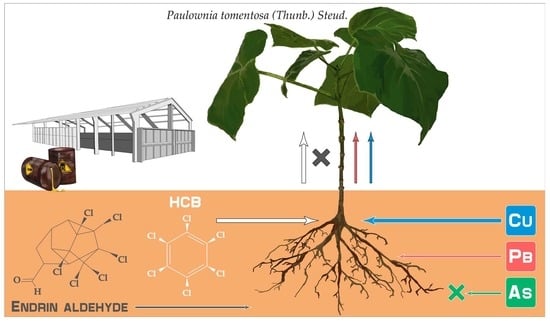
Phytoremediation of Soil Contaminated by Organochlorine Pesticides and Toxic Trace Elements: Prospects and Limitations of Paulownia tomentosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection
2.2. Experiment Design
2.3. Biomass Collection at Harvest
2.4. Chemical Analysis
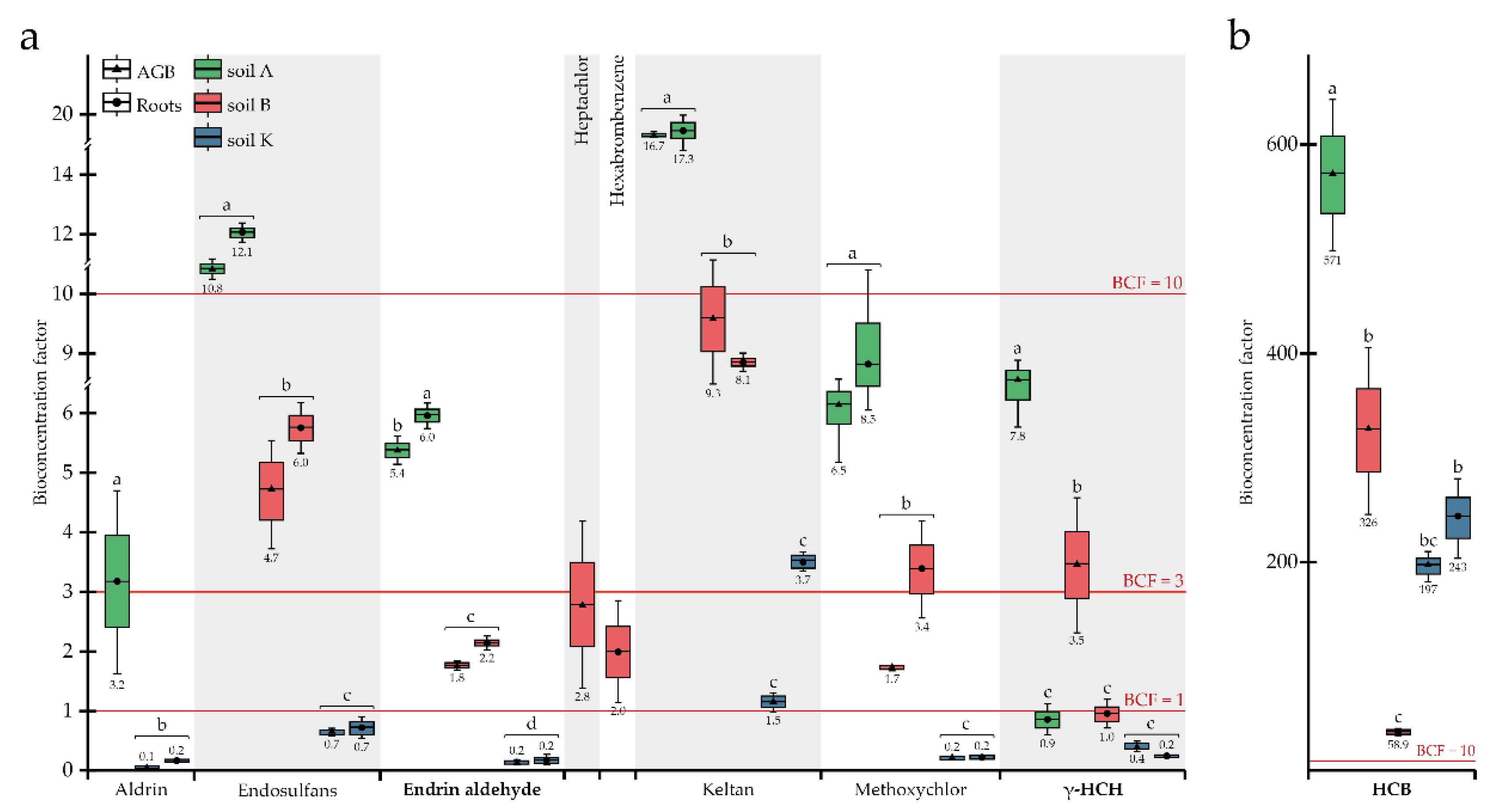
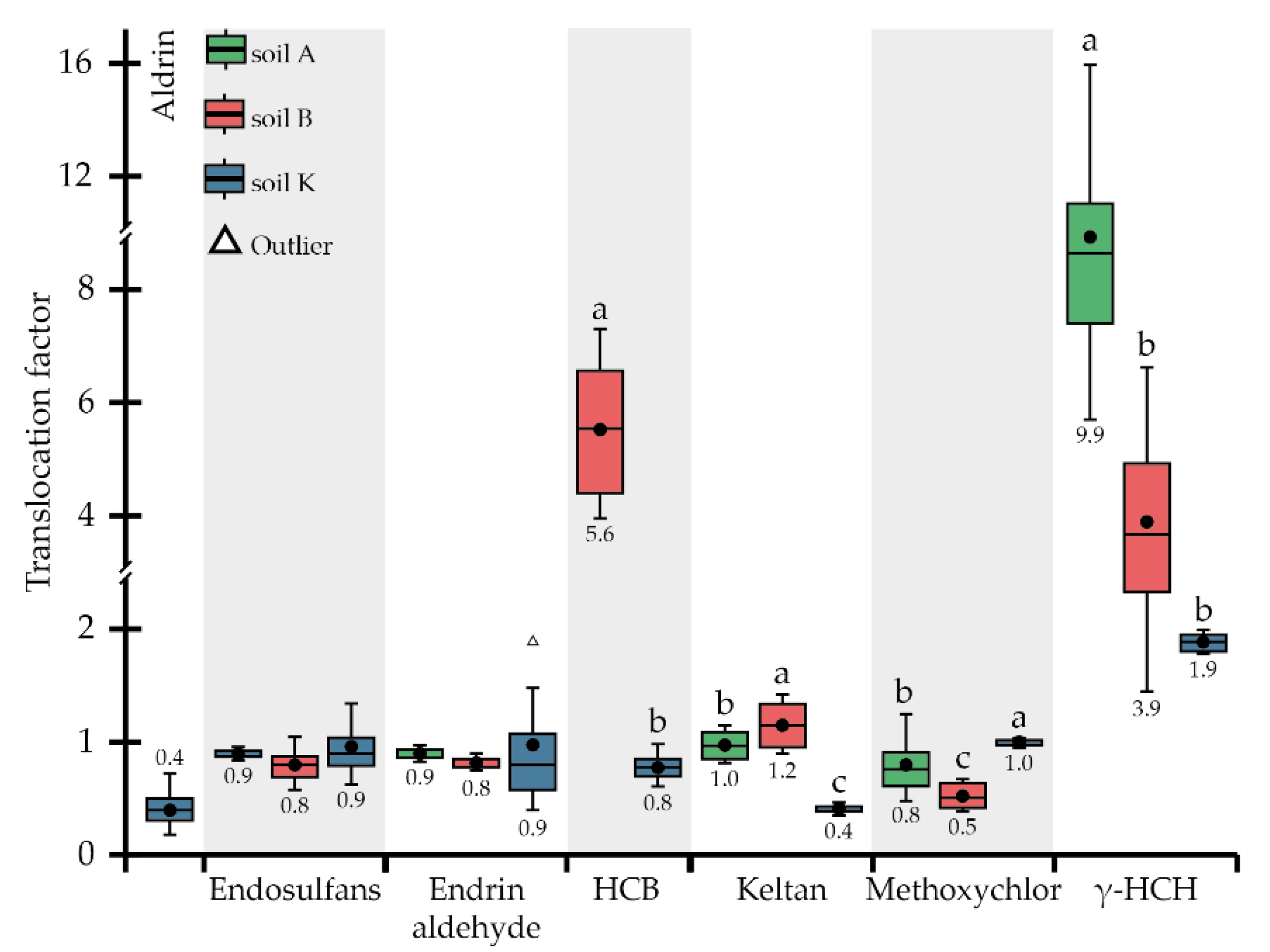
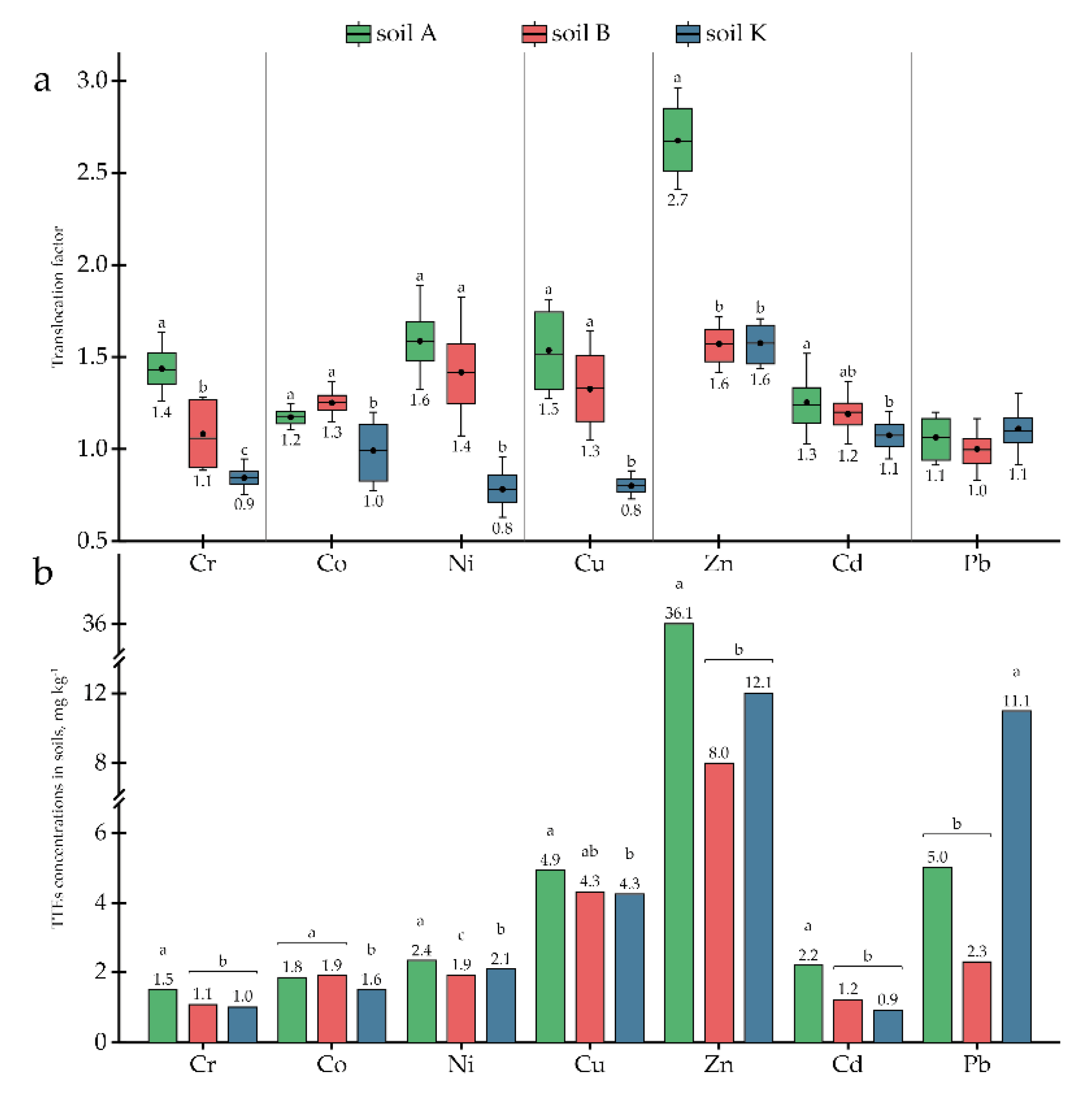
2.5. Calculation of Phytoremediation Coefficients
2.6. Statistical Analysis
3. Results
3.1. Contamination of the Research Soils
3.2. Phytoremediation Potential of P. tomentosa Utilised in Complex OCP- and TTE-Contaminated Soils
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cameselle, C.; Gouveia, S. Phytoremediation of Mixed Contaminated Soil Enhanced with Electric Current. J. Hazard. Mater. 2019, 361, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, T.; Zha, Y.; Du, W.; Yin, Y.; Guo, H. Urea-Enhanced Phytoremediation of Cadmium with Willow in Pyrene and Cadmium Contaminated Soil. J. Hazard. Mater. 2021, 405, 124257. [Google Scholar] [CrossRef] [PubMed]
- Macci, C.; Peruzzi, E.; Doni, S.; Masciandaro, G. Monitoring of a Long Term Phytoremediation Process of a Soil Contaminated by Heavy Metals and Hydrocarbons in Tuscany. Environ. Sci. Pollut. Res. 2020, 27, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Baubekova, A.; Akindykova, A.; Mamirova, A.; Dumat, C.; Jurjanz, S. Evaluation of Environmental Contamination by Toxic Trace Elements in Kazakhstan Based on Reviews of Available Scientific Data. Environ. Sci. Pollut. Res. 2021, 28, 43315–43328. [Google Scholar] [CrossRef] [PubMed]
- Nurzhanova, A.; Kulakow, P.; Rubin, E.; Rakhimbayev, I.; Sedlovskiy, A.; Zhambakin, K.; Kalugin, S.; Kolysheva, E.; Erickson, L. Obsolete Pesticides Pollution and Phytoremediation of Contaminated Soil in Kazakhstan. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Kulakow, P.A., Pidlisnyuk, V.V., Eds.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2010; pp. 87–111. ISBN 978-90-481-3592-9. [Google Scholar]
