Dithiocarbamates: Properties, Methodological Approaches and Challenges to Their Control
Abstract
:1. Introduction
- ❖
- Methyl-dithiocarbamates (MDTCs), including metam sodium;
- ❖
- Dimethyl-dithiocarbamates (DMDTCs), including ziram, thiram and ferbam;
- ❖
- Ethylene-bis-dithiocarbamates (EBDTCs), including mancozeb, maneb, zineb and metiram;
- ❖
- Propylene-bis-dithiocarbamates (PBDTCs), including propineb.
2. Materials and Methods
3. Uses and Applications
- (i)
- Anticancer. Compounds containing dithiocarbamates have been evaluated as anticancer drugs as they can inhibit catalase (an enzyme responsible for cancer growth) and induce apoptosis in the mitochondria [15]. For example, a pyrrolidine dithiocarbamate (PyDT)-zinc(II) complex and a PyDT-copper(II) complex were compared to treat breast and prostate cancer. Of the two, the copper complex was more potent in inhibiting the proteasome and inducing apoptosis [16]. Gold(III) compounds are also used as anticancer agents, and the characteristics of some gold(III) dithiocarbamate derivatives with cisplatin were compared. They are more cytotoxic, highly reactive towards some biological macromolecules and inhibit DNA and RNA synthesis much faster than cisplatin [17,18,19].
- (ii)
- Alcoholism. The best-known DTC derivative is the diethyldithiocarbamate disulfiram [15]. Disulfiram (tetraethylthiuram disulfide) is a drug that has been used for 60 years to treat alcoholism as an aldehyde dehydrogenase inhibitor, which leads to the accumulation of acetaldehyde in the blood [20,21]. The main adverse side effects include flushing, nausea and tachycardia. Disulfiram also acts on the central nervous system by inhibiting dopamine-β-hydroxylase, which causes an increase in dopamine concentration in the brain. This can cause schizophrenia and, rarely, psychosis in otherwise healthy individuals [22]. However, the results obtained from the use of disulfiram are conflicting. In a controlled context, the results obtained are positive, even if it is often prescribed for short periods. The reasons are not entirely known, but the researchers think the side effects are usually minor and severe unpleasant reactions are uncommon, although monitoring should be undertaken [20,23,24].
- (iii)
- Treatment of tuberculosis. Some N-mono- and N,N-di-substituted dithiocarbamates have been used to treat tuberculosis, acting through inhibition of the enzyme carbonic anhydrase. Both enzymes, mtCA 1 (Rv1284) and mtCA 3 (Rv3273), were inhibited using dithiocarbamates of formula R1R2N-CSSM where R1 is H, alkyl and substituted alkyl; R2 is alkyl, aryl and heterocycle and M is Na, K or triethylammonium. These DTCs were more effective than commonly used drugs [25,26].
- (iv)
- Alzheimer’s treatment. Several coumarin–dithiocarbamate hybrids have been synthesised and evaluated to treat Alzheimer′s disease. Several compounds were tested, and it was found that the terminal amino group associated with the dithiocarbamate moiety inhibit acetylcholinesterase (AChE), and the cyclic amine substituents have more potent activity than the alkyl amines [15,27]. At the same time, the piperidinyl group proved to be more beneficial than the pyrrolidinyl group. However, the best compound is obtained with a four-carbon linker between coumarin and the dithiocarbamates′ fraction. The compound obtained has the maximum ability to inhibit the enzyme. It could interact simultaneously with the catalytic active site (CAS) and the peripheral anionic site (PAS) of AChE, reversing cognitive dysfunction [28].
- (v)
- SARS-CoV-2 treatment. Dithiocarbamates have been used to treat MERS (Middle East respiratory syndrome) and SARS (severe acute respiratory syndrome) coronaviruses [29,30]. Specifically, disulfiram, a drug used to treat alcoholism, has been shown to inactivate viral coronavirus proteins (thioprotease and RNA replicase) [15]. Both disulfiram and some of its derivatives (tyram and dipentamethylenethiuram disulfide (DPTD)) are capable of inhibiting PLpro, a papain-like protease, through allosteric inhibition [31].
