The Determination of Polycyclic Aromatic Hydrocarbons (PAHs) with HPLC-DAD-FLD and GC-MS Techniques in the Dissolved and Particulate Phase of Road-Tunnel Wash Water: A Case Study for Cross-Array Comparisons and Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sampling Locations and Sample Treatment
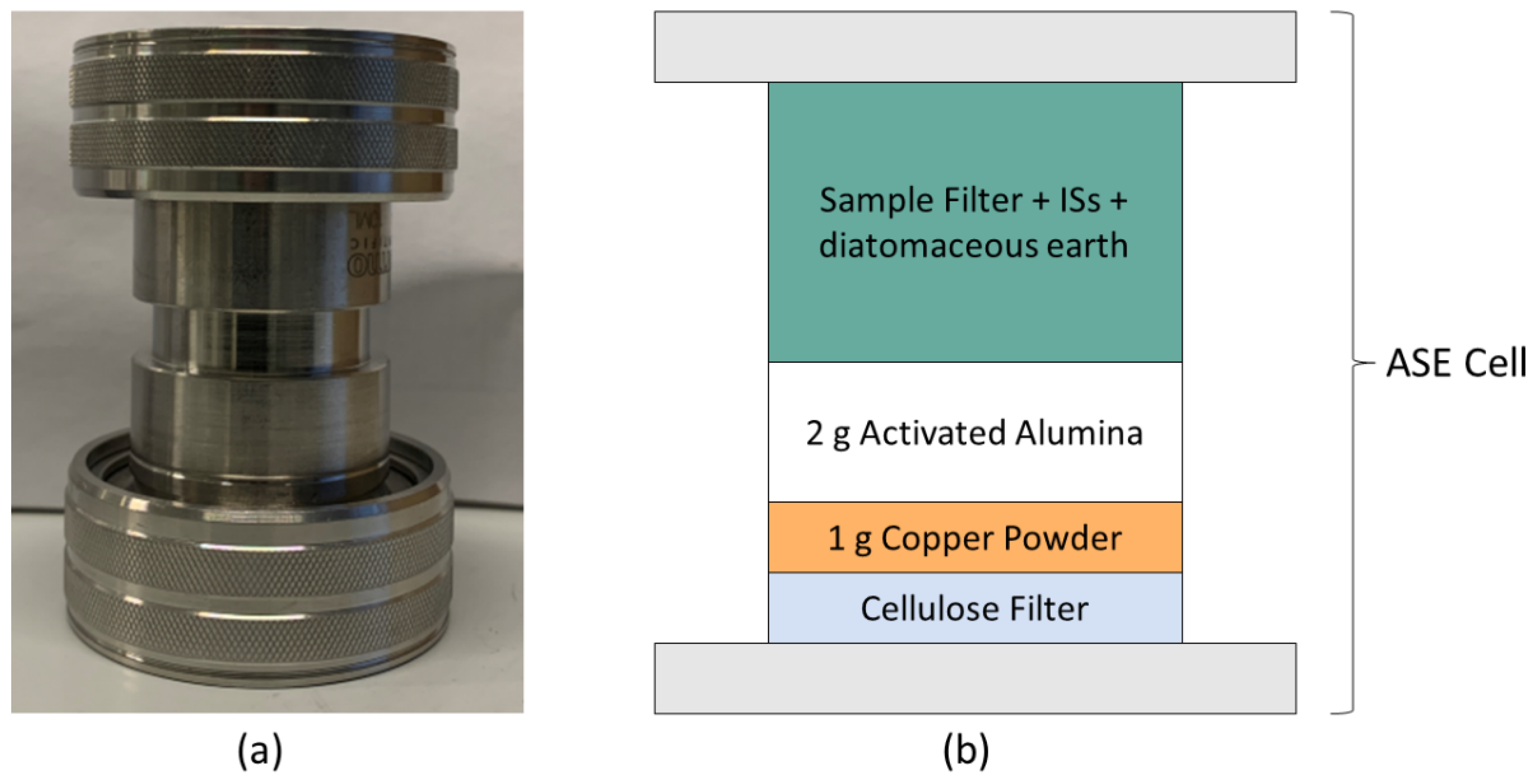
2.3. Sample Preparation: Particulate Phase
2.4. Sample Preparation: Dissolved Phase
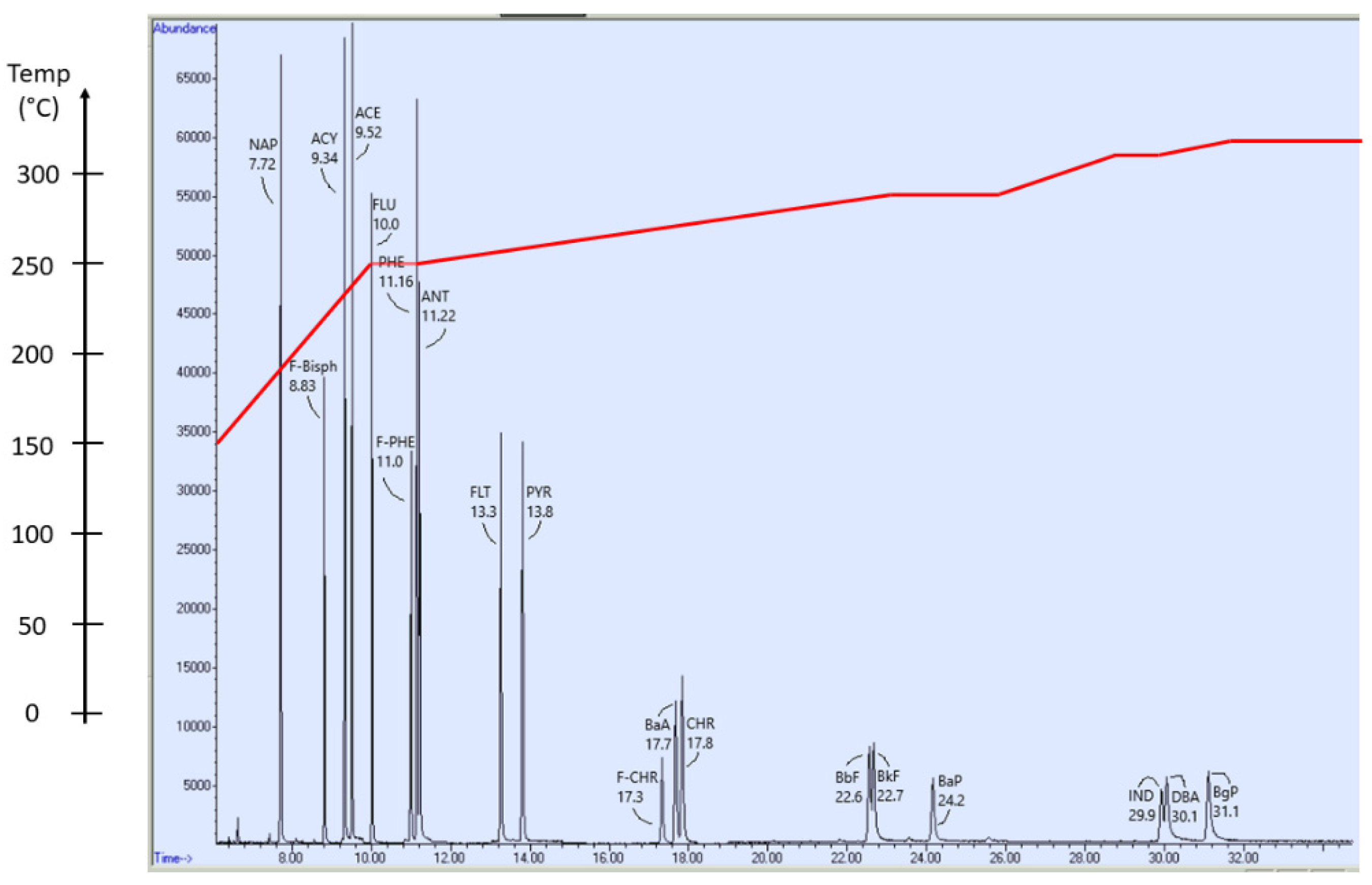
2.5. GC-MS Determination
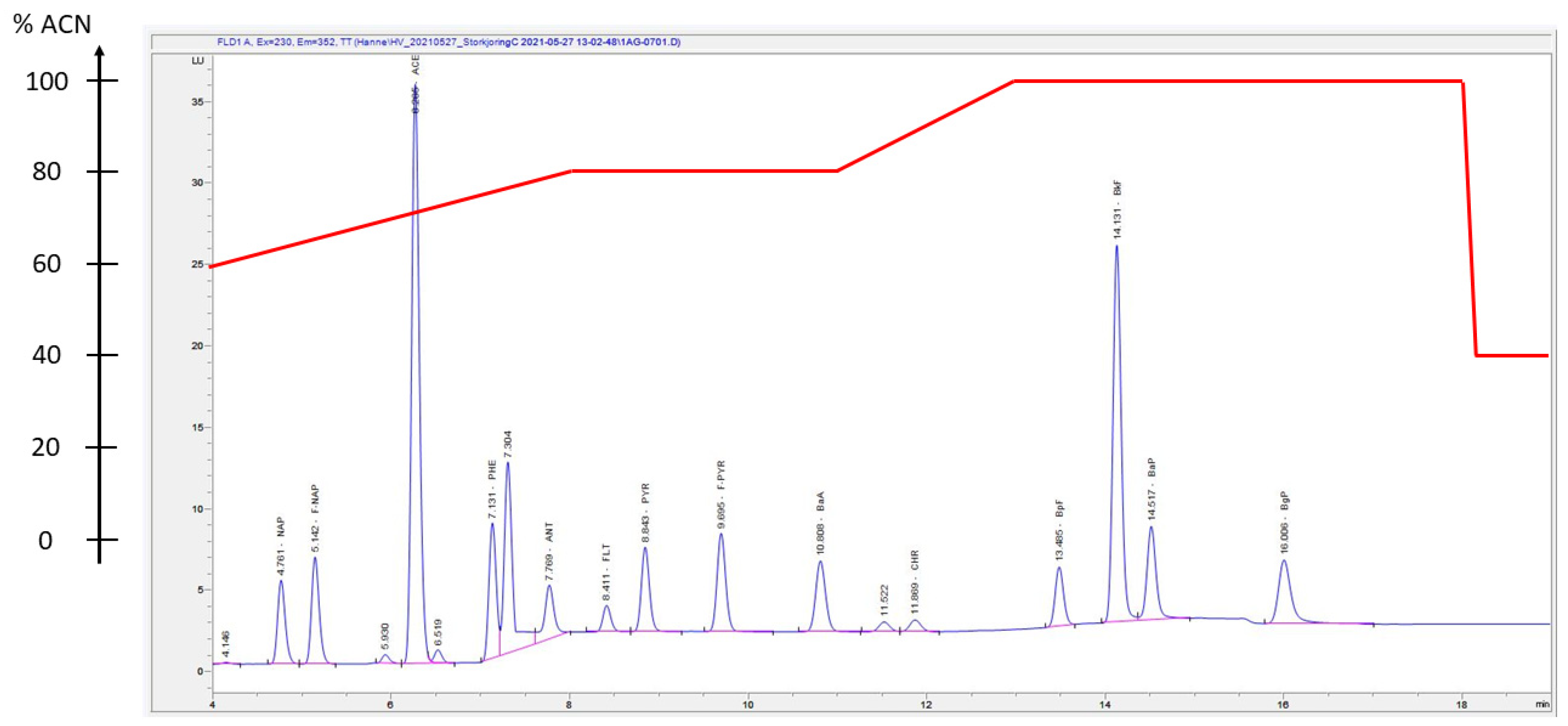
2.6. HPLC-DAD-FLD Determination
2.7. Extraction Performance
2.8. Performance Characteristics of the HPLC-DAD-FLD and GC-MS Method
2.9. Calculation of Specific PAH Forensic Ratios
2.10. Data Analysis
3. Results and Discussion
3.1. Method Performance
3.2. Application of Methods
3.2.1. Performance
3.2.2. Concentrations in Particulate and Dissolved Phases
3.2.3. Forensic Ratios
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markiewicz, A.; Björklund, K.; Eriksson, E.; Kalmykova, Y.; Strömvall, A.-M.; Siopi, A. Emissions of Organic Pollutants from Traffic and Roads: Priority Pollutants Selection and Substance Flow Analysis. Sci. Total Environ. 