Alleviation of Ammonium Toxicity in Salvia splendens ‘Vista Red’ with Silicon Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Treatments, and Experimental Conditions
2.2. Measurement of Plant Growth Parameters and Destructive Sampling
2.3. Calculation of the Ammonium Toxicity Ratio (%)
2.4. Estimation of the Photosynthetic Capacity
2.5. Determinations of Si, K, Ca, and Mg Concentrations
2.6. Analysis of Antioxidant Enzyme Activities in Leaf Samples
2.7. Quantifications of O2·−, H2O2, MDA, and Carotenoids in Leaf Samples
2.8. Statistical Analysis and Graphing
3. Results
3.1. Effects of the Three NH4+:NO3− Ratios and Si Supplementation on the Plant Growth Attributes
3.2. NH4+ Toxicity Ratio as Influenced by Si Application
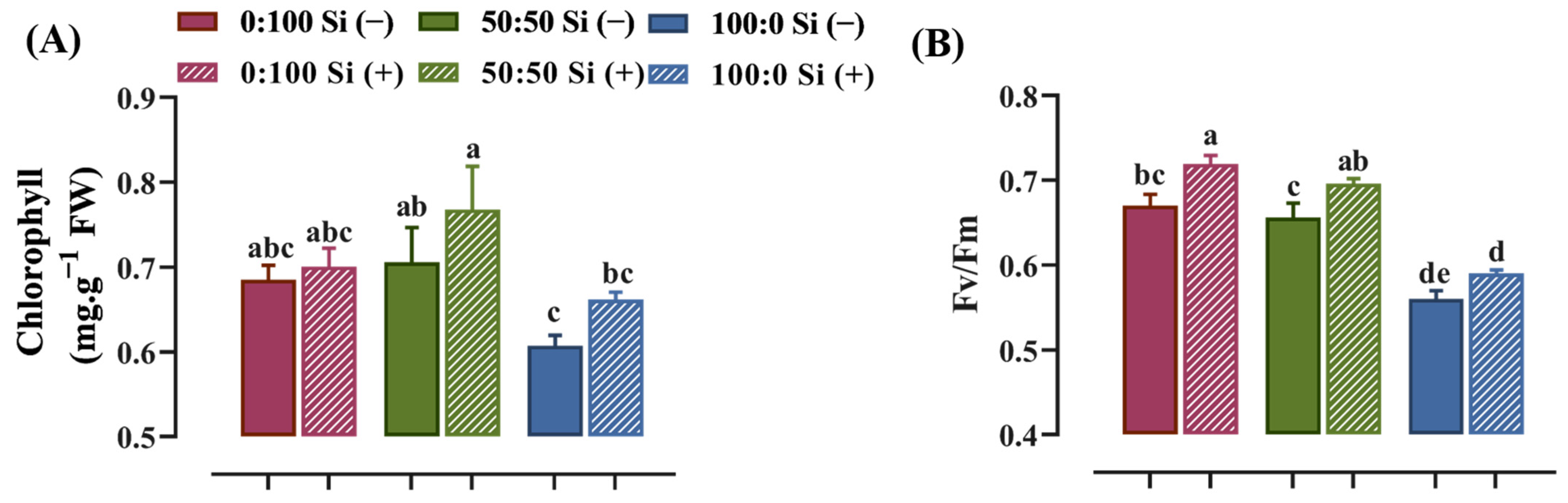
3.3. Photosynthetic Ability as Affected by the N Form and Si Supplementation
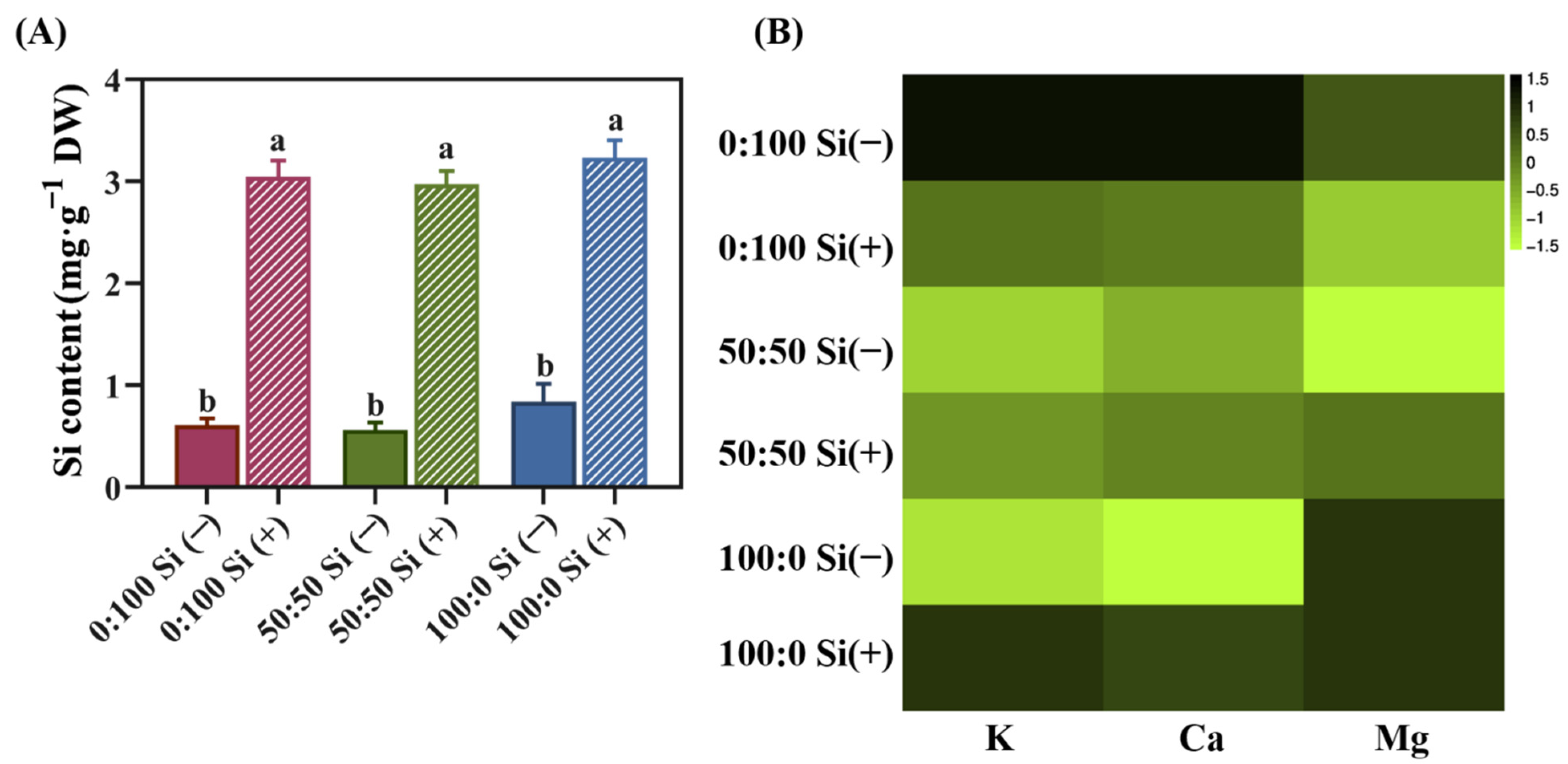
3.4. The Accumulation of Silicon (Si), Potassium (K), Calcium (Ca), and Magnesium (Mg)
3.5. Responses of Antioxidant Capacity to N Forms and Si Application
3.6. Oxidative Damage as Affected by the Three NH4+:NO3− Ratios and Si Supplementations
3.7. Responses between Antioxidant Capacity and Si Supplementation Are Supported by PCA Analysis
4. Discussion
4.1. Si Promoted Plant Growth and Alleviated the NH4+ Toxicity Degree
4.2. Si Ameliorated Damaged Photosynthetic Capacity Caused by NH4+ Toxicity
4.3. Si Alleviated the Inhibition of Key Cation Uptakes under NH4+ Toxicity
4.4. Improved Antioxidative Enzyme Activities by Si Contributed to the Mitigation of NH4+ Toxicity
4.5. Si Decreased the ROS Accumulation, Lipid Peroxidation, and Pigment Degradation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xing, S.; Wang, J.; Zhou, Y.; Bloszies, S.A.; Tu, C.; Hu, S. Effects of NH4+–N/NO3−–N ratios on photosynthetic characteristics, dry matter yield and nitrate concentration of spinach. Exp. Agric. 2015, 51, 151–160. [Google Scholar] [CrossRef]
- Cruz, C.; Bio, A.; Domínguez-Valdivia, M.; Aparicio-Tejo, P.M.; Lamsfus, C.; Martins-Louçao, M.A. How does glutamine synthetase activity determine plant tolerance to ammonium? Planta 2006, 223, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Mokhele, B.; Zhan, X.; Yang, G.; Zhang, X. Nitrogen assimilation in crop plants and its affecting factors. Can. J. Plant Sci. 2012, 92, 399–405. [Google Scholar] [CrossRef]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-X.; Wang, Z.-H.; Stewart, B. Responses of crop plants to ammonium and nitrate N. Adv. Agron. 2013, 118, 205–397. [Google Scholar]
- Song, J.; Yang, J.; Jeong, B.R. Root GS and NADH-GDH play important roles in enhancing the ammonium tolerance in three bedding plants. Int. J. Mol. Sci. 2022, 23, 1061. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, T.; Inaba, J.; Wakazaki, M.; Sato, M.; Toyooka, K.; Miyagi, A.; Kawai-Yamada, M.; Sugiura, D.; Nakagawa, T.; Kiba, T.; et al. Excessive ammonium assimilation by plastidic glutamine synthetase causes ammonium toxicity in Arabidopsis thaliana. Nat. Commun. 2021, 12, 4944. [Google Scholar] [CrossRef] [PubMed]