Latest Trends in the World Usage of Dithiocarbamates
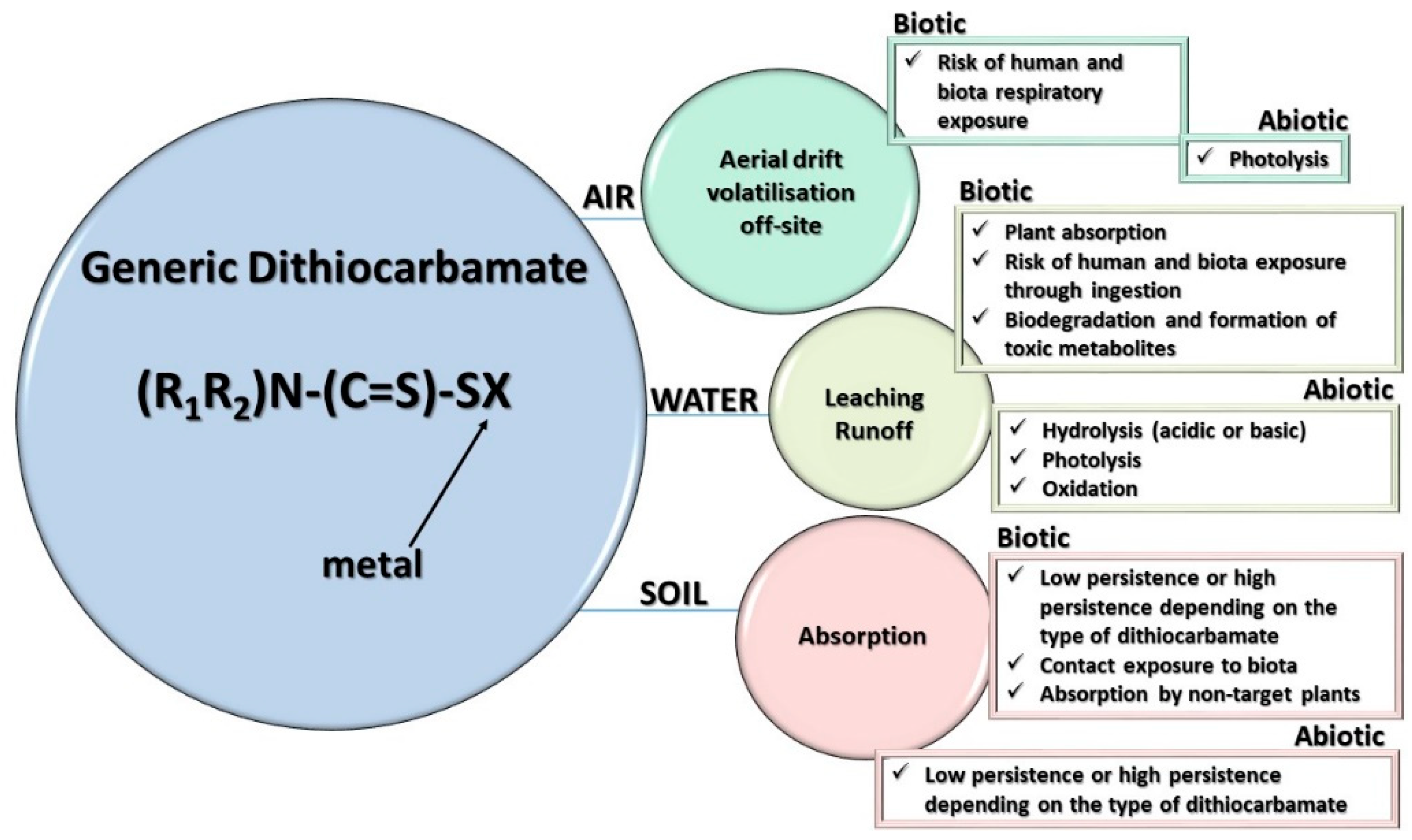
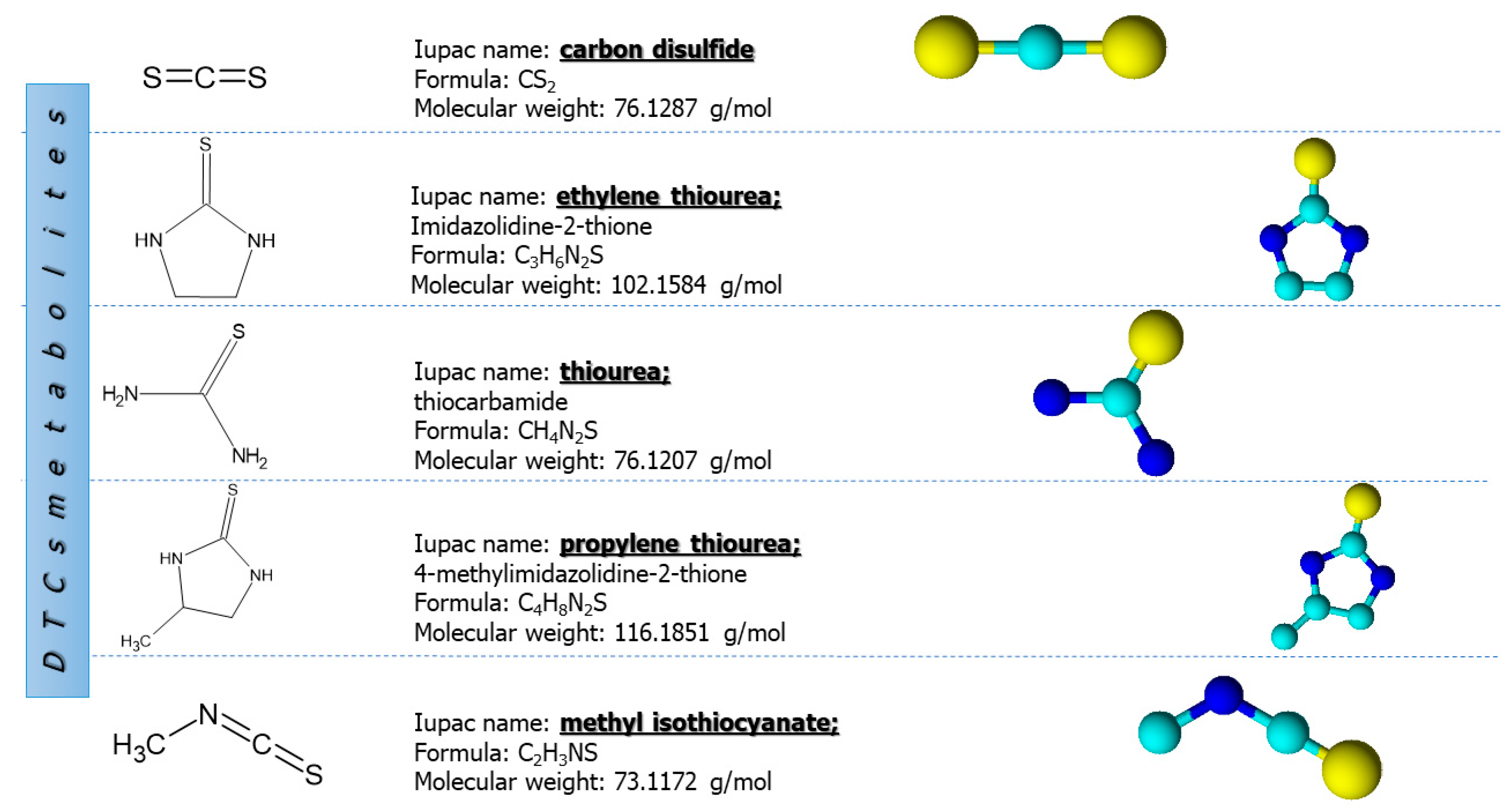
4. Metabolism and Environmental Fate
5. Toxicity of DTCs and Their Metabolites
6. Analytical Methods for Dithiocarbamate Detection
6.1. Hot Acid Digestion-Based Methods
6.2. Gas Chromatography-Based Methods
6.3. Liquid Chromatography-Based Methods
| Researched Active Principle | Investigated Matrix | Principle of the Method | Equipment | LOD and LOQ | References |
|---|---|---|---|---|---|
| DTCs as sum of CS2 | Fruits and vegetables | DTCs are reduced to CS2 | LC-MS/MS | LOD: 0.02–1.19 mg/kg LOQ: 0.03–2.69 mg/kg | [86] |
| DTCs as sum of CS2 | Soy (leaves, pods, seeds, soil) | DTCs are reduced to CS2 | GC-MS | LOQ: 0.32, 0.18, 0.19, 0.1 mg/kg | [87] |
| Maneb, Zineb, Propineb Mancozeb | Water and soil | DTCs are reduced to CS2 complexed with a copper acetate solution in the presence of diethanolamine | Spectrophotometer | n.a. | [88] |
| Mancozeb | Chamomile | The preparation involves the use of QuEChERS | LC-MS/MS | LOQ: 0.05 mg/kg | [89] |
| Propineb, mancozeb, Thiuram | Beer, malt and fruit juice | DTCs are methylated and subsequently analysed | LC-MS/MS | LOQ: <0.007 mg/kg | [90] |
| DTCs as sum of CS2 | Foods | The evolved carbon disulfide is collected and reacted to form the yellow cupric salt of N,N-bis(2-hydroxyethyl) dithiocarbamic acid which can be measured colorimetrically | Spectrophotometer | n.a. | [91] |
| DTCs as sum of CS2 | Lettuce | The purpose of this study was to compare the performance of GC-ECD, GC-PFPD and GC-MS and UV-VIS spectrophotometric methods | GC-ECD GC-PFPD GC-MS Spectrophotometer | LOD: 0.02–0.28 mg/kg LOQ: 0.05–0.40 mg/kg | [61] |
| DTCs as sum of CS2 | Soybean | DTC are determined as CS2 using acidic hydrolysis and isooctane partitioning, followed by GC-PFPD and GC-ITD-MS analyses | GC-PFPD GC-ITD-MS | LOD: 0.02 mg/kg LOQ: 0.05 mg/kg | [75] |
| DMDC-methyl EBDC-dimethyl | Tap water | The samples were prepared by a modified version of the pre-treatment method for polycarbamate analysis by HPLC | TPI on-column GC/MS | LOQ: 0.3 g/L | [76] |
