Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation
Abstract
:1. Introduction
2. Water Quality Assessment
3. Heavy Metal Contamination and Water Quality Degradation
3.1. Anthropogenic Source of Heavy Metal Contamination
3.1.1. Industrial Sources
3.1.2. Domestic Sources
3.1.3. Agricultural Sources of Pollution
3.1.4. Dumping of Waste and Landfills
3.2. Natural Source of Heavy Metal Contamination
4. Toxicity of Heavy Metals and Human Health
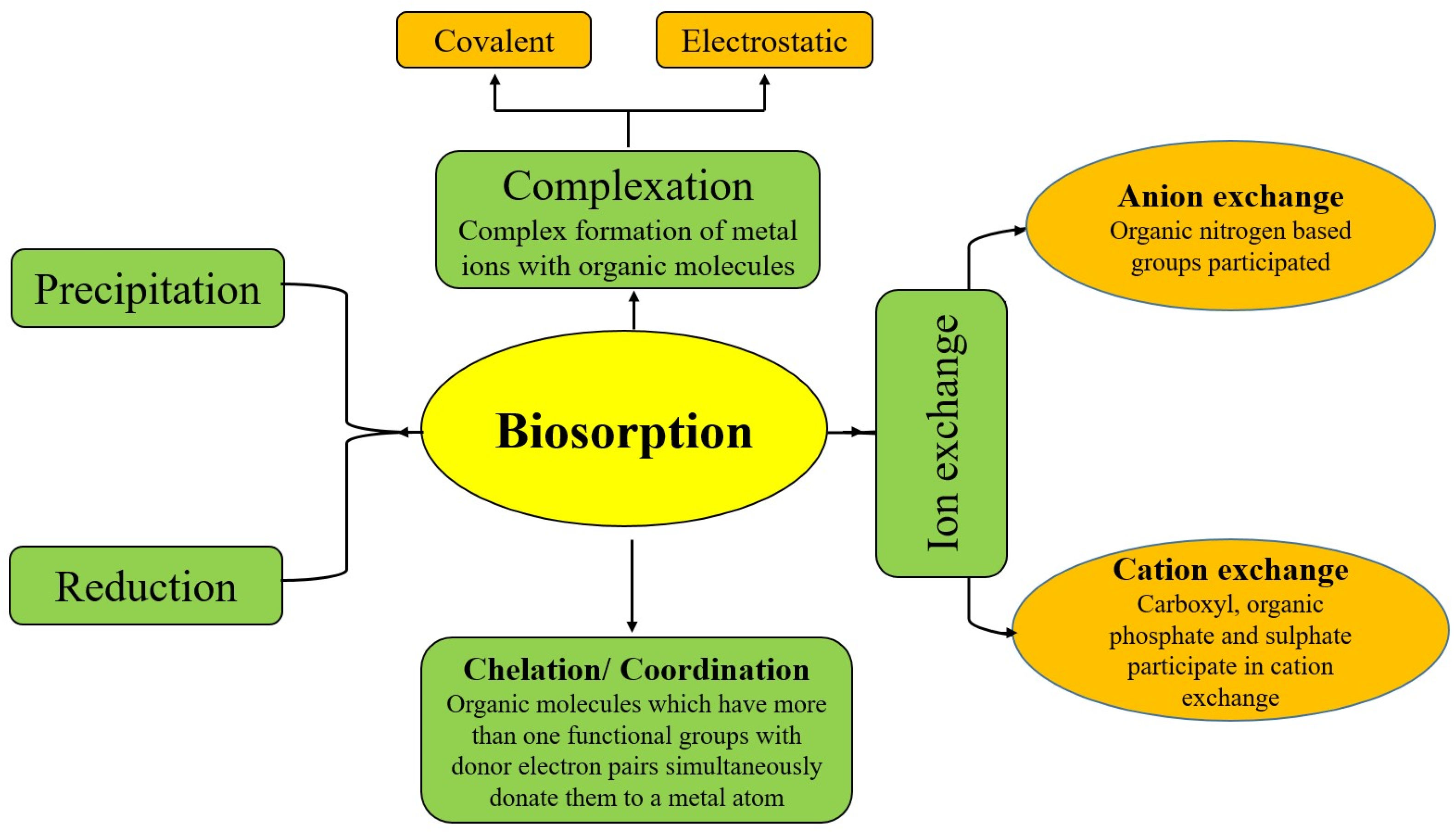
5. Removal of Heavy Metal Ions
5.1. Metabolically Independent Methods for Heavy Metal Removal
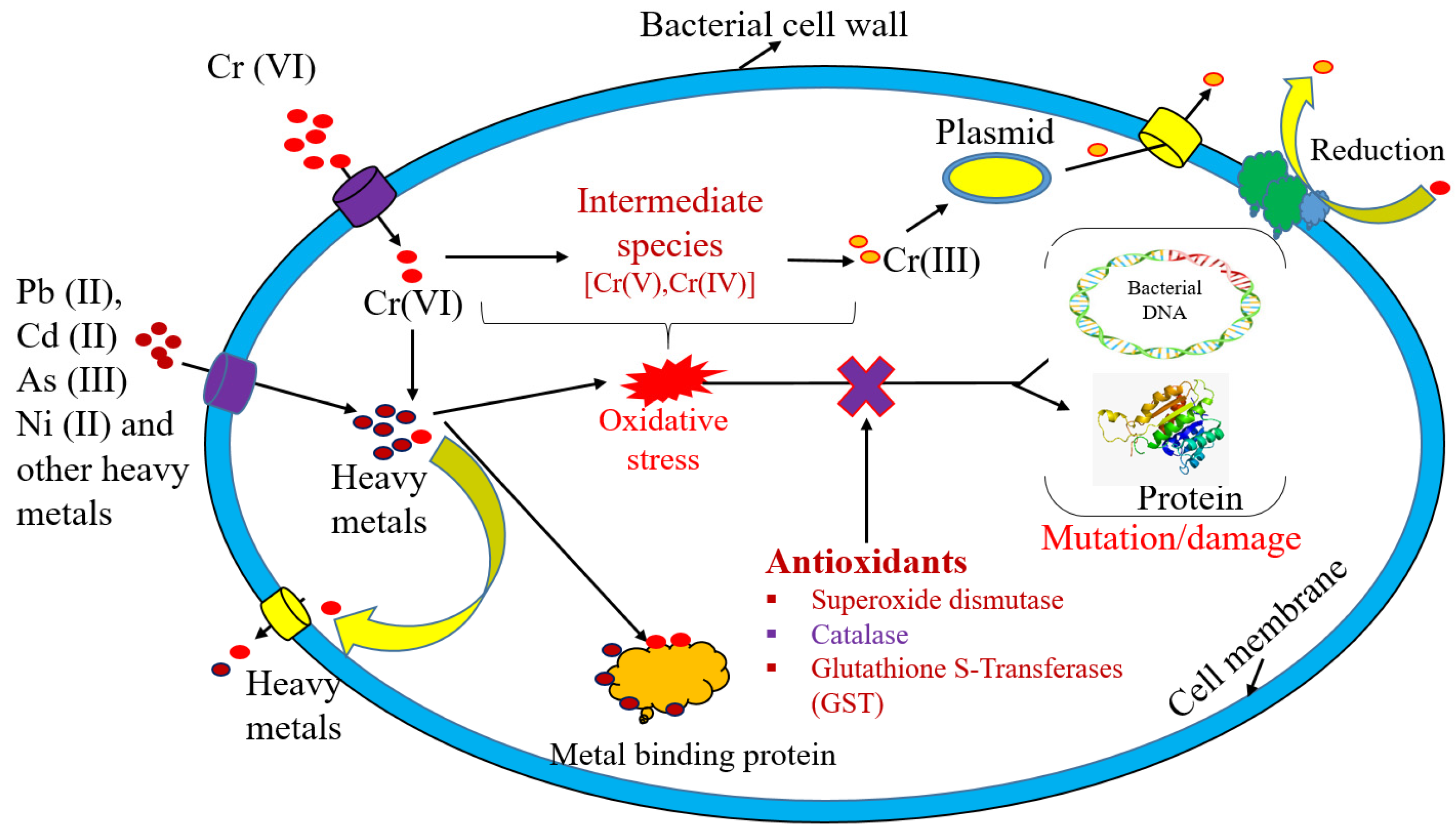
5.2. Metabolically Dependent Approaches for Heavy Metal Removal
6. Technology Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, H.-G.; Zeng, E.Y.; Wong, M.H. Environmental Contamination: Health Risks and Ecological Restoration. Ecotoxicology 2013, 22, 1183–1184. [Google Scholar] [CrossRef]
- Nathanson, J.A. Pollution. Encyclopedia Britannica. 11 February 2021. Available online: https://www.britannica.com/science/pollution-environment (accessed on 31 July 2021).