- Moklyachuk, L.; Gorodiska, I.; Slobodenyuk, O.; Petryshyna, V. Phytoremediation of Soil Polluted with Obsolete Pesticides in Ukraine. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Kulakow, P.A., Pidlisnyuk, V.V., Eds.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2010; pp. 113–124. ISBN 978-90-481-3592-9. [Google Scholar]
- Bogdevich, O.; Cadocinicov, O. Elimination of Acute Risks from Obsolete Pesticides in Moldova: Phytoremediation Experiment at a Former Pesticide Storehouse. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Kulakow, P.A., Pidlisnyuk, V.V., Eds.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2010; pp. 61–85. ISBN 978-90-481-3592-9. [Google Scholar]
- Kaimi, E.; Mukaidani, T.; Tamaki, M. Screening of Twelve Plant Species for Phytoremediation of Petroleum Hydrocarbon-Contaminated Soil. Plant Prod. Sci. 2007, 10, 211–218. [Google Scholar] [CrossRef]
- Rissato, S.R.; Galhiane, M.S.; Fernandes, J.R.; Gerenutti, M.; Gomes, H.M.; Ribeiro, R.; de Almeida, M.V. Evaluation of Ricinus communis L. for the Phytoremediation of Polluted Soil with Organochlorine Pesticides. BioMed Res. Int. 2015, 2015, 549863. [Google Scholar] [CrossRef]
- Dudai, N.; Tsion, I.; Shamir, S.Z.; Nitzan, N.; Chaimovitsh, D.; Shachter, A.; Haim, A. Agronomic and Economic Evaluation of Vetiver Grass (Vetiveria zizanioides L.) as Means for Phytoremediation of Diesel Polluted Soils in Israel. J. Environ. Manag. 2018, 211, 247–255. [Google Scholar] [CrossRef]
- Huang, Y.; Song, Y.; Johnson, D.; Huang, J.; Dong, R.; Liu, H. Selenium Enhanced Phytoremediation of Diesel Contaminated Soil by Alternanthera philoxeroides. Ecotoxicol. Environ. Saf. 2019, 173, 347–352. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Mamirova, A.; Pranaw, K.; Shapoval, P.Y.; Trögl, J.; Nurzhanova, A. Potential Role of Plant Growth-Promoting Bacteria in Miscanthus × giganteus Phytotechnology Applied to the Trace Elements Contaminated Soils. Int. Biodeterior. Biodegrad. 2020, 155, 105103. [Google Scholar] [CrossRef]