- Roosta, H.R.; Sajjadinia, A.; Rahimi, A.; Schjoerring, J.K. Responses of cucumber plant to NH4+ and NO3− nutrition: The relative addition rate technique vs. cultivation at constant nitrogen concentration. Sci. Hortic. 2009, 121, 397–403. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Growth, quality, and nitrogen assimilation in response to high ammonium or nitrate supply in cabbage (Brassica campestris L.) and lettuce (Lactuca sativa L.). Agronomy 2021, 11, 2556. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Dong, A.-X.; Xin, H.-B.; Li, Z.-J.; Liu, H.; Sun, Y.-Q.; Nie, S.; Zhao, Z.-N.; Cui, R.-F.; Zhang, R.-G.; Yun, Q.-Z.; et al. High-quality assembly of the reference genome for scarlet sage, Salvia splendens, an economically important ornamental plant. GigaScience 2018, 7, giy068. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.-L.; Kriebel, R.; Drummond, C.P.; Walked, J.B.; Sytsma, K.J. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.R.; Lee, C.W. Influence of ammonium, nitrate, and chloride on solution pH and ion uptake by ageratum and salvia in hydroponic culture. J. Plant Nutr. 1996, 19, 1343–1360. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Decreased solution pH and increased K+ uptake are related to ammonium tolerance in hydroponically cultured plants. Horticulturae 2022, 8, 228. [Google Scholar] [CrossRef]
- Pirooz, P.; Amooaghaie, R.; Ahadi, A.; Sharififar, F.; Torkzadeh-Mahani, M. Silicon and nitric oxide synergistically modulate the production of essential oil and rosmarinic acid in Salvia officinalis under Cu stress. Protoplasma 2021, 259, 905–916. [Google Scholar] [CrossRef]
- Campos, C.N.S.; Silva Júnior, G.B.D.; Prado, R.D.M.; David, C.H.O.D.; Souza Junior, J.P.D.; Teodoro, P.E. Silicon mitigates ammonium toxicity in plants. Agron. J. 2020, 112, 635–647. [Google Scholar] [CrossRef]
- Viciedo, D.O.; de Mello Prado, R.; Lizcano Toledo, R.; dos Santos, L.C.N.; Calero Hurtado, A.; Nedd, L.L.T.; Castellanos Gonzalez, L. Silicon supplementation alleviates ammonium toxicity in sugar beet (Beta vulgaris L.). J. Soil Sci. Plant Nutr. 2019, 19, 413–419. [Google Scholar] [CrossRef]
- Song, X.-P.; Verma, K.K.; Tian, D.-D.; Zhang, X.-Q.; Liang, Y.-J.; Huang, X.; Li, C.-N.; Li, Y.-R. Exploration of silicon functions to integrate with biotic stress tolerance and crop improvement. Biol. Res. 2021, 54, 19. [Google Scholar] [CrossRef]
- El-Ashry, R.M.; El-Saadony, M.T.; El-Sobki, A.E.; El-Tahan, A.M.; Al-Otaibi, S.; El-Shehawi, A.M.; Saad, A.M.; Elshaer, N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 2022, 29, 920–932. [Google Scholar] [CrossRef]