2017, 580, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.H.; Harrison, R.M.; Harrad, S. The Contribution of Traffic to Atmospheric Concentrations of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Technol. 1999, 33, 3538–3542. [Google Scholar] [CrossRef]
- Meland, S. Tunnel wash water—A source to aquatic pollution. VANN 2012, 47, 182–193. [Google Scholar]
- Petersen, K.; Bæk, K.; Grung, M.; Meland, S.; Ranneklev, S.B. In Vivo and in Vitro Effects of Tunnel Wash Water and Traffic Related Contaminants on Aquatic Organisms. Chemosphere 2016, 164, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.T. Polycyclic Aromatic Hydrocarbons. A Review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of Environmental Exposure to Polycyclic Aromatic Hydrocarbons: A Review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, B.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung Cancer Risk after Exposure to Polycyclic Aromatic Hydrocarbons: A Review and Meta-Analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef]
- Giergielewicz-Możajska, H.; Dąbrowski, Ł.; Namieśnik, J. Accelerated Solvent Extraction (ASE) in the Analysis of Environmental Solid Samples—Some Aspects of Theory and Practice. Crit. Rev. Anal. Chem. 2001, 31, 149–165. [Google Scholar] [CrossRef]
- Díaz-Moroles, N.E.; Garza-Ulloa, H.J.; Castro-Ríos, R.; Ramírez-Villarreal, E.G.; Barbarín-Castillo, J.M.; dela Luz Salazar-Cavazos, M.; Waksman-de Torres, N. A Comparison of the Performance of Two Chromatographic and Three Extraction Techniques for the Analysis of PAHs in Sources of Drinking Water. J. Chromatogr. Sci. 2007, 45, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Emsbo-Mattingly, S.; Litman, E. Chapter 34—Forensic Identification of Historical and Ongoing Tar Oil Releases in Nearshore Environments. In Oil Spill Environmental Forensics Case Studies; Stout, S.A., Wang, Z., Eds.; Butterworth-Heinemann: Rockland, MA, USA, 2018; pp. 785–826. [Google Scholar] [CrossRef]
- Pies, C.; Hoffmann, B.; Petrowsky, J.; Yang, Y.; Ternes, T.A.; Hofmann, T. Characterization and Source Identification of Polycyclic Aromatic Hydrocarbons (PAHs) in River Bank Soils. Chemosphere 2008, 72, 1594–1601. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River Basin: A Critical Appraisal of PAH Ratios as Indicators of PAH Source and Composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Aguilar-Alarcón, P.; Gonzalez, S.V.; Simonsen, M.A.; Borrero-Santiago, A.R.; Sanchís, J.; Meriac, A.; Kolarevic, J.; Asimakopoulos, A.G.; Mikkelsen, Ø. Characterizing Changes of Dissolved Organic Matter Composition with the Use of Distinct Feeds in Recirculating Aquaculture Systems via High-Resolution Mass Spectrometry. Sci. Total Environ. 2020, 749, 142326. [Google Scholar] [CrossRef] [PubMed]
- Sporring, S.; Bøwadt, S.; Svensmark, B.; Björklund, E. Comprehensive Comparison of Classic Soxhlet Extraction with Soxtec Extraction, Ultrasonication Extraction, Supercritical Fluid Extraction, Microwave Assisted Extraction and Accelerated Solvent Extraction for the Determination of Polychlorinated Biphenyls in Soil. J. Chromatogr. A 2005, 1090, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A. Use of Accelerated Solvent Extraction with In-Cell Cleanup to Eliminate Sample Cleanup during Sample Preparation; White Paper 70632; Thermo Fisher Scientific: Sunnyvale, CA, USA, 2013; pp. 1–7. [Google Scholar]
- EPA. Test Method 8310: Polynuclear Aromatic Hydrocarbons; EPA: Washington, DC, USA, 1986; pp. 1–13.