- Yan, L.; Le, Q.V.; Sonne, C.; Yang, Y.; Yang, H.; Gu, H.; Ma, N.L.; Lam, S.S.; Peng, W. Phytoremediation of Radionuclides in Soil, Sediments and Water. J. Hazard. Mater. 2021, 407, 124771. [Google Scholar] [CrossRef]
- Mendes, C.V.T.; Carvalho, M.G.V.S.; Baptista, C.M.S.G.; Rocha, J.M.S.; Soares, B.I.G.; Sousa, G.D.A. Valorisation of Hardwood Hemicelluloses in the Kraft Pulping Process by Using an Integrated Biorefinery Concept. Food Bioprod. Process. 2009, 87, 197–207. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Preethi; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic Biomass Based Biorefinery: A Successful Platform towards Circular Bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- Gołąb-Bogacz, I.; Helios, W.; Kotecki, A.; Kozak, M.; Jama-Rodzeńska, A. The Influence of Three Years of Supplemental Nitrogen on Above- and Belowground Biomass Partitioning in a Decade-Old Miscanthus × giganteus in the Lower Silesian Voivodeship (Poland). Agriculture 2020, 10, 473. [Google Scholar] [CrossRef]
- Porvaz, P.; Tóth, Š.; Marcin, A. Cultivation of Chinese Silvergrass (Miscanthus sinensis Anderss.) On the East Slovak Lowland as a Potential Source of Raw Material for Energy Purposes. Agriculture 2012, 58, 146–153. [Google Scholar] [CrossRef]
- Zachar, M.; Lieskovský, M.; Majlingová, A.; Mitterová, I. Comparison of Thermal Properties of the Fast-Growing Tree Species and Energy Crop Species to Be Used as a Renewable and Energy-Efficient Resource. J. Therm. Anal. Calorim. 2018, 134, 543–548. [Google Scholar] [CrossRef]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of Heavy Metal-Contaminated Soils Using the Perennial Energy Crops Miscanthus spp. and Arundo donax L. BioEnergy Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Vasco-Correa, J.; Li, Y. Giant Reed: A Competitive Energy Crop in Comparison with Miscanthus. Renew. Sustain. Energy Rev. 2016, 54, 350–362. [Google Scholar] [CrossRef]
- Hauptvogl, M.; Kotrla, M.; Prčík, M.; Pauková, Ž.; Kováčik, M.; Lošák, T. Phytoremediation Potential of Fast-Growing Energy Plants: Challenges and Perspectives—A Review. Pol. J. Environ. Stud. 2019, 29, 505–516. [Google Scholar] [CrossRef]