| Milneb | Foods | DTCs and milneb were extracted from foods with cysteineῌEDTA solution as sodium salts, and methylated with methyl iodide | GC-MS | LOQ: 0.01 mg/kg | [77] |
| Ziram | Spinach | First method: extraction of 1 g of lyophilised sample with a 1:1 (v/v) aqueous EDTA-methanol solution and later partition with hexane as clean up; Second method: involves supercritical carbon dioxide, with the addition of methanol as an organic modifier, to perform the extraction of 0.25 g of lyophilised sample | HPLC-UV | LOQ: 0.05 mg/kg | [80] |
| N-methyl-DTC N,N-dimethyl-DTC Ethylenebis-DTC Propylenebis-DTC | Fruits and vegetables | A new reversed-phase ion-pair chromatographic method was developed, consisting of surface extraction followed by direct injection into a liquid chro- matographic system equipped with UV and electrochemical detectors, connected in series | LC-UV LC-ED | LOD: 4–7 µg/L LOQ: 8–18 µg/L | [82] |
| EBDC, PBDC | Fruits, vegetables and mushrooms | EBDCs and PBDCs were decomposed in an alkaline medium and derivatised with dimethyl sulfate to EBDC-dimethyl and PBDC-dimethyl, respectively | UPLC-MS/MS | LOQ: 0.0004–0.0015 mg/kg | [84] |
| Manzeb, Maneb, Zineb | Environmental water | EBDCs were transformed into water-soluble sodium salts by adding an alkaline EDTA solution. Subsequently extraction and derivation are carried out | LC-MS | LOD: 0.043 µg/L | [85] |
| Disulfiram, Dazomet, Thiram, Metabolites | Fruits and vegetables | First method: MSPD + LC-APCI- MS Second method: SPE + LC-APCI-MS | LC-APCI-MS | LOQ: 0.25–2.5 mg/kg | [92] |
6.4. SPE Extraction
6.5. Alternative Analytical Approaches
7. Analysis of Dithiocarbamate Metabolites
8. Monitoring of Dithiocarbamates
| Human Monitoring | |||||
| Sample Type | Analytes | Analytical Method | Time of Monitoring | Monitoring Results | References |
| 4727 people (ages 1–79) Survey on health effects of human fruit and vegetable consumption containing residues of DTCs/ETU and other pesticides | DTCs/ETU | Risk/benefit assessment through the use of real databases and mathematical models. | n.a. |
| [114] |