- Khan, W.; Aziz, T.; Maqsood, M.; Farooq, M.; Abdullah, Y.; Ramzani, P.; Bilal, H. Silicon nutrition mitigates salinity stress in maize by modulating ion accumulation, photosynthesis, and antioxidants. Photosynthetica 2018, 56, 1047–1057. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Farooq, M.A.; Detterbeck, A.; Clemens, S.; Dietz, K.-J. Silicon-induced reversibility of cadmium toxicity in rice. J. Exp. Bot. 2016, 67, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.S.; de Mello Prado, R.; Hurtado, A.C.; De Andrade, R.A.; Da Silva, G.P. Ammonia toxicity affect cations uptake and growth in papaya plants inclusive with silicon addition. Acta Biol. Colomb. 2020, 25, 345–353. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Jiang, W.; Liu, D. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ. Sci. Pollut. Res. 2013, 20, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alam, P.; Alyemeni, M.N.; Wijaya, L.; Ali, S.; Ashraf, M. Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J. Plant Growth Regul. 2019, 38, 70–82. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Jeong, B.R. Silicon alleviates temperature stresses in poinsettia by regulating stomata, photosynthesis, and oxidative damages. Agronomy 2020, 10, 1419. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Hu, J.; Lee, J.; Jeong, B.R. Pre-and/or postharvest silicon application prolongs the vase life and enhances the quality of cut peony (Paeonia lactiflora Pall.) flowers. Plants 2021, 10, 1742. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Biju, S.; Fuentes, S.; Gupta, D. Silicon modulates nitro-oxidative homeostasis along with the antioxidant metabolism to promote drought stress tolerance in lentil plants. Physiol. Plant. 2021, 172, 1382–1398. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Amako, K.; Chen, G.-X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar]
- Mavis, R.D.; Stellwagen, E. Purification and subunit structure of glutathione reductase from bakers’ yeast. J. Biol. Chem. 1968, 243, 809–814. [Google Scholar] [CrossRef]
- Wu, Y.-X.; von Tiedemann, A. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ. Pollut. 2002, 116, 37–47. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choudhuri, M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Knight, J.A.; Pieper, R.K.; McClellan, L. Specificity of the thiobarbituric acid reaction: Its use in studies of lipid peroxidation. Clin. Chem. 1988, 34, 2433–2438. [Google Scholar] [CrossRef]
- Li, N.; Wang, K.; Lv, Y.; Zhang, Z.; Cao, B.; Chen, Z.; Xu, K. Silicon enhanced the resistance of Chinese cabbage (Brassica rapa L. ssp. pekinensis) to ofloxacin on the growth, photosynthetic characteristics and antioxidant system. Plant Physiol. Biochem. 2022, 175, 44–57. [Google Scholar]