- Pogrzeba, M.; Rusinowski, S.; Sitko, K.; Krzyżak, J.; Skalska, A.; Małkowski, E.; Ciszek, D.; Werle, S.; McCalmont, J.P.; Mos, M.; et al. Relationships between Soil Parameters and Physiological Status of Miscanthus × giganteus Cultivated on Soil Contaminated with Trace Elements under NPK Fertilisation vs. Microbial Inoculation. Environ. Pollut. 2017, 225, 163–174. [Google Scholar] [CrossRef]
- Nurzhanova, A.; Pidlisnyuk, V.; Abit, K.; Nurzhanov, C.; Kenessov, B.; Stefanovska, T.; Erickson, L. Comparative Assessment of Using Miscanthus × giganteus for Remediation of Soils Contaminated by Heavy Metals: A Case of Military and Mining Sites. Environ. Sci. Pollut. Res. 2019, 26, 13320–13333. [Google Scholar] [CrossRef]
- Zgorelec, Z.; Bilandzija, N.; Knez, K.; Galic, M.; Zuzul, S. Cadmium and Mercury Phytostabilization from Soil Using Miscanthus × giganteus. Sci. Rep. 2020, 10, 6685. [Google Scholar] [CrossRef] [PubMed]
- Pidlisnyuk, V.; Hettiarachchi, G.M.; Zgorelec, Z.; Prelac, M.; Bilandžija, N.; Davis, L.C.; Erickson, L.E. Phytotechnologies for Site Remediation. In Phytotechnology with Biomass Production: Sustainable Management of Contaminated Sites; Erickson, L.E., Pidlisnyuk, V., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2021; pp. 5–36. ISBN 1-00-308261-0. [Google Scholar]
- El-Ramady, H.R.; Abdalla, N.; Alshaal, T.; Elhenawy, A.S.; Shams, M.S.; Faizy, S.E.-D.A.; Belal, E.-S.B.; Shehata, S.A.; Ragab, M.I.; Amer, M.M.; et al. Giant Reed for Selenium Phytoremediation under Changing Climate. Environ. Chem. Lett. 2015, 13, 359–380. [Google Scholar] [CrossRef]
- Nurzhanova, A.; Pidlisnyuk, V.; Sailaukhanuly, Y.; Kenessov, B.; Trogl, J.; Aligulova, R.; Kalugin, S.; Nurmagambetova, A.; Abit, K.; Stefanovska, T. Phytoremediation of Military Soil Contaminated by Metals and Organochlorine Pesticides Using Miscanthus. Commun. Agric. Appl. Biol. Sci. 2017, 82, 61–68. [Google Scholar]
- GISD Global Invasive Species Database. Available online: http://www.iucngisd.org/gisd/search.php (accessed on 27 April 2021).