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An Overview on the Accumulation, Distribution, Transformations, Toxicity and Analytical Methods for the Monitoring of Persistent Organic Pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.V.; Park, D.; Lee, Y.-C. Indoor Air Pollution, Related Human Diseases, and Recent Trends in the Control and Improvement of Indoor Air Quality. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M. Biological Pollutants and Biological Pollution––an Increasing Cause for Concern. Mar. Pollut. Bull. 2003, 46, 275–280. [Google Scholar] [CrossRef]
- Lawson, L. Restorative Commons: Creating Health and Well-Being through Urban Landscape. Ecol. Restor. 2012, 30, 244–245. [Google Scholar] [CrossRef]
- United Nations World Water Development Report (UNWWDR). 2014. Available online: https://www.unesco.org/en/wwap/wwdr (accessed on 28 July 2023).
- Dewata, I.; Adri, Z. Water Quality Assessment and Determining the Carrying Capacity of Pollution Load Batang Kuranji River. IOP Conf. Ser. Mater. Sci. Eng. 2018, 335, 012027. [Google Scholar] [CrossRef]
- Akhigbe, S.; GJ, U.; HO, N. Impact of Domestic and Industrial Waste on Surface and Ground Water Quality Within Slaughter Area, Trans-Amadi Industrial Layout, Port Harcourt, Nigeria. Int. J. Waste Resour. 2018, 8, 1000327. [Google Scholar] [CrossRef]
- Ternes, T.; Joss, A.; Oehlmann, J. Occurrence, Fate, Removal and Assessment of Emerging Contaminants in Water in the Water Cycle (from Wastewater to Drinking Water). Water Res. 2015, 72, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Bisimwa, A.M.; Amisi, F.M.; Bamawa, C.M.; Muhaya, B.B.; Kankonda, A.B. Water Quality Assessment and Pollution Source Analysis in Bukavu Urban Rivers of the Lake Kivu Basin (Eastern Democratic Republic of Congo). Environ. Sustain. Indic. 2022, 14, 100183. [Google Scholar] [CrossRef]
- Fu, L.; Jiang, Y.; Ding, J.; Liu, Q.; Peng, Q.-Z.; Kang, M.-Y. Impacts of Land Use and Environmental Factors on Macroinvertebrate Functional Feeding Groups in the Dongjiang River Basin, Southeast China. J. Freshw. Ecol. 2015, 31, 21–35. [Google Scholar] [CrossRef]
- Beck, S.M.; McHale, M.R.; Hess, G.R. Beyond Impervious: Urban Land-Cover Pattern Variation and Implications for Watershed Management. Environ. Manag. 2016, 58, 15–30. [Google Scholar] [CrossRef]
- Mockler, E.M.; O’Loughlin, F.E.; Bruen, M. Understanding Hydrological Flow Paths in Conceptual Catchment Models Using Uncertainty and Sensitivity Analysis. Comput. Geosci. 2016, 90, 66–77. [Google Scholar] [CrossRef]
- Zou, L.; Xia, J.; She, D. Analysis of Impacts of Climate Change and Human Activities on Hydrological Drought: A Case Study in the Wei River Basin, China. Water Resour. Manag. 2017, 32, 1421–1438. [Google Scholar] [CrossRef]
- Akdogan, A.; Soylak, M. Assessment of Heavy Metal Levels in Street Dust Samples from Denizli, Turkey, and Analysis by Flame Atomic Absorption Spectrometry. Atomic Spectrosc. 2016, 37, 25–29. [Google Scholar] [CrossRef]
- Kumar, U.; Garg, A.P. Bisorption of Heavy Metals Oxides by Heavy Metals Resis-Tant Microorganism in Liquid Culture-ICP-MS Technique. Progress. Agric. 2018, 18, 66. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Verma, M.; Kamal, R.; Tiwari, R.; Sanjay Chivate, M.; Rai, S.N.; Kumar, A.; Singh, A.; Singh, M.P.; et al. Hexavalent-Chromium-Induced Oxidative Stress and the Protective Role of Antioxidants against Cellular Toxicity. Antioxidants 2022, 11, 2375. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Rai, S.N.; Kumar, A.; Singh, A.K.; Singh, M.P.; Sahoo, A.; Shekhar, S.; Vamanu, E.; Mishra, V. Heavy Metal Contamination in the Aquatic Ecosystem: Toxicity and Its Remediation Using Eco-Friendly Approaches. Toxics 2023, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean. Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S. Physico–chemical treatment techniques for wastewater. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Sreekumari, S.S. Adsorptive removal of heavy metal ions from industrial effluents usingactivated carbon derived from waste coconut buttons. J. Environ. Sci. 2011, 23, 1989–1998. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef]
- Singh, V.; Singh, J.; Singh, N.; Rai, S.N.; Verma, M.K.; Verma, M.; Singh, V.; Chivate, M.S.; Bilal, M.; Mishra, V. Simultaneous removal of ternary heavy metal ions by a newly isolated Microbacterium paraoxydans strain VSVM IIT(BHU) from coal washery effluent. BioMetals 2022, 36, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2007, 84, 13–28. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M. Metals, Toxicity and Oxidative Stress. Current Medicinal Chemistry 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Aqil, F.; Ahmad, I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour. Technol. 2007, 98, 2557–2561. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I. Water Quality Assessment: A Case Study of the Jhenai River in Bangladesh. RA J. Appl. Res. 2018, 4, 1884–1888. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality Third Edition Incorporating the First and Second Addenda Volume-1. Recommendations. 2008. Available online: https://www.who.int/water_sanitation_health/dwq/fulltext.pdf (accessed on 21 June 2023).
