07-2 | Saccharin | Neg | NA | NA | TN | NA | NA | TN |
| 69-72-7 | Salicylic Acid | Neg | 1795 | 513 | TN | NA | NA | TN |
| 599-79-1 | Sulfasalazine | Neg | NA * | NA | TN | NA | NA | TN |
| 68-26-8 | Retinol | Neg | NA | NA | TN | 12.63 | 13.05 | FP |
| 94-26-8 | Butylparaben | Neg | NA | NA | TN | NA | 34.47 | FP |
| 51-52-5 | 6-Propyl-2-thiouracil | Pos | NA | NA | FN | NA | NA | FN |
| 10043-35-3 | Boric acid | Pos | NA | NA | FN | NA | NA | FN |
| 4449-51-8 | Cyclopamine | Pos | NA | NA | FN | NA | NA | FN |
| 6055-19-2 | Cyclophosphamide | Pos | NA * | NA | FN | 100.19 | NA | FN |
| 2392-39-4 | Dexamethasone | Pos | 21.8 | 37.7 | TP | 13.83 | NA | FN |
| 107-21-1 | Ethylene Glycol | Pos | NA | NA | FN | NA | NA | FN |
| 127-07-1 | Hydroxyurea | Pos | 237 | 74.9 | TP | 64.54 | NA | FN |
| 133073-73-1 | Methotrexate | Pos | 0.062 | 0.059 | TP | 0.35 | NA | FN |
| 3056-17-5 | Stavudine | Pos | NA | 32.5 | TP | 90.28 | NA | FN |
| Sensitivity | 65.4% | 65.38% | ||||||
| Specificity | 100.0% | 85.7% | ||||||
| Balanced Accuracy | 82.7% | 75.5% | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamble, J.T.; Hopperstad, K.; Deisenroth, C. The DevTox Germ Layer Reporter Platform: An Assay Adaptation of the Human Pluripotent Stem Cell Test. Toxics 2022, 10, 392. https://doi.org/10.3390/toxics10070392
Gamble JT, Hopperstad K, Deisenroth C. The DevTox Germ Layer Reporter Platform: An Assay Adaptation of the Human Pluripotent Stem Cell Test. Toxics. 2022; 10(7):392. https://doi.org/10.3390/toxics10070392
Chicago/Turabian StyleGamble, John T., Kristen Hopperstad, and Chad Deisenroth. 2022. "The DevTox Germ Layer Reporter Platform: An Assay Adaptation of the Human Pluripotent Stem Cell Test" Toxics 10, no. 7: 392. https://doi.org/10.3390/toxics10070392