- Prabakaran, K.; Li, J.; Anandkumar, A.; Leng, Z.; Zou, C.B.; Du, D. Managing Environmental Contamination through Phytoremediation by Invasive Plants: A Review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Jiménez, L.; Rodríguez, A.; Ferrer, J.; Pérez, A.; Angulo, V. La Paulownia: Una Planta de Rápido Crecimiento como Materia Prima para la Fabricación de Papel. Afinidad 2005, 62, 100–105. [Google Scholar]
- López, F.; Pérez, A.; Zamudio, M.A.M.; De Alva, H.E.; García, J.C. Paulownia as Raw Material for Solid Biofuel and Cellulose Pulp. Biomass Bioenergy 2012, 45, 77–86. [Google Scholar] [CrossRef]
- Kajba, D.; Andrić, I. Selection of Willows (Salix sp.) for Biomass Production. SEEFOR 2014, 5, 145–151. [Google Scholar] [CrossRef]
- Marsal, F.; Thevathasan, N.V.; Guillot, S.; Mann, J.; Gordon, A.M.; Thimmanagari, M.; Deen, W.; Silim, S.; Soolanayakanahally, R.; Sidders, D. Biomass Yield Assessment of Five Potential Energy Crops Grown in Southern Ontario, Canada. Agrofor. Syst. 2016, 90, 773–783. [Google Scholar] [CrossRef]
- El-Showk, S.; El-Showk, N. The Paulownia Tree. In An Alternative for Sustainable Forestry; Crop Development: Rabat, Morocco, 2003; pp. 1–8. [Google Scholar]
- Ye, X.; Zhang, Z.; Chen, Y.; Cheng, J.; Tang, Z.; Hu, Y. Physico-Chemical Pretreatment Technologies of Bioconversion Efficiency of Paulownia tomentosa (Thunb.) Steud. Ind. Crops Prod. 2016, 87, 280–286. [Google Scholar] [CrossRef]
- Buzan, R.L.; Maxim, A.; Odagiu, A.; Balint, C.; Hărțăgan, R.M. Paulownia sp. Used as an Energetic Plant, for the Phytoremediation of Soils and in Agroforestry Systems. ProEnviron. Promed. 2018, 11, 76–85. [Google Scholar]
- Doumett, S.; Lamperi, L.; Checchini, L.; Azzarello, E.; Mugnai, S.; Mancuso, S.; Petruzzelli, G.; Del Bubba, M. Heavy Metal Distribution between Contaminated Soil and Paulownia tomentosa, in a Pilot-Scale Assisted Phytoremediation Study: Influence of Different Complexing Agents. Chemosphere 2008, 72, 1481–1490. [Google Scholar] [CrossRef]
- Doumett, S.; Fibbi, D.; Azzarello, E.; Mancuso, S.; Mugnai, S.; Petruzzelli, G.; Bubba, M.D. Influence of the Application Renewal of Glutamate and Tartrate on Cd, Cu, Pb and Zn Distribution Between Contaminated Soil and Paulownia tomentosa in a Pilot-Scale Assisted Phytoremediation Study. Int. J. Phytoremediat. 2010, 13, 1–17. [Google Scholar] [CrossRef]
- Bahri, N.B.; Laribi, B.; Soufi, S.; Rezgui, S.; Bettaieb, T. Growth Performance, Photosynthetic Status and Bioaccumulation of Heavy Metals by Paulownia tomentosa (Thunb.) Steud Growing on Contaminated Soils. Int. J. Agron. Agric. Res. 2015, 6, 32–43. [Google Scholar]
- Bahri, N.B.; Rezgui, S.; Bettaieb, T. Physiological Responses of Paulownia tomentosa (Thunb.) Steud Grown on Contaminated Soils with Heavy Metals. J. New Sci. 2015, 23, 1064–1070. [Google Scholar]
- Grama, M.; Adams, F.; Siretanu, L.; Cincilei, A.; Bulmaga, P. Analytical Study of Obsolete Pesticides Stockpiles in the Republic of Moldova into NATO Science for Peace Project “Clean-Up Chemicals—Moldova”. In Environmental Security Assessment and Management of Obsolete Pesticides in Southeast Europe; Simeonov, L.I., Macaev, F.Z., Simeonova, B.G., Eds.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2013; pp. 381–395. ISBN 978-94-007-6461-3. [Google Scholar]
- Nurzhanova, A.; Kalugin, S.; Zhambakin, K. Obsolete Pesticides and Application of Colonizing Plant Species for Remediation of Contaminated Soil in Kazakhstan. Environ. Sci. Pollut. Res. 2013, 20, 2054–2063. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of pH. ISO/TC 190/SC 3 Chemical and Physical Characterization; ISO: Bern, Switzerland, 2021; p. 8.