- Bogardi, J.J.; Leentvaar, J.; Sebesvári, Z. Biologia Futura: Integrating Freshwater Ecosystem Health in Water Resources Management. Biol. Futur. 2020, 71, 337–358. [Google Scholar] [CrossRef]
- Waage, M.D.; Kaatz, L. Nonstationary Water Planning: An Overview of Several Promising Planning Methods1. JAWRA J. Am. Water Resour. Assoc. 2011, 47, 535–540. [Google Scholar] [CrossRef]
- Ragas, A.; Leuven, R. Modelling of Water Quality-Based Emission Limits for Industrial Discharges in Rivers. Water Sci. Technol. 1999, 39, 185–192. [Google Scholar] [CrossRef]
- Heathwaite, L.; Sharpley, A. Evaluating Measures to Control the Impact of Agricultural Phosphorus on Water Quality. Water Sci. Technol. 1999, 39, 149–155. [Google Scholar] [CrossRef]
- Jahan, S.; Strezov, V. Water Quality Assessment of Australian Ports Using Water Quality Evaluation Indices. PLoS ONE 2017, 12, e0189284. [Google Scholar] [CrossRef] [PubMed]
- Drinking Water Quality Monitoring & Surveillance Framework. Available online: https://jaljeevanmission.gov.in/sites/default/files/guideline/WQMS-Framework.pdf (accessed on 27 July 2023).
- Ogwueleka, T.C. Assessment of the Water Quality and Identification of Pollution Sources of Kaduna River in Niger State (Nigeria) Using Exploratory Data Analysis. Water Environ. J. 2012, 28, 31–37. [Google Scholar] [CrossRef]
- Banerjee, P.; Prasad, B. Determination of Concentration of Total Sodium and Potassium in Surface and Ground Water Using a Flame Photometer. Appl. Water Sci. 2020, 10, 113. [Google Scholar] [CrossRef]
- Gomes, C.H.; Schmidt, A.M.; Dessart, R.L.; Casa Nova, G.P. Geochemical Analyses of Water and Public Health of the Mangueirão and Salso Streams in Caçapava Do Sul, RS, Brazil. Ambiente E Agua-Interdiscip. J. Appl. Sci. 2017, 12, 760. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in Metal-Induced Oxidative Stress and Human Disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, M.; Wei, J.; Qi, J. Pollution Load Estimation and Control Countermeasures of Zhangze Reservoir. Front. Environ. Sci. 2022, 10, 874124. [Google Scholar] [CrossRef]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of Industries in Water Scarcity and Its Adverse Effects on Environment and Human Health. Environ. Concerns Sustain. Dev. 2019, 2029, 235–256. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Chen, B.; Wang, M.; Duan, M.; Ma, X.; Hong, J.; Xie, F.; Zhang, R.; Li, X. In Search of Key: Protecting Human Health and the Ecosystem from Water Pollution in China. J. Clean. Prod. 2019, 228, 101–111. [Google Scholar] [CrossRef]
- Wu, H.; Gai, Z.; Guo, Y.; Li, Y.; Hao, Y.; Lu, Z.-N. Does Environmental Pollution Inhibit Urbanization in China? A New Perspective through Residents’ Medical and Health Costs. Environ. Res. 2020, 182, 109128. [Google Scholar] [CrossRef] [PubMed]
- Parris, K. Impact of Agriculture on Water Pollution in OECD Countries: Recent Trends and Future Prospects. Int. J. Water Resour. Dev. 2011, 27, 33–52. [Google Scholar] [CrossRef]
- Singh Shekhawat, S.; Verma, K.; Gupta, A. Status, Challenges and Future Prospects of Wastewater Reuse for Agricultural Irrigation in Developing Countries: A Mini Review. Acta Sci. Agric. 2020, 4, 21–30. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Natasha; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A Review of Environmental Contamination and Health Risk Assessment of Wastewater Use for Crop Irrigation with a Focus on Low and High-Income Countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Lai, W. Pesticide Use and Health Outcomes: Evidence from Agricultural Water Pollution in China. J. Environ. Econ. Manag. 2017, 86, 93–120. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, L.; Deng, L.; Jin, Z. Characteristics, Sources, Water Quality and Health Risk Assessment of Trace Elements in River Water and Well Water in the Chinese Loess Plateau. Sci. Total Environ. 2019, 650, 2004–2012. [Google Scholar] [CrossRef]
- Blanco, A.; Roper, W.E. Remote Sensing Techniques to Detect Surface Water Quality Constituents in Coastal and Inland Water Bodies from Point or Non Point Pollution Sources. Proc. Water Environ. Fed. 2007, 2007, 2039–2067. [Google Scholar] [CrossRef]
- Lancaster, M. Green Chemistry: An Introductory Text; RSC Paperbacks Ser.; RSC: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Zacchaeus, O.O.; Adeyemi, M.B.; Azeem Adedeji, A.; Adegoke, K.A.; Anumah, A.O.; Taiwo, A.M.; Ganiyu, S.A. Effects of Industrialization on Groundwater Quality in Shagamu and Ota Industrial Areas of Ogun State, Nigeria. Heliyon 2020, 6, e04353. [Google Scholar] [CrossRef]
- Dutta, V.; Dubey, D.; Kumar, S. Cleaning the River Ganga: Impact of Lockdown on Water Quality and Future Implications on River Rejuvenation Strategies. Sci. Total Environ. 2020, 743, 140756. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Singh, M.P.; Mishra, V. Bioremediation of Toxic Metal Ions from Coal Washery Effluent. Desalinat. Water Treat. 2020, 197, 300–318. [Google Scholar] [CrossRef]
- John, N.U.; Chimka, E.I. Effect of Discharge of Partially Treated Refinery Effluent on the Okrika River. J. Environ. Stud. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Liu, Z. A Global Energy Outlook. Glob. Energy Interconnect. 2015, 91–100. [Google Scholar] [CrossRef]
- Singh, V.; Mishra, V. Microbial Removal of Cr (VI) by a New Bacterial Strain Isolated from the Site Contaminated with Coal Mine Effluents. J. Environ. Chem. Eng. 2021, 9, 106279. [Google Scholar] [CrossRef]
- Annual Report. Central Pollution Control Board, India. Available online: https://yamunariverproject.wp.tulane.edu/wp-content/uploads/sites/507/2021/01/cpcb_2009-water-quality-status.pdf (accessed on 27 July 2023).