| Urine | ETU | APCI-LC-MS | Approximately 2 months |
| [119] |
| Blood, urine and environmental samples | ETU | HPLC-UV for ETU/medical examinations for exposed, no exposed and control workers | Approximately 1 year |
| [121] |
| Maternal urine | Mancozeb/ETU | LC-MS/MS | 15 months |
| [96] |
| Food Monitoring | |||||
| Sample Type | Analytes | Analytical Method | Time of Monitoring | Monitoring Results | References |
| Fruit and vegetable | DTCs and other pesticides | CS2 analysis with spectrophotometry/gas chromatography (GC/FPD) | 10 years (from 2001 to 2010) |
| [112] |
| Food (biological and animal food; baby and young food) | DTCs and other pesticides | Evaluation and statistical analysis of data derived from official controls of the Member States of the European Union, of Norway and Iceland | 1 year (2018) |
| [118] |
| Fruit (tangerines, oranges, peaches, nectarines, khakis) | DTCs | CS2 analysis with GC-MS | 20 months |
| [113] |
| Young seedlings and leaves of corn, lettuce, pepper and tomato | [4,5-14C] ETU | Liquid scintillation counting | more than 20 days |
| [128]; [129] |
| Animals Monitoring | |||||
| Sample Type | Analytes | Analytical Method | Time of Monitoring | Monitoring Results | References |
| Daphnia magna | Thiram | Acute and chronic toxicity tests | 21 days | Increase in the immobilisation rate;
| [122] |
| Zebrafish (Danio rerio) | Propineb | Acute toxicity tests [126] | 72 h |
| [127] |
| Male rats | Mancozeb | Histological studies and Hormone assay and liver function test | 4 weeks |
| [123] |
| Wistar Rats | Mancozeb | Comet assay in total blood and the micronucleus test in bone marrow | 18 days |
| [130] |
9. Conclusions
- ❖
- an environmentally friendly pest control based on pest prevention and prioritises alternative pest control methods, considering the chemical pesticides option as a last resort;
- ❖
- a ban for all plant protection products in sensitive areas such as urban green areas (e.g., public parks, gardens, playgrounds, recreation or sports grounds, public paths), protected areas that fall within the network of Natura 2000 sites and any ecologically sensitive area for pollinators;