- FAO. World Reference Base for Soil Resources 2014: International Soil Classification Systems for Naming Soils and Creating Legends for Soil Maps (Update 2015); World Soil Resources: Rome, Italy, 2014. [Google Scholar]
- GOST 26213-91; Soil. Determination of Humus by the Tyurin Method. GosStandard: Moscow, Russia, 1991.
- GOST 26207-91; Soil. Determination of the Mobile Compounds of Phosphorus and Potassium by Kirsanov Method, Modified by CRIAAS. GosStandard: Moscow, Russia, 1991.
- GOST 26423-85; Methods for Determination of Electrical Conductivity, pH of Salt Regime and Solid Residue of Salt Extract. GosStandard: Moscow, Russia, 1985.
- GOST 17.4.3.01-2017; Nature Protection. Soils. General Requirement for Sampling. GosStandard: Moscow, Russia, 2019.
- ST RK 2131-2011; Soil Quality. Determination of Organochlorine Pesticides and Polychlorinated Biphenyls Content. Gas Chromatographic Method with Electron Capture Detection. GosStandard: Astana, Kazakhstan, 2012.
- ST RK 2011-2010; Water, Food, Feed and Tobacco. Determination of Organochlorine Pesticides by Chromatographic Methods. GosStandard: Astana, Kazakhstan, 2010.
- Mamirova, A.; Pidlisnyuk, V.; Amirbekov, A.; Ševců, A.; Nurzhanova, A. Phytoremediation Potential of Miscanthus sinensis And. in Organochlorine Pesticides Contaminated Soil Amended by Tween 20 and Activated Carbon. Environ. Sci. Pollut. Res. 2021, 28, 16092–16106. [Google Scholar] [CrossRef]
- ST RK ISO 11047-2008; Soil Quality. Determination of the Content of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc in Soil Extracts in Aqua Regia. Spectrophotometric Methods of Atomic Absorption in a Flame and with Electrothermal Spray. ICS 13.080 Soil Quality; “Sonar Consulting and Trading Company Ltd” LPP; GosStandard: Astana, Kazakhstan, 2008; p. 52.
- GOST 23581.8-79; Iron Ores, Concentrates, Agglomerates and Pellets. Methods for the Determination of Arsenic Content. ICS 73.060.10 Iron Ores; GosStandard: Moscow, Russia, 1981; p. 12.
- GOST 26930-86; Raw Material and Food-Stuffs. Method for Determination of Arsenic. ICS 67.050 General Methods of Inspection and Analysis of Food; GosStandard: Moscow, Russia, 1987; p. 6.
- GOST 30178-96; Raw Material and Food-Stuffs. Atomic Absorption Method for Determination of Toxic Elements. ICS 67.050 General Methods of Inspection and Analysis of Food; GosStandard: Moscow, Russia, 1996; p. 11.
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of Trace Elements by Wetland Plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Yanqun, Z.; Yuan, L.; Jianjun, C.; Haiyan, C.; Li, Q.; Schvartz, C. Hyperaccumulation of Pb, Zn and Cd in Herbaceous Grown on Lead–Zinc Mining Area in Yunnan, China. Environ. Int. 2005, 31, 755–762. [Google Scholar] [CrossRef]
- MHRK and MEPRK Standards for Maximum Permissible Concentrations of Harmful Substances, Pests and Other Biological Substances Polluting the Soil, Approved by a Joint Order of the Ministry of Health of the Republic of Kazakhstan Dated January 30, 2004 No. 99 and the Ministry of Environmental Protection of the Republic of Kazakhstan Dated January 27, 2004 No. 21-P; Ministry of Environmental Protection: Nur-Sultan, Kazakhstan, 2004.
- KSES Kazakh Standard for Environmental Safety. Approval of Hygienic Standards for Environmental Safety (Soil). Order of the Minister of Health of the Republic of Kazakhstan Dated April 21, 2021 No. 452. Registered with the Ministry of Justice of the Republic of Kazakhstan on April 22, 2021 No. 22595; Ministry of Justice: Nur-Sultan, Kazakhstan, 2021; p. 5.