- Kowalik-Klimczak, A.; Stanislawek, E. Reclamation of Water from Dairy Wastewater Using Polymeric Nanofiltration Membranes. Desalinat. Water Treat. 2018, 128, 364–371. [Google Scholar] [CrossRef]
- Koul, B.; Yadav, D.; Singh, S.; Kumar, M.; Song, M. Insights into the Domestic Wastewater Treatment (DWWT) Regimes: A Review. Water 2022, 14, 3542. [Google Scholar] [CrossRef]
- Alvarez, S.; Asci, S.; Vorotnikova, E. Valuing the Potential Benefits of Water Quality Improvements in Watersheds Affected by Non-Point Source Pollution. Water 2016, 8, 112. [Google Scholar] [CrossRef]
- Straškraba, M. Ecotechnological Methods for Managing Non-Point Source Pollution in Watersheds, Lakes and Reservoirs. Water Sci. Technol. 1996, 33, 73–80. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Lateef, M.A.; Mohammed, S.A.S.; Ahmad, M.; Usman, A.R.A.; Almajed, A. Heavy Metal Immobilization Studies and Enhancement in Geotechnical Properties of Cohesive Soils by EICP Technique. Appl. Sci. 2020, 10, 7568. [Google Scholar] [CrossRef]
- Pal, D.; Maiti, S.K. An approach to counter sediment toxicity by immobilization of heavy metals using waste fish scale derived biosorbent. Ecotoxicol. Environ. Saf. 2020, 187, 109833. [Google Scholar] [CrossRef] [PubMed]
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of Microbes for Bioremediation of Crude Oil Polluted Environments: A Mini Review. Open Microbiol. J. 2015, 9, 48–54. [Google Scholar] [PubMed]
- Bhattacharya, P.; Banerjee, P.; Mallick, K.; Ghosh, S.; Majumdar, M.A.; Bandyopadhyay, S. Potential of biosorbent developed from fruit peel of Trewianudiflora for removal of hexavalent chromium from synthetic and industrial effluent: Analyzing phytotoxicity in germinating Vigna seeds. J. Environ. Sci. Health 2013, 48, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.; Tuzen, M. Biosorption of total chromium from aqueous solution by red algae (Ceramiumvigatum): Equilibrium, kinetics and thermodynamics studies. J. Hazard. Mater. 2008, 160, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Hadjmohammadi, M.R.; Salary, M.; Biparva, P. Removal of Cr (VI) from aqueous solution using pine needles powder as a biosorbent. J. Appl. Sci. Environ. Sanit. 2010, 6, 1–13. [Google Scholar]
- Nandhagopal, K.; Munuswamy, E.; Krishnan, V. Biosorption of chromium vi by ubiquitous dictyota biomass. Int. J. Pharm. Biol. Sci. 2018, 8, 127–131. [Google Scholar]
- Hiew, B.Y.Z.; Lee, L.Y.; Lee, X.J.; Thangalazhy-Gopakumar, S.; Gan, S. Utilisation of environmentally friendly okara-based biosorbent for cadmium(II) removal. Environ. Sci. Pollut. Res. 2021, 28, 40608–40622. [Google Scholar] [CrossRef]
- Bundschuh, J.; Schneider, J.; Alam, M.A.; Niazi, N.K.; Herath, I.; Parvez, F.; Tomaszewska, B.; Guilherme, L.R.G.; Maity, J.P.; López, D.L.; et al. Seven Potential Sources of Arsenic Pollution in Latin America and Their Environmental and Health Impacts. Sci. Total Environ. 2021, 780, 146274. [Google Scholar] [CrossRef]
- Suhrhoff, T.J. Phytoprevention of Heavy Metal Contamination From Terrestrial Enhanced Weathering: Can Plants Save the Day? Front. Clim. 2022, 3, 820204. [Google Scholar] [CrossRef]
- Wen, J.; Wu, Y.; Li, X.; Lu, Q.; Luo, Y.; Duan, Z.; Li, C. Migration Characteristics of Heavy Metals in the Weathering Process of Exposed Argillaceous Sandstone in a Mercury-Thallium Mining Area. Ecotoxicol. Environ. Saf. 2021, 208, 111751. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zheng, X.; Chen, C. Leaching Behavior of Heavy Metals and Transformation of Their Speciation in Polluted Soil Receiving Simulated Acid Rain. PLoS ONE 2012, 7, e49664. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Ko, T.-H. Evaluation of Acid Leaching on the Removal of Heavy Metals and Soil Fertility in Contaminated Soil. J. Chem. 2018, 2018, 5036581. [Google Scholar] [CrossRef]
- Sasakova, N.; Gregova, G.; Takacova, D.; Mojzisova, J.; Papajova, I.; Venglovsky, J.; Szaboova, T.; Kovacova, S. Pollution of Surface and Ground Water by Sources Related to Agricultural Activities. Front. Sustain. Food Syst. 2018, 2, 42. [Google Scholar] [CrossRef]
- Sullivan, P.J.; Agardy, F.J.; Clark, J.J.J. The Water We Drink. Environ. Sci. Drink. Water 2005, 2005, 1–28. [Google Scholar] [CrossRef]
- Ji, L.; Li, Y.; Zhang, G.; Bi, Y. Anthropogenic Disturbances Have Contributed to Degradation of River Water Quality in Arid Areas. Water 2021, 13, 3305. [Google Scholar] [CrossRef]
- Jabeen, F.; Aslam, A. Heavy Metals Toxicity and Associated Health Risks in Vegetables Grown under Soil Irrigated with Sewage Water. Univers. J. Agric. Res. 2018, 6, 173–180. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The Relative Impact of Toxic Heavy Metals (THMs) (Arsenic (As), Cadmium (Cd), Chromium (Cr)(VI), Mercury (Hg), and Lead (Pb)) on the Total Environment: An Overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Boufekane, A.; Saighi, O. Assessing Groundwater Quality for Irrigation Using Geostatistical Method—Case of Wadi Nil Plain (North-East Algeria). Groundw. Sustain. Dev. 2019, 8, 179–186. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Yosim, A.; Fry, R.C. Incorporating Epigenetic Data into the Risk Assessment Process for the Toxic Metals Arsenic, Cadmium, Chromium, Lead, and Mercury: Strategies and Challenges. Front. Genet. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud. Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of Lead (Pb) and Its Effects on Human: A Review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Arsenic. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic#:~:text=Long%2Dterm%20exposure%20to%20arsenic,increased%20deaths%20in%20young%20adults (accessed on 27 July 2023).