- Chen, T.; Liu, Y.X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Liu, Y.; von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 2017, 68, 2581–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, G.P.; de Mello Prado, R.; Ferreira, R.P.S. Absorption of nutrients, growth and nutritional disorders resulting from ammonium toxicity in rice and spinach plants. Emir. J. Food Agric. 2016, 28, 882–889. [Google Scholar] [CrossRef]
- Barreto, R.F.; Júnior, A.A.S.; Maggio, M.A.; de Mello Prado, R. Silicon alleviates ammonium toxicity in cauliflower and in broccoli. Sci. Hortic. 2017, 225, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.N.S.; de Mello Prado, R.; Caione, G. Silicon and excess ammonium and nitrate in cucumber plants. Afr. J. Agric. Res. 2016, 11, 276–283. [Google Scholar]
- Barreto, R.; Prado, R.; Leal, A.; Troleis, M.; Junior, G.S.; Monteiro, C.; Santos, L.; Carvalho, R. Mitigation of ammonium toxicity by silicon in tomato depends on the ammonium concentration. Acta Agric. Scand. B-Soil Plant Sci. 2016, 66, 483–488. [Google Scholar] [CrossRef]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Effect of silicon on crop growth, yield and quality. In Silicon in Agriculture; Springer: Dordrecht, The Netherlands, 2015; pp. 209–223. [Google Scholar]
- Vasanthi, N.; Saleena, L.M.; Raj, S.A. Silicon in crop production and crop protection—A review. Agric. Rev. 2014, 35, 14–23. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Silicon mitigates ammonium toxicity in cabbage (Brassica campestris L. ssp. pekinensis) ‘Ssamchu’. Front. Sustain. Food Syst. 2022, 6, 922666. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R. Combined effects of phosphorus nutrition and elevated carbon dioxide concentration on chlorophyll fluorescence, photosynthesis, and nutrient efficiency of cotton. J. Plant Nutr. Soil Sci. 2014, 177, 892–902. [Google Scholar] [CrossRef] [Green Version]
- Cetin, O.; Uzen, N.; Temiz, M. Effect of N-fertigation frequency on the lint yield, chlorophyll, and photosynthesis rate of cotton. J. Agric. Sci. Technol. 2015, 17, 909–920. [Google Scholar]
- Kura-Hotta, M.; Satoh, K.; Katoh, S. Relationship between photosynthesis and chlorophyll content during leaf senescence of rice seedlings. Plant Cell Physiol. 1987, 28, 1321–1329. [Google Scholar]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.-O.; Wu, Z. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ. Exp. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Singh, S.K.; Badgujar, G.; Reddy, V.R.; Fleisher, D.H.; Bunce, J.A. Carbon dioxide diffusion across stomata and mesophyll and photo-biochemical processes as affected by growth CO2 and phosphorus nutrition in cotton. J. Plant Physiol. 2013, 170, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Li, Z.; Ning, T.; Zheng, Y. Responses of photosynthesis, chlorophyll fluorescence, and grain yield of maize to controlled-release urea and irrigation after anthesis. J. Plant Nutr. Soil Sci. 2013, 176, 595–602. [Google Scholar] [CrossRef]