- UCT. EPA Method 8310: Solid Phase Extraction of Polynuclear Aromatic Hydrocarbons (PAHs) in Water; UCT: Washington, DC, USA, 2016; pp. 1–12. [Google Scholar]
- Henderson, J.W., Jr.; Biazzo, W.; Long, W. Polycyclic Aromatic Hydrocarbon (PAH) Separations Using ZORBAX Eclipse PAH Columns—Analyses from Six to 24 PAHs; 5989-7968EN; Agilent Technologies: Wilmington, NC, USA, 2008; pp. 1–8. [Google Scholar]
- Fu, R.; Zou, Y. Analysis of Polynuclear Aromatic Hydrocarbons (PAHs) in Water with ZORBAX Eclipse PAH Column; 5989-7953EN; Agilent Technologies: Shanghai, China, 2008; pp. 1–8. [Google Scholar]
- Henderson, J.W., Jr.; Woodward, C. Robustness of Eclipse PAH Columns for HPLC Analysis of Polycyclic Aromatic Hydrocarbons; 5989-7828EN; Agilent Technologies: Wilmington, NC, USA, 2008; pp. 1–6. [Google Scholar]
- Pule, B.O.; Mmualefe, L.C.; Totro, N. Analysis of Polycyclic Aromatic Hydrocarbons in Soil with Agilent Bond Elut HPLC-FLD; 5990-5452EN; Department of Chemistry, Rhodes University: Grahamstown, South Africa, 2012; pp. 1–8. [Google Scholar]
- EPA. Method 610: Polynuclear Aromatic Hydrocarbons; 610; United States Environmental Protection Agency: Washington, DC, USA, 1984; pp. 1–25.
- Gratz, S.; Gamble, A.M.B.; Gracie, J.; Jackson, D.; Roetting, J.; Ciolino, L.; McCauley, H.; Schneider, G.; Crockett, D.; Heitkemper, D.; et al. Screening and Determination of Polycyclic Aromatic Hydrocarbons in Seafoods Using QuEChERS-Based Extraction and High Performance Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2011, 94, 1601–1616. [Google Scholar] [CrossRef]
- Schuhn, B. Sensitive Determination of Polycyclic Aromatic Hydrocarbons in Tap Water by Online Solid Phase Extraction and UHPLC; 5991-6288EN; Agilent Technologies: Waldbronn, Germany, 2015; pp. 1–8. [Google Scholar]
- Camargo, M.C.R.; Antoniolli, P.R.; Vicente, E. HPLC-FLD Simultaneous Determination of 13 Polycyclic Aromatic Hydrocarbons: Validation of an Analytical Procedure for Soybean Oils. J. Braz. Chem. Soc. 2011, 22, 1354–1361. [Google Scholar] [CrossRef]
- Flock, M.; Herbert, M.-P.; Fischer, I. The Excited-State Structure and Photophysics of Isolated Acenaphthylene. Chem. Phys. 2018, 515, 744–749. [Google Scholar] [CrossRef]
- Joseph, M. HPLC Detector Options for the Determination of Polynuclear Aromatic Hydrocarbons; Application Note 7; Varian Chromatography Systems; Agilent Technologies: Wilmington, NC, USA; pp. 1–4.