- Gannon, N.; Decker, G.C. The Conversion of Heptachlor to Its Epoxide on Plants. J. Econ. Entomol. 1958, 51, 3–7. [Google Scholar] [CrossRef]
- NCBI PubChem Annotation Record for Hexabromobenzene. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2912#section=LogP (accessed on 2 August 2021).
- Blaylock, B.L. Aldrin. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier: New York, NY, USA, 2005; pp. 66–68. ISBN 978-0-12-369400-3. [Google Scholar]
- Sojinu, O.S.; Sonibare, O.O.; Ekundayo, O.O.; Zeng, E.Y. Assessment of Organochlorine Pesticides Residues in Higher Plants from Oil Exploration Areas of Niger Delta, Nigeria. Sci. Total Environ. 2012, 433, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.J. Unusual Accumulations of Elements by Plants and Animals. Sci. Prog. 1971, 59, 505–526. [Google Scholar]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal Hyperaccumulator Plants: A Review of the Ecology and Physiology of a Biological Resource for Phytoremediation of Metal-Polluted Soils. In Phytoremediation of Contaminated Soil and Water; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-0-367-80314-8. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-429-19203-6. [Google Scholar]

| Parameter | Unit | Soil A | Soil B | Soil K |
|---|---|---|---|---|
| C | % | 4.44 ± 0.11 | 5.27 ± 0.10 | 6.10 ± 0.02 |
| pH (water) | 7.48 ± 0.01 | 7.49 ± 0.02 | 7.85 ± 0.02 | |
| P2O5 | mg kg−1 | 353 ± 15 | 71 ± 0 | 400 ± 5.0 |
| K2O | mg kg−1 | 965 ± 15 | 740 ± 0 | 885 ± 25 |
| Ca | meq/100 g | 16.4 ± 0.50 | 19.2 ± 0 | 20.8 ± 0.70 |
| Mg | meq/100 g | 7.75 ± 1.40 | 5.05 ± 0.72 | 2.70 ± 1.23 |
| Na | meq/100 g | 0.16 ± 0 | 0.16 ± 0 | 0.38 ± 0.01 |
| K | meq/100 g | 1.31 ± 0 | 0.80 ± 0.03 | 1.04 ± 0.03 |
| Contaminant | Pesticide Type a | MPC b,c | Soil A | Soil B | Soil K | p-Value | Root MSE |
|---|---|---|---|---|---|---|---|
| OCPs, µg kg−1 | |||||||
| Aldrin | I | 2.5 | 12.2 b | 96.0 b | 345.2 a | <0.01 | 59.7 |
| Chlordane | I | 100 | 30.1 | <LOD | 72.1 | 0.34 | 47.1 |
| Chlorobenzilate | I | 20 | 277.6 | 5509 | 32,242 | 0.45 | 31,134 |
| DDD | I | 100 | 1153 | 2976 | 25,506 | 0.44 | 24,241 |
| DDE | I | 100 | 9709 | 69,847 | 777,967 | 0.40 | 716,310 |
| DDT | I | 100 | 1237 | 6274 | 10,023 | 0.33 | 6613 |
| Dibutyl chlorendate | H | - | 511.1 | 1285 | 2135 | 0.33 | 1208 |
| Dieldrin | I | 0.5 | 42.3 | 291.3 | <LOD | 0.18 | 185 |
| Endosulfans | I | 100 | 83.2 b | 124.1 b | 759.2 a | <0.001 | 63.0 |
| Endosulfan sulfate | mI | - | 654.5 | 265.7 | 356.0 | 0.46 | 373 |
| Endrin | I | 1 | 1289 | 181.3 | 44,085 | 0.41 | 42,462 |
| Endrin aldehyde | mI | - | 62.4 b | 130.8 ab | 1088 a | <0.05 | 394 |
| HCB | F | 500 | 21.3 | 41.6 | 14.0 | 0.07 | 11.7 |
| Heptachlor | I | 50 | <LOD | 118.4 b | 269.0 a | <0.001 | 17.1 |
| Heptachlorepoxide | I | 50 | 190.3 | <LOD | 3029 | 0.39 | 3580 |
| Hexabromobenzene | F | 30 | 39.8 c | 187.6 b | 604.0 a | <0.001 | 54.0 |
| Keltan (Dicofol) | I | 100 | 11.9 | 22.1 | 32.9 | 0.10 | 10.7 |
| Methoxychlor | I | 1600 | 11.1 c | 137.2 b | 1307 a | <0.001 | 43.9 |
| γ-HCH | I | 100 | 19.3 b | 20.1 b | 76.4 a | <0.001 | 3.0 |
| HCH isomers | mI | 100 | 162.7 | 258.9 | 600.4 | 0.25 | 299.2 |
| TTEs, mg kg−1 | |||||||
| Cr | 6 | 1.53 a | 1.12 b | 0.98 b | <0.01 | 0.12 | |
| Co | 5 | 1.84 a | 1.89 a | 1.55 b | <0.001 | 0.06 | |
| Ni | 4 | 2.36 a | 1.85 c | 2.08 b | <0.001 | 0.08 | |