- Liu, G.; Du, Q.; Li, J. Interactive effects of nitrate-ammonium ratios and temperatures on growth, photosynthesis, and nitrogen metabolism of tomato seedlings. Sci. Hortic. 2017, 214, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.E.A.; Rodrigues, F.Á.; Moreira, W.R.; DaMatta, F.M. Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae. Phytopathology 2014, 104, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Consistent alleviation of abiotic stress with silicon addition: A meta-analysis. Funct. Ecol. 2016, 30, 1340–1357. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, W.; Chen, Q.; Liu, Y.; Ding, R. Effect of exogenous silicon (Si) on H+-ATPase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environ. Exp. Bot. 2006, 57, 212–219. [Google Scholar] [CrossRef]
- Zhang, L.; Song, H.; Li, B.; Wang, M.; Di, D.; Lin, X.; Kronzucker, H.J.; Shi, W.; Li, G. Induction of S-nitrosoglutathione reductase protects root growth from ammonium toxicity by regulating potassium homeostasis in Arabidopsis and rice. J. Exp. Bot. 2021, 72, 4548–4564. [Google Scholar] [CrossRef] [PubMed]
- Balkos, K.D.; Britto, D.T.; Kronzucker, H.J. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant Cell Environ. 2010, 33, 23–34. [Google Scholar] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Silva, J.A.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Ueda, M.; Kitajima, S.; Takeda, T.; Shigeoka, S.; Kurano, N.; Miyachi, S.; Miyake, C.; Yokota, A. Characterization of ascorbate peroxidases from unicellular red alga Galdieria partita. Plant Cell Physiol. 2001, 42, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Liu, Y.; Gong, X.; Zeng, G.; Zheng, B.; Wang, D.; Sun, Z.; Zhou, L.; Zeng, X. Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 9999–10008. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and plants: Current knowledge and future prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Han, L.-M.; Hua, W.-P.; Cao, X.-Y.; Yan, J.-A.; Chen, C.; Wang, Z.-Z. Genome-wide identification and expression analysis of the superoxide dismutase (SOD) gene family in Salvia miltiorrhiza. Gene 2020, 742, 144603. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Wang, Y.-K.; Lu, X.-M.; Jia, S.-S. Effects of exogenous silicon on physiological characteristics of cucumber seedlings under ammonium stress. Yingyong Shengtai Xuebao 2014, 25, 1395–1400. (In Chinese) [Google Scholar] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y.; Zheng, X.; Wang, Y. Silicon dioxide nanoparticles improve plant growth by enhancing antioxidant enzyme capacity in bamboo (Pleioblastus pygmaeus) under lead toxicity. Trees 2020, 34, 469–481. [Google Scholar] [CrossRef]
- Rahman, S.U.; Xuebin, Q.; Zhao, Z.; Du, Z.; Imtiaz, M.; Mehmood, F.; Hongfei, L.; Hussain, B.; Ashraf, M.N. Alleviatory effects of silicon on the morphology, physiology, and antioxidative mechanisms of wheat (Triticum aestivum L.) roots under cadmium stress in acidic nutrient solutions. Sci. Rep. 2021, 11, 1–12. [Google Scholar]