- Mandal, S.C.; Mandal, V.; Das, A.K. Chapter 6—Classification of Extraction Methods. In Essentials of Botanical Extraction; Mandal, S.C., Mandal, V., Das, A.K., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 83–136. [Google Scholar] [CrossRef]
- Røsvik, A. Profile and Sources of PAHs in Freshwaters, Sediments and Plastic Debris in Mausund, Norway. Development of a HPLC-FLD Method for Analysing 15 PAHs. Master’s Thesis, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 2021. [Google Scholar]
- Alexandrou, N.; Smith, M.; Park, R.; Lumb, K.; Brice, K. The Extraction of Polycyclic Aromatic Hydrocarbons from Atmospheric Particulate Matter Samples by Accelerated Solvent Extraction (ASE). Int. J. Environ. Anal. Chem. 2001, 81, 257–280. [Google Scholar] [CrossRef]
- Wang, W.; Meng, B.; Lu, X.; Liu, Y.; Tao, S. Extraction of Polycyclic Aromatic Hydrocarbons and Organochlorine Pesticides from Soils: A Comparison between Soxhlet Extraction, Microwave-Assisted Extraction and Accelerated Solvent Extraction Techniques. Anal. Chim. Acta 2007, 602, 211–222. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Baran, S. Application of Solid-Phase Extraction to Determination of Polycyclic Aromatic Hydrocarbons in Sewage Sludge Extracts. J. Hazard. Mater. 2004, 113, 237–245. [Google Scholar] [CrossRef]
- Kootstra, P.R.; Straub, M.H.C.; Stil, G.H.; van der Velde, E.G.; Hesselink, W.; Land, C.C.J. Solid-Phase Extraction of Polycyclic Aromatic Hydrocarbons from Soil Samples. J. Chromatogr. A 1995, 697, 123–129. [Google Scholar] [CrossRef]
- Concetta Bruzzoniti, M.; Fungi, M.; Sarzanini, C. Determination of EPA’s Priority Pollutant Polycyclic Aromatic Hydrocarbons in Drinking Waters by Solid Phase Extraction-HPLC. Anal. Methods 2010, 2, 739–745. [Google Scholar] [CrossRef]
- Xu, T. Rapid Determination of Trace Polycyclic Aromatic Hydrocarbons in Particulate Matter Using Accelerated Solvent Extraction Followed by Ultra High Performance Liquid Chromatography with Fluorescence Detection. J. Sep. Sci. 2016, 39, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Haines, P.J. Chapter 8 Molecular Spectrometry. In Environmental Analytical Chemistry; Blackwell Science Ltd.: Oxford, UK, 2000; p. 470. [Google Scholar]
- Grung, M.; Kringstad, A.; Bæk, K.; Allan, I.J.; Thomas, K.V.; Meland, S.; Ranneklev, S.B. Identification of Non-Regulated Polycyclic Aromatic Compounds and Other Markers of Urban Pollution in Road Tunnel Particulate Matter. J. Hazard. Mater. 2017, 323, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rabodonirina, S.; Net, S.; Ouddane, B.; Merhaby, D.; Dumoulin, D.; Popescu, T.; Ravelonandro, P. Distribution of Persistent Organic Pollutants (PAHs, Me-PAHs, PCBs) in Dissolved, Particulate and Sedimentary Phases in Freshwater Systems. Environ. Pollut. 2015, 206, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Allan, I.J.; O’Connell, S.G.; Meland, S.; Bæk, K.; Grung, M.; Anderson, K.A.; Ranneklev, S.B. PAH Accessibility in Particulate Matter from Road-Impacted Environments. Environ. Sci. Technol. 2016, 50, 7964–7972. [Google Scholar] [CrossRef] [Green Version]
- Meland, S. Chemical Characterisation of Sediment from Vassum Sedimentation Basin; NPRA Reports; 94; Norwegian Public Roads Administration: Oslo, Norway, 2012; pp. 1–22.