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Yan, G.; Gao, Y.; Xue, K.; Qi, Y.; Fan, Y.; Tian, X.; Wang, J.; Zhao, R.; Zhang, P.; Liu, Y.; et al. Toxicity Mechanisms and Remediation Strategies for Chromium Exposure in the Environment. Front. Environ. Sci. 2023, 11, 161. [Google Scholar] [CrossRef]
- Johnson-Arbor, K.; Tefera, E.; Farrell, J. Characteristics and Treatment of Elemental Mercury Intoxication: A Case Series. Health Sci. Rep. 2021, 4, e293. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Comparative Review of Different Adsorption Techniques Used in Heavy Metals Removal in Water. Biointerface Res. Appl. Chem. 2022, 13, 397. [CrossRef]
- Devi, B.D.; Thatheyus, A.J.; Ramya, D. Bioremoval of hexavalent chromium, using Pseudomonas fluorescens. J. Microbiol. Biotechnol. Res. 2012, 2, 727–735. [Google Scholar]
- Machida, M.; Amano, Y.; Aikawa, M. Adsorptive Removal of Heavy Metal Ions by Activated Carbons. Carbon 2011, 49, 3393. [Google Scholar] [CrossRef]
- Na Nagara, V.; Sarkar, D.; Elzinga, E.J.; Datta, R. Removal of Heavy Metals from Stormwater Runoff Using Granulated Drinking Water Treatment Residuals. Environ. Technol. Innov. 2022, 28, 102636. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.M.A.B.; Mohd Arif Zainol, M.R.R.; Murshed, M.F.; Hariz-Zain; Faris, M.A.; Bayuaji, R. Review on Adsorption of Heavy Metal in Wastewater by Using Geopolymer. MATEC Web Conf. 2017, 97, 01023. [Google Scholar] [CrossRef]
- Al-Enezi, G.; Hamoda, M.F.; Fawzi, N. Ion Exchange Extraction of Heavy Metals from Wastewater Sludges. J. Environ. Sci. Health Part A 2004, 39, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Djedidi, Z.; Bouda, M.; Souissi, M.A.; Cheikh, R.B.; Mercier, G.; Tyagi, R.D.; Blais, J.-F. Metals Removal from Soil, Fly Ash and Sewage Sludge Leachates by Precipitation and Dewatering Properties of the Generated Sludge. J. Hazard. Mater. 2009, 172, 1372–1382. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Thirukumaran, P.; Yoon, D.H.; Lee, Y.R.; Cheong, I.W. Simultaneous Removal of Heavy Metal Ions Using Carbon Dots-Doped Hydrogel Particles. Chemosphere 2022, 286, 131760. [Google Scholar] [CrossRef] [PubMed]
- Blöcher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.K.; Matis, K.A. Hybrid Flotation—Membrane Filtration Process for the Removal of Heavy Metal Ions from Wastewater. Water Res. 2003, 37, 4018–4026. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, A.Q.; Telfah, D.B.; Ismail, R. Heavy Metals Removal from Landfill Leachate by Coagulation/Flocculation Process Combined with Continuous Adsorption Using Eggshell Waste Materials. Water Sci. Technol. 2021, 84, 3817–3832. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Hu, J.; Sun, Y.; Zhu, Q.; Wu, L.; Liu, B.; Xiao, K.; Liang, S.; Yang, J.; Hou, H. Simultaneous Heavy Metal Removal and Sludge Deep Dewatering with Fe(II) Assisted Electrooxidation Technology. J. Hazard. Mater. 2021, 405, 124072. [Google Scholar] [CrossRef] [PubMed]
- Agnello, A.C.; Bagard, M.; van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative Bioremediation of Heavy Metals and Petroleum Hydrocarbons Co-Contaminated Soil by Natural Attenuation, Phytoremediation, Bioaugmentation and Bioaugmentation-Assisted Phytoremediation. Sci. Total Environ. 2016, 563–564, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Sharma, R. Bioremediation of Toxic Heavy Metals: A Patent Review. Recent. Pat. Biotechnol. 2017, 11, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Al-Qodah, Z.; Al-Shannag, M. Heavy Metal Ions Removal from Wastewater Using Electrocoagulation Processes: A Comprehensive Review. Sep. Sci. Technol. 2017, 52, 2649–2676. [Google Scholar] [CrossRef]
- Kumar, S.; Shahnaz, T.; Selvaraju, N.; Rajaraman, P.V. Kinetic and Thermodynamic Studies on Biosorption of Cr(VI) on Raw and Chemically Modified Datura Stramonium Fruit. Environ. Monit. Assess. 2020, 192, 248. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8, 782. [Google Scholar] [CrossRef]
- Mishra, A.; Dubey, A.; Shinghal, S. Biosorption of Chromium(VI) from Aqueous Solutions Using Waste Plant Biomass. Int. J. Environ. Sci. Technol. 2014, 12, 1415–1426. [Google Scholar] [CrossRef]
- Khalid, M.; Hassani, S.; Abdollahi, M. Metal-Induced Oxidative Stress: An Evidence-Based Update of Advantages and Disadvantages. Curr. Opin. Toxicol. 2020, 20–21, 55–68. [Google Scholar] [CrossRef]
- Aksu, Z.; Sag, Y.; Kutsal, T. The Biosorpnon of Copperod by C. vulgaris and Z. ramigera. Environ. Technol. 1992, 13, 579–586. [Google Scholar] [CrossRef]
- Silva, E.A.; Cossich, E.S.; Tavares, C.G.; Cardozo Filho, L.; Guirardello, R. Biosorption of Binary Mixtures of Cr(III) and Cu(II) Ions by Sargassum sp. Braz. J. Chem. Eng. 2003, 20, 213–227. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, C.; Berkhouse, H.; Zhang, Y.; Lv, Y.; Lu, W.; Yang, Y.; Zhou, J. Removal of Cadmium by Bioflocculant Produced by Stenotrophomonas Maltophilia Using Phenol-Containing Wastewater. Chemosphere 2016, 155, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, D.; Hu, K.; Deng, H.; Zhang, M.; Wang, A.; Qiu, R.; Yan, K. Coupling Adsorption-Photocatalytic Reduction of Cr(VI) by Metal-Free N-Doped Carbon. Sci. Total Environ. 2020, 704, 135284. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption and me. Water Res. 2007, 41, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshanee, M.; Das, S. Biosorption and Removal of Toxic Heavy Metals by Metal Tolerating Bacteria for Bioremediation of Metal Contamination: A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 104686. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef]
- Zhang, W.; An, Y.; Li, S.; Liu, Z.; Chen, Z.; Ren, Y.; Wang, S.; Zhang, X.; Wang, X. Enhanced heavy metal removal from an aqueous environment using an eco-friendly and sustainable adsorbent. Sci. Rep. 2020, 10, 16453. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and bioaccumulation-the prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef]