- Ahmad, A.; Khan, W.U.; Shah, A.A.; Yasin, N.A.; Naz, S.; Ali, A.; Tahir, A.; Batool, A.I. Synergistic effects of nitric oxide and silicon on promoting plant growth, oxidative stress tolerance and reduction of arsenic uptake in Brassica juncea. Chemosphere 2021, 262, 128384. [Google Scholar] [CrossRef]
- Koca, H.; Bor, M.; Özdemir, F.; Türkan, İ. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007, 60, 344–351. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Li, W.; Lu, J. Effects of ammonium on the antioxidative response in Hydrilla verticillata (Lf) Royle plants. Ecotoxicol. Environ. Safe 2010, 73, 189–195. [Google Scholar] [CrossRef]
- Yang, S.; Hao, D.; Jin, M.; Li, Y.; Liu, Z.; Huang, Y.; Chen, T.; Su, Y. Internal ammonium excess induces ROS-mediated reactions and causes carbon scarcity in rice. BMC Plant Biol. 2020, 20, 143. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Zhang, Y.; Chai, T. Silicon attenuates cadmium toxicity in Solanum nigrum L. by reducing cadmium uptake and oxidative stress. Plant Physiol. Biochem. 2013, 68, 1–7. [Google Scholar] [CrossRef]





| Nutrient Source | Ammonium to Nitrate Ratio Combined with (+) or without (−) Si | |||||
|---|---|---|---|---|---|---|
| 0:100 Si (−) | 0:100 Si (+) | 50:50 Si (−) | 50:50 Si (+) | 100:0 Si (−) | 100:0 Si (+) | |
| NH4H2PO4 | - | - | 2.0 | 2.0 | - | - |
| (NH4)2SO4 | - | - | 4.5 | 4.5 | 13.0 | 13.0 |
| K2SO4 | - | - | 4.5 | 3.5 | 1.2 | 0.2 |
| CaCl2·6H2O | - | - | - | - | 4.9 | 4.9 |
| Ca(NO3)2·4H2O | 6.9 | 6.9 | 5.9 | 5.9 | - | - |
| KNO3 | 4.8 | 3.8 | - | - | - | - |
| Mg(NO3)2·6H2O | 1.3 | 1.3 | 0.6 | 0.6 | - | - |
| MgSO4·7H2O | 1.0 | 1.0 | 1.4 | 1.4 | 1.7 | 1.7 |
| KH2PO4 | 1.0 | 1.0 | - | - | 2.0 | 2.0 |
| K2SiO3 | - | 1.0 | - | 1.0 | - | 1.0 |
| NH4+:NO3− Ratio (A) | Si Supply (B) | Dry Weight (mg) | Shoot Length (cm) | Leaf Length (cm) | Leaf Width (cm) |
|---|---|---|---|---|---|
| 0:100 | − | 10.1 z | 2.4 | 2.4 | 0.9 |
| + | 11.0 | 2.5 | 3.0 | 1.0 | |
| 50:50 | − | 10.3 | 2.3 | 2.9 | 0.9 |
| + | 11.9 | 2.5 | 3.0 | 0.9 | |
| 100:0 | − | 5.6 | 1.8 | 1.2 | 0.5 |
| + | 7.9 | 2.2 | 1.9 | 0.7 | |
| F-test | A | ** y | *** | * | ** |
| B | ** | ** | * | * | |
| A × B | * | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Yang, J.; Jeong, B.R. Alleviation of Ammonium Toxicity in Salvia splendens ‘Vista Red’ with Silicon Supplementation. Toxics 2022, 10, 446. https://doi.org/10.3390/toxics10080446
Song J, Yang J, Jeong BR. Alleviation of Ammonium Toxicity in Salvia splendens ‘Vista Red’ with Silicon Supplementation. Toxics. 2022; 10(8):446. https://doi.org/10.3390/toxics10080446
Chicago/Turabian StyleSong, Jinnan, Jingli Yang, and Byoung Ryong Jeong. 2022. "Alleviation of Ammonium Toxicity in Salvia splendens ‘Vista Red’ with Silicon Supplementation" Toxics 10, no. 8: 446. https://doi.org/10.3390/toxics10080446