- Mackay, D.; Shiu, W.-Y.; Shiu, W.-Y.; Lee, S.C. Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, 2nd ed.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; Volume I. [Google Scholar]
- Paruch, A.M.; Roseth, R. Treatment of Tunnel Wash Water—Experiments with Organic Sorbent Materials. Part II: Removal of Toxic Metals. J. Environ. Sci. 2008, 20, 1042–1045. [Google Scholar] [CrossRef]
- Beckius, J.; Keilen, H.B. Quality Standards for Water, Sediment and Biota—Revised 2020.10.30; M608; The Norwegian Environment Agency: Oslo, Norway, 2016; pp. 1–13.
- Budzinski, H.; Jones, I.; Bellocq, J.; Piérard, C.; Garrigues, P. Evaluation of Sediment Contamination by Polycyclic Aromatic Hydrocarbons in the Gironde Estuary. Mar. Chem. 1997, 58, 85–97. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. PAH Diagnostic Ratios for the Identification of Pollution Emission Sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]


| Time Interval (min) | FLD Excitation (nm) | FLD Emission (nm) | PMT |
|---|---|---|---|
| 0.00–6.75 | 230 | 352 | 10 |
| 6.75–7.50 | 260 | 352 | 10 |
| 7.50–15.50 | 230 | 420 | 10 |
| 15.50–16.80 | 290 | 430 | 10 |
| 16.80–22.50 | 230 | 460 | 10 |
| Recoveries ASE (%) for Particle Phase | Recoveries SPE (%) for the Dissolved Phase | ||||||
|---|---|---|---|---|---|---|---|
| Present Study [29] | Alexandrou et al. [30], n = 5 | Wang et al. [31], n = 3 | Present Study | Oleszczuk and Baran [32], n = 9 | Kootstra et al. [33], n = 3 | Bruzzoniti et al. [34], n = 6 | |
| NAP | 57 | n.a. | 70 | 54 | 99 a | 88 a | n.d. |
| ACY | n.d. | 92 | 85 | 60 | 104 a | 82 | n.a. |
| ACE | 73 | 83 | 91 | 51 | 64 | 81 | 21 a |
| FLU | 78 | 89 | 89 | n.a. | 93 | 84 | 67 |
| PHE | 82 | 94 | 96 | 44 | 72 a | 83 a | 92 a |
| ANT | 83 | 85 | 93 | 42 | 82 a | 77 a | 81 a |
| FLT | 87 | 90 | 84 | 47 | 81 a | 71 | 85 a |
| PYR | 88 | 90 | 97 | 46 | 90 a | 69 | 88 a |
| BaA | 91 | 97 | 90 | 59 | 106 a | 68 | 84 |
| CHR | 91 | 99 | 77 | 65 | 96 a | 75 | 82 |
| BbF | 91 | 102 | 94 | 68 | 91 | 64 | 82 |
| BkF | 91 | 109 | 105 | 71 | 87 | 66 | 77 |
| BaP | 92 | 96 | 110 | 68 | 77 | 51 | 74 |
| DBA | 88 | 105 | 112 | n.d. | 72 | 64 | 70 |
| BgP | 92 | 107 | 111 | 79 | 69 | 65 | 74 |
| IND | 104 | 99 | 73 | n.d. | 81 | 58 | 70 |
| PAH | BT | Str | Sme | Gra | Gri | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| GC-MS µg/L | HPLC-FLD µg/L | GC-MS µg/L | HPLC-FLD µg/L | GC-MS µg/L | HPLC-FLD µg/L | GC-MS µg/L | HPLC-FLD µg/L | GC-MS µg/L | HPLC-FLD µg/L | |
| NAP | 0.25 | 0.50 | n.d. | 0.031 | n.d. | 0.10 | 0.074 | 0.47 | n.d. | n.d. |