- Tsezos, M.; Remoudaki, E.; Angelatou, V. Biosorption sites of selected metals using Electron microscopy. Comp. Biochem. Phys. A 1997, 118, 481–487. [Google Scholar] [CrossRef]
- Duwiejuah, A.B.; Abubakari, A.H.; Quainoo, A.K.; Amadu, Y. Review of Biochar Properties and Remediation of Metal Pollution of Water and Soil. J. Health Pollut. 2020, 10, 200902. [Google Scholar] [CrossRef]
- Dias, M.A.; Rosa, C.A.; Linardi, V.R.; Conte, R.A.; De Castro, H.F. Application of factorial design to study of heavy metals biosorption by waste biomass from beverage distillery. Appl. Biochem. Biotechnol. 2001, 91, 413–422. [Google Scholar] [CrossRef]
- Naja, G.M.; Volesky, B. Treatment of Metal-Bearing Effluents: Removal and Recovery. In Handbook on Heavy Metals in the Environment; Wang, L.K., Chen, J.P., Hung, Y.T., Shammas, N.K., Eds.; Taylor & Francis and CRC Press: Boca Raton, FL, USA, 2010; p. 46. [Google Scholar]
- Saranya, N.; Ajmani, A.; Sivasubramanian, V.; Selvaraju, N. Hexavalent Chromium Removal from Simulated and Real Effluents Using Artocarpus Heterophyllus Peel Biosorbent - Batch and Continuous Studies. J. Mol. Liq. 2018, 265, 779–790. [Google Scholar] [CrossRef]
- Baldovi, A.A.; Ayvazian, A.P.; Coelho, L.H.G.; de Jesus, T.A. Biosorption of Pb(II) by Unmodified Banana Peel in Batch and Column Experiments: A Potential Green and Low-Cost Technology for Industrial Effluent Treatment. Water Air Soil. Pollut. 2022, 233, 490. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Waheed-uz-Zaman, U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb(II) and Cd(II) from Water by Adsorption on Peels of Banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Ahmad, S.R.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Selective Removal of Hexavalent Chromium from Wastewater by Rice Husk: Kinetic, Isotherm and Spectroscopic Investigation. Water 2021, 13, 263. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Krishnaiah, N.; Subbaiah, M.V.; Krishnaiah, A. Biosorption of Pb(II) from Aqueous Solution by Solanum Melongena Leaf Powder as a Low-Cost Biosorbent Prepared from Agricultural Waste. Colloids Surf. B Biointerfaces 2014, 114, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Heraldy, E.; Lestari, W.W.; Permatasari, D.; Arimurti, D.D. Biosorbent from Tomato Waste and Apple Juice Residue for Lead Removal. J. Environ. Chem. Eng. 2018, 6, 1201–1208. [Google Scholar] [CrossRef]
- Letechipia, J.O.; González-Trinidad, J.; Júnez–Ferreira, H.E.; Bautista–Capetillo, C.; Robles Rovelo, C.O.; Contreras Rodríguez, A.R. Removal of Arsenic from Semiarid Area Groundwater Using a Biosorbent from Watermelon Peel Waste. Heliyon 2023, 9, e13251. [Google Scholar] [CrossRef]
- Abid, M.; Niazi, N.K.; Bibi, I.; Farooqi, A.; Ok, Y.S.; Kunhikrishnan, A.; Ali, F.; Ali, S.; Igalavithana, A.D.; Arshad, M. Arsenic(V) Biosorption by Charred Orange Peel in Aqueous Environments. Int. J. Phytoremediat. 2015, 18, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Amar, M.B.; Walha, K.; Salvadó, V. Valorisation of Pine Cone as an Efficient Biosorbent for the Removal of Pb(II), Cd(II), Cu(II), and Cr(VI). Adsorpt. Sci. Technol. 2021, 2021, 6678530. [Google Scholar] [CrossRef]
- d’Halluin, M.; Rull-Barrull, J.; Bretel, G.; Labrugère, C.; Le Grognec, E.; Felpin, F.X. Chemically modified cellulose filter paper for heavy metal remediation in water. ACS Sustain. Chem. Eng. 2017, 5, 1965–1973. [Google Scholar] [CrossRef]
- Abu-Danso, E.; Peräniemi, S.; Leiviskä, T.; Bhatnagar, A. Synthesis of S-ligand tethered cellulose nanofibers for efficient removal of Pb(II) and Cd(II) ions from synthetic and industrial wastewater. Environ. Pollut. 2018, 242, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, T.; Huang, Q.; Wang, J.; Lu, S.; Yan, J. Enhanced adsorption for Pb(II) and Cd(II) of magnetic rice husk biochar by KMnO4 modification. Environ. Sci. Pollut. Res. 2019, 26, 8902–8913. [Google Scholar] [CrossRef] [PubMed]
- Changmai, M.; Banerjee, P.; Nahar, K.; Purkait, M.K. A Novel Adsorbent from Carrot, Tomato and Polyethylene Terephthalate Waste as a Potential Adsorbent for Co (II) from Aqueous Solution: Kinetic and Equilibrium Studies. J. Environ. Chem. Eng. 2018, 6, 246–257. [Google Scholar] [CrossRef]
- Meng, F.; Yang, B.; Wang, B.; Duan, S.; Chen, Z.; Ma, W. Novel Dendrimerlike Magnetic Biosorbent Based on Modified Orange Peel Waste: Adsorption–Reduction Behavior of Arsenic. ACS Sustain. Chem. Eng. 2017, 5, 9692–9700. [Google Scholar] [CrossRef]
- Singh, V.; Singh, J.; Mishra, V. Development of a Cost-Effective, Recyclable and Viable Metal Ion Doped Adsorbent for Simultaneous Adsorption and Reduction of Toxic Cr (VI) Ions. J. Environ. Chem. Eng. 2021, 9, 105124. [Google Scholar] [CrossRef]
- Salman, M.; Rehman, R.; Farooq, U.; Tahir, A.; Mitu, L. Biosorptive Removal of Cadmium(II) and Copper(II) Using Microwave-Assisted Thiourea-Modified Sorghum Bicolor Agrowaste. J. Chem. 2020, 2020, 8269643. [Google Scholar] [CrossRef]
- Hassan, P.B.; Rasheed, R.O.; Zargoosh, K. Cadmium and Lead Removal from Aqueous Solution Using Magnetite Nanoparticles Biofabricated from Portulaca Oleracea Leaf Extract. J. Nanomater. 2022, 2022, 1024554. [Google Scholar] [CrossRef]
- Diep, P.; Mahadevan, R.; Yakunin, A.F. Heavy Metal Removal by Bioaccumulation Using Genetically Engineered Microorganisms. Front. Bioeng. Biotechnol. 2018, 2018, 157. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Yu, Q.; Huang, J.; Yu, G. Removal of perfluorooctane sulfonate from wastewater by anion exchange resins: Effects of resin properties and solution chemistry. Water Res. 2010, 18, 5188–5195. [Google Scholar] [CrossRef] [PubMed]