| Cu | 3 | 4.93 a | 4.34 ab | 4.28 b | <0.05 | 0.26 | |
| Zn | 23 | 36.07 a | 7.99 b | 12.07 b | <0.001 | 4.15 | |
| As | 2 | 0.32 b | 0.67 a | 0.27 b | <0.001 | 0.06 | |
| Cd | 0.5 | 2.17 a | 1.17 b | 0.85 b | <0.001 | 0.23 | |
| Pb | 32 | 5.01 b | 2.25 b | 11.11 a | <0.01 | 2.12 | |
| Contaminant | Soil A | Soil B | Soil K | p-Value | Root MSE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AGB | Roots | AGB | Roots | AGB | Roots | SO Effect | PP Effect | Cumulative Effect | ||
| HCB | 12,170 a | <LOD | 13,572 a | 2449 b | 2760 b | 3395 b | <0.001 | <0.001 | <0.001 | 1679 |
| Keltan | 198 a | 206 a | 206 a | 179 a | 50.5 c | 121 b | <0.001 | 0.11 | <0.01 | 21.0 |
| Methoxychlor | 71.7 c | 94.0 c | 235 b | 464 a | 236 b | 237 b | <0.001 | <0.01 | <0.01 | 47.8 |
| γ-HCH | 151 a | 16.3 c | 70.0 b | 19.0 c | 29.5 bc | 15.5 c | <0.001 | <0.001 | <0.001 | 15.9 |
| Cr | 5.04 a | 3.52 c | 3.90 bc | 3.69 bc | 3.83 bc | 4.45 ab | <0.10 | <0.05 | <0.001 | 0.33 |
| Co | 1.87 ab | 1.60 b | 2.10 a | 1.67 b | 1.56 b | 1.57 b | <0.01 | <0.01 | <0.05 | 0.13 |
| Ni | 8.17 ab | 5.18 c | 8.65 a | 6.08 bc | 5.48 c | 6.81 abc | <0.10 | <0.01 | <0.01 | 0.83 |
| Cu | 19.1 bcd | 12.7 d | 30.1 a | 22.6 b | 15.6 cd | 19.4 bc | <0.001 | <0.01 | <0.01 | 2.39 |
| Zn | 56.0 a | 21.0 d | 46.6 b | 29.7 c | 55.8 a | 35.4 c | <0.001 | <0.001 | <0.001 | 2.37 |
| Only SO Effect | ||||||||||
| Endosulfans | 902 | 1007 a | 588 | 739 b | 493 | 546 c | <0.001 | <0.05 | 0.61 | 83.5 |
| Endrin aldehyde | 336 | 372 a | 231 | 281 b | 159 | 199 c | <0.001 | <0.10 | 0.96 | 42.9 |
| Cd | 0.74 | 0.60 b | 0.63 | 0.53 b | 1.29 | 1.20 a | <0.001 | <0.01 | 0.72 | 0.08 |
| Pb | 3.87 | 3.65 b | 4.24 | 4.26 ab | 4.49 | 4.09 a | <0.05 | 0.25 | 0.59 | 0.34 |
| Not Available for Statistical Analysis | ||||||||||
| Aldrin | <LOD | 39.0 | <LOD | <LOD | 22.5 | 57.5 | ||||
| Heptachlor | <LOD | <LOD | 331 | <LOD | <LOD | <LOD | ||||
| Hexabromobenzene | <LOD | <LOD | <LOD | 374 | <LOD | <LOD | ||||
| Pollutant | Current Data | Data of Sojinu et al. [64] | |||
|---|---|---|---|---|---|
| P. tomentosa | C. colocynthis | M. esculenta | Z. mays | P. purpureum | |
| Aldrin | ND | 0.04 | 0.05 | 0.02 | 0.12 |
| Endosulfans | 10.84 | 0.38 | 0.99 | 0.57 | 3.46 |
| Endrin aldehyde | 5.38 | 0.51 | ND | 0.77 | 0.53 |
| Heptachlor | ND | 0.33 | 4.07 | 0.74 | 19.95 |
| Methoxychlor | 6.46 | ND | 0.70 | 0.51 | 0.38 |
| γ-HCH | 7.82 | 0.59 | 1.10 | 0.55 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamirova, A.; Baubekova, A.; Pidlisnyuk, V.; Shadenova, E.; Djansugurova, L.; Jurjanz, S. Phytoremediation of Soil Contaminated by Organochlorine Pesticides and Toxic Trace Elements: Prospects and Limitations of Paulownia tomentosa. Toxics 2022, 10, 465. https://doi.org/10.3390/toxics10080465
Mamirova A, Baubekova A, Pidlisnyuk V, Shadenova E, Djansugurova L, Jurjanz S. Phytoremediation of Soil Contaminated by Organochlorine Pesticides and Toxic Trace Elements: Prospects and Limitations of Paulownia tomentosa. Toxics. 2022; 10(8):465. https://doi.org/10.3390/toxics10080465
Chicago/Turabian StyleMamirova, Aigerim, Almagul Baubekova, Valentina Pidlisnyuk, Elvira Shadenova, Leyla Djansugurova, and Stefan Jurjanz. 2022. "Phytoremediation of Soil Contaminated by Organochlorine Pesticides and Toxic Trace Elements: Prospects and Limitations of Paulownia tomentosa" Toxics 10, no. 8: 465. https://doi.org/10.3390/toxics10080465