| ACY | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| ACE | n.d. | n.d. | n.d. | n.d. | n.d. | 0.0098 | n.d. | 0.21 | n.d. | 0.012 |
| FLU | n.d. | n.a | 1.1 | n.a | 0.44 | n.a | n.d. | n.a | 0.58 | n.a |
| PHE | 0.091 | 0.022 | n.d. | n.d. | 0.026 | n.d. | 0.032 | n.d. | 0.034 | n.d. |
| ANT | n.d. | n.d. | 0.12 | n.d. | n.d. | n.d. | 9.3 | n.d. | n.d. | n.d. |
| FLT | 0.15 | n.d. | n.d. | n.d. | n.d. | n.d. | 3.9 | n.d. | n.d. | n.d. |
| PYR | 6.3 | 5.1 | n.d. | n.d. | 0.088 | 6.3 | 1.4 | n.d. | 1.5 | 18 |
| BaA | 1.84 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| CHR | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 6.3 | n.d. | n.d. | n.d. |
| BbF | n.d. | 4.3 * | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| BkF | n.d. | n.d. | n.d. | 0.0069 | n.d. | 0.038 | n.d. | 0.0075 | n.d. | 3.0 |
| BaP | n.d. | n.a | n.d. | n.a | n.d. | n.a | n.d. | n.a | n.d. | n.a |
| BgP | n.d. | 5.2 * | n.d. | n.d. | n.d. | 280 * | n.d. | 230 * | n.d. | 230 * |
| IND | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| DBA | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 400 * |
| Total PAH | 0.52 | 0.52 | 0.12 | 0.038 | 0.11 | 0.15 | 0.11 | 0.69 | 0.034 | 0.022 |
| Sampling Site | ANT/(ANT + PHE) | FLT/(FLT + PYR) | BaA/(BaA + CHR) |
|---|---|---|---|
| BT | 0.00 | 0.02 | 1.00 |
| Str | 1.00 | n.d. | n.d. |
| Sme | 0.00 | 0.00 | n.d. |
| Gra | 1.00 | 0.73 | 0.00 |
| Gri | 0.00 | 0.00 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vistnes, H.; Sossalla, N.A.; Røsvik, A.; Gonzalez, S.V.; Zhang, J.; Meyn, T.; Asimakopoulos, A.G. The Determination of Polycyclic Aromatic Hydrocarbons (PAHs) with HPLC-DAD-FLD and GC-MS Techniques in the Dissolved and Particulate Phase of Road-Tunnel Wash Water: A Case Study for Cross-Array Comparisons and Applications. Toxics 2022, 10, 399. https://doi.org/10.3390/toxics10070399
Vistnes H, Sossalla NA, Røsvik A, Gonzalez SV, Zhang J, Meyn T, Asimakopoulos AG. The Determination of Polycyclic Aromatic Hydrocarbons (PAHs) with HPLC-DAD-FLD and GC-MS Techniques in the Dissolved and Particulate Phase of Road-Tunnel Wash Water: A Case Study for Cross-Array Comparisons and Applications. Toxics. 2022; 10(7):399. https://doi.org/10.3390/toxics10070399
Chicago/Turabian StyleVistnes, Hanne, Nadine A. Sossalla, Anna Røsvik, Susana V. Gonzalez, Junjie Zhang, Thomas Meyn, and Alexandros G. Asimakopoulos. 2022. "The Determination of Polycyclic Aromatic Hydrocarbons (PAHs) with HPLC-DAD-FLD and GC-MS Techniques in the Dissolved and Particulate Phase of Road-Tunnel Wash Water: A Case Study for Cross-Array Comparisons and Applications" Toxics 10, no. 7: 399. https://doi.org/10.3390/toxics10070399