- Timkova, I.; Sedláková-Kaduková, J.; Pristaš, P. Biosorption and Bioaccumulation Abilities of Actinomycetes/Streptomycetes Isolated from Metal Contaminated Sites. Separations 2018, 5, 54. [Google Scholar] [CrossRef]
- Ahluwalia, S.S. Microbial removal of hexavalent chromium and scale up potential. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 383–398. [Google Scholar]
- Shi, J.; Cai, Y. Environmental Chemistry and Toxicology of Heavy Metals. Ecotoxicol. Environ. Saf. 2020, 202, 110926. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer, P.; Saracoglu, N. Bioaccumulation and biosorption of copper (II) and chromium (III) from aqueous solutions by Pichia stipitis yeast. J. Chem. Technol. Biotechnol. 2009, 84, 604–610. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, A.; Rehman, A. Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J. Environ. Sci. 2009, 21, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Choinska-Pulit, A.; Sobolczyk-Bednarek, J.; Łaba, W. Optimization of copper, lead and cadmium biosorption onto newly isolated bacterium using a Box-Behnken design. Ecotoxicol. Environ. Saf. 2018, 149, 275–283. [Google Scholar] [CrossRef]
- Dey, U.; Chatterjee, S.; Mondal, N.K. Isolation and Characterization of Arsenic-Resistant Bacteria and Possible Application in Bioremediation. Biotechnol. Rep. 2016, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Heidari, P.; Panico, A. Sorption Mechanism and Optimization Study for the Bioremediation of Pb(II) and Cd(II) Contamination by Two Novel Isolated Strains Q3 and Q5 of Bacillus sp. Int. J. Environ. Res. Public Health 2020, 17, 4059. [Google Scholar] [CrossRef]
- Shardendu; Tripti, K.; Singh, D.N.; Sayantan, D. Evaluation of arsenic removal potential of arsenic resistant bacteria with the role of physiological and genomic factors. Indian J. Exp. Biol. 2017, 55, 251–261. [Google Scholar]
- Olubode, T.P.; Amusat, A.I.; Olawale, B.R.; Adekola, F.F. Biosorption of heavy metals using bacterial isolates from e-waste soil. Afr. J. Microbiol. Res. 2022, 16, 268–272. [Google Scholar]
- Little, P.; Martin, M.H. Biological Monitoring of Heavy Metal Pollution. Environ. Pollut. (1970) 1974, 6, 1–19. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; El-Khateeb, A.Y.; Ghoniem, A.A.; El-Hersh, M.S.; Saber, W.I.A. Innovative low-cost biosorption process of Cr6+ by Pseudomonas alcaliphila NEWG-2. Sci. Rep. 2010, 10, 14043–14061. [Google Scholar] [CrossRef] [PubMed]
- Tekerlekopoulou, A.G.; Tsiflikiotou, M.; Akritidou, L.; Viennas, A.; Tsiamis, G.; Pavlou, S.; Bourtzis, K.; Vayenas, D.V. Modelling of biological Cr (VI) removal in draw-fill reactors using microorganisms in suspended and attached growth systems. Water Res. 2013, 47, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Humphries, A.C.; Nott, K.P.; Hall, L.D.; Macaskie, L.E. Reduction of Cr (VI) by immobilized cells of Desulfovibrio vulgaris NCIMB 8303 and Microbacterium sp. NCIMB 13776. Biotechnol. Bioeng. 2005, 90, 589–596. [Google Scholar] [CrossRef]
- Ibrahim, A.S.S.; El-Tayeb, A.M.; Elbadawi, B.Y.; Al-Salamah, A.A.; Antranikian, G. Hexavalent chromate reduction by alkaliphilic Amphibacillus sp. KSUCr3 is mediated by copper-dependent membrane-associated Cr (VI). Extremophiles 2012, 16, 659–668. [Google Scholar] [CrossRef]
- Akpomie, O.O.; Ejechi, B.O. Removal of Cr (VI) from Tannery Effluents with Mixed Cultures of Bacteria and Fungi Isolated from Soils Contaminated with Tropical Tannery Effluents. Int. J. Environ. Waste Manag. 2016, 17, 60. [Google Scholar] [CrossRef]
- Henson, M.W.; Santo Domingo, J.W.; Kourtev, P.S.; Jensen, R.V.; Dunn, J.A.; Learman, D.R. Metabolic and genomic analysis elucidates strain-level variation in Microbacterium spp. isolated from chromate contaminated sediment. PeerJ 2015, 3, 1395–1412. [Google Scholar] [CrossRef]
- Ahemad, M. Enhancing phytoremediation of chromium-stressed soils through plant-growth-promoting bacteria. J. Genet. Eng. Biotechnol. 2015, 13, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, O.I.; Lushchak, O.V.; Lushchak, J.V.; Torous, I.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Chromium effects on free radical processes in goldfish tissues: Comparison of Cr (III) and Cr (VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Praveenkumar, R.; Ilavarasi, A.; Rajeshwari, K.; Thajuddin, N. Biochemical changes of fresh water cyanobacteria Dolichospermum flos-aquae NTMS07 to chromium-induced stress with special reference to antioxidant enzymes and cellular fatty acids. Bull. Environ. Contam. Toxicol. 2013, 90, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Joutey, N.T.; Sayel, H.; Bahafid, W.; El Ghachtouli, N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol. 2015, 233, 45–69. [Google Scholar] [PubMed]
- Elbasiouny, H.; Darwesh, M.; Elbeltagy, H.; Abo-Alhamd, F.G.; Amer, A.A.; Elsegaiy, M.A.; Khattab, I.A.; Elsharawy, E.A.; Ebehiry, F.; El-Ramady, H.; et al. Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: A review. Environ. Monit. Assess. 2021, 193, 449. [Google Scholar] [CrossRef] [PubMed]
- Ethaib, S.; Al-Qutaifia, S.; Al-Ansari, N.; Zubaidi, S.L. Function of Nanomaterials in Removing Heavy Metals for Water and Wastewater Remediation: A Review. Environments 2022, 9, 123. [Google Scholar] [CrossRef]
- Trivunac, K.; Stevanovic, S. Removal of Heavy Metal Ions from Water by Complexation-Assisted Ultrafiltration. Chemosphere 2006, 64, 486–491. [Google Scholar] [CrossRef]
- Singh, V.; Mishra, V. Sustainable reduction of Cr (VI) and its elemental mapping on chitosan coated citrus limetta peels biomass in synthetic wastewater. Sep. Sci. Technol. 2021, 57, 1609–1626. [Google Scholar] [CrossRef]

| Parameters | Permissible Limits |
|---|---|
| pH | 6–9 |
| Temperature | 25 |
| Total solids (mg/L) | 1500 |
| Nitrate (mg/L) | 50 |
| Ammonia (mg/L) | 1.5 |
| Ni (II) (mg/L) | 0.07 |
| Zn (II) (mg/L) | 0.05 |
| Cd (II) (mg/L) | 0.03 × 10−1 |
| Pb (II) (mg/L) | 0.01 |
| Ti (II) (mg/L) | 0.05 |
| Cr (VI) (mg/L) | 0.05 |
| As (V and III) (mg/L) | 0.01 |
| Parameters | Unit | Method or Equipment Used | References |
|---|---|---|---|
| Temperature | °C | Thermometer | [39] |
| pH | pH meter | [39] | |
| Nitrate | mg/L | UV spectrophotometer | [39] |
| Chloride | mg/L | Argentometric method (silver nitrate method) or Mohr’s method | [39] |
| Sodium | mg/L | Flame photometer | [40] |
| Total hardness | mg/L | EDTA titrimetric method | [41] |
| Dissolve oxygen (DO) | mg/L | Winkler method | [41] |
| Alkalinity | mg/L | Titrimetric method | [40,41] |
| Conductivity | µs/cm | Conductivity meters | [41] |
| Sulphate | mg/L | UV spectrophotometer | [40,41] |
| Phosphate | mg/L | UV spectrophotometer | [40] |
| Magnesium | mg/L | EDTA titrimetric method | [41] |
| Minerals and heavy metals | mg/L | Inductively coupled plasma optical emission spectroscopy (ICP-OES), ICP-MS, or atomic absorption spectrophotometer | [42] |
| Pollutants | Health Impacts | References |
|---|---|---|
| Pb (II) | Pb (II) is toxic to health by accumulating in the body and damages the central nervous system. Most risky relative to children and pregnant women. | [94] |
| As (II) | Risk of cancer and cause skin lesions. As (II) toxicity is also associated with cardiovascular diseases and diabetes. | [95] |
| Cd (II) | Cd (II) exposure causes reproductive, cardiovascular, pulmonary, and gastrointestinal disorders. | [96] |
| Cr (VI) | Cr (VI) can be responsible for acute and chronic toxicity in the living organism. It also has carcinogenic effects. | [97] |
| Hg (II) | It causes harmful effects on the living system, including headaches, anorexia, and rash. It also affects the digestive system, reproductive system, kidney, and respiratory system. | [98] |
| Ni (II) | Depending on the dosage and duration of exposure, various health issues can arise, including dermatitis, asthma, and cancer of the respiratory tract. | [99] |
| Methods | Advantage | Limitations | References |
|---|---|---|---|
| Oxidation | A rapid process for heavy metal removal | Expensive and generates by-products | [104] |
| Ion exchange | Effective removal of a wide range of heavy metals | Adsorbents require regeneration or disposal | [104,105] |
| Chemical precipitation | An effective method for the removal of heavy metals | Production of a large amount of sludge | [104,106,107] |
| Adsorption | Flexibility and simplicity of method design and insensitivity to toxic metals | Regeneration required after adsorption | [104,108] |
| Membrane filtration | An effective method for the removal of heavy metal ions | High operation cost and concentrated sludge production | [104,109] |
| Photochemical | No production of sludge | Formation of by-products | [104] |
| Coagulation/flocculation | Economically feasible | Formation of large particles and production of sludge | [104,110] |
| Electrochemical coagulation | Economically feasible | A large amount of sludge production | [111] |
| Biological treatment | Eco-friendly, inexpensive, and effective removal of heavy metals | Biological methods have yet to be established and commercialized | [112,113] |
| Biosorbent | Heavy Metal | Biosorption Capacity (mg/g) | References |
|---|---|---|---|
| Trewia nudiflora fruit peels powder | 294.12 | [132] | |
| Banana peel | Pb (II) | 0.5 | [133] |
| Banana peel | Cd (II) | 5.71 | [134] |
| Rice husk | Cr (VI) | 33.68 | [135] |
| Solanum melongena leaf powder | Pb (II) | 71.42 | [136] |
| Tomato waste Apple huice residue | Pb (II) | 152 108 | [137] |
| watermelon peel waste | As (III and V) | 2.42 | [138] |
| Orange peels | As (V) | 32.7 | [139] |
| Pine cone | Pb (II) Cd (II) Cu (II) Cr (VI) | 100.01 78.73 33.55 57.36 | [140] |
| Modified Biosorbent | Heavy Metals | Biosorption Capacity (mg/g) | References |
|---|---|---|---|
| treated magnetic biochar | Pb (II) Cd (II) | 148 79 | [143] |
| Composite adsorbent of carrot, tomato and PET | Co (II) | 312.50 | [144] |
| Sulfuric-acid-treated orange peels | As (V) | 60.09 | [139] |
| Iron nanoparticles modified orange peels | As (V) | 81.30 | [145] |
| Ferrous-ion-doped rice husk | Cr (VI) | 11.14 | [146] |
| Microwave-assisted thiourea-modified Sorghum bicolor | Cu (II) Cd (II) | 15.15 17.24 | [147] |
| Portulaca oleracea extract fabricated Fe3O4 NPs | Cd (II) Pb (II) | 177.48 108.22 | [148] |
| Microorganism | Heavy Metals | Removal Efficiency (%) | Optimum pH | Optimum Temperature | Initial Metal Concentration (mg/L) | References |
|---|---|---|---|---|---|---|
| Pseudomonas azotoformans strain JAW1 | Cd (II) | 44.67 | 6 | 30 | 25 | [157] |
| Bacillus sp. SW2 Bacillus sp. SW4 | As (V) As (III) As (V) As (III) | 53.29 51.45 51.99 50.37 | - | - | 100 | [158] |
| Bacillus sp. Strain Q3 | Pb (II) | 76.4 | 6.2 | 34.3 | 127.4 | [159] |
| Paracoccus sp. strain NC-A Alcaligenes faecalis strain NC-B Stenotrophomonas sp. strain NC-C | As (V) As (V) As (III) | 84.50 93.00 79.60 | 7 7 7 | 35 35 35 | - - - | [160] |
| Bacillus cereus S13 Bacillus cereus S25 | Pb (II) Co (II) Cr (VI) | 98.00 93.70 93.90 | - - - | - - - | 10 10 10 | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. https://doi.org/10.3390/toxics11100828
Zhang P, Yang M, Lan J, Huang Y, Zhang J, Huang S, Yang Y, Ru J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics. 2023; 11(10):828. https://doi.org/10.3390/toxics11100828
Chicago/Turabian StyleZhang, Peng, Mingjie Yang, Jingjing Lan, Yan Huang, Jinxi Zhang, Shuangshuang Huang, Yashi Yang, and Junjie Ru. 2023. "Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation" Toxics 11, no. 10: 828. https://doi.org/10.3390/toxics11100828