- ❖
- support farmers and professional pesticide workers to choose alternative and sustainable pest-control methods.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubino, F.M.; Mrema, E.J.; Colosio, C. Pesticide Residues: Dithiocarbamates. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 5–10. [Google Scholar]
- Marina Fonseca Almeida, E.; De Souza, D. Current electroanalytical approaches in the carbamates and dithiocarbamates determination. Food Chem. 2023, 417, 135900. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.K.; Faubel, W. Methods of analysis of dithiocarbamate pesticides: A review. Pestic. Sci. 1999, 55, 965–970. [Google Scholar] [CrossRef]
- Janz, D.M. Dithiocarbamates. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 212–214. [Google Scholar] [CrossRef]
- Chung, S.W.C.; Wong, W.W.K. Chromatographic analysis of dithiocarbamate residues and their metabolites in foods employed in dietary exposure studies—A review. Food Addit. Contam.-Part A 2022, 39, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Szolar, O.H.J. Environmental and pharmaceutical analysis of dithiocarbamates. Anal. Chim. Acta 2007, 582, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Barcelò, D.; Marie-Claire, H. Trace Determination of Pesticides and their Degradation Products in Water; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Caldas, E.D.; Tressou, J.; Boon, P.E. Dietary exposure of Brazilian consumers to dithiocarbamate pesticides—A probabilistic approach. Food Chem. Toxicol. 2006, 44, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Chia-Fu, Y.; Sun-Dsong, C.; Wei-Shi, C.; Jia-Der, F.; Chuen-Ying, L. Application of dithiocarbamate resin-metal complexes as stationary phases in gas chromatography. J. Chromatogr. A 1993, 630, 275–285. [Google Scholar] [CrossRef]
- Aghbash, K.O.; Alamgholiloo, H.; Pesyan, N.N.; Khaksar, S.; Rostamnia, S. Gold nanoparticle stabilized dithiocarbamate functionalized magnetite carbon as promise clean nanocatalyst for A3-coupling organic transformation. Mol. Catal. 2021, 499, 111252. [Google Scholar] [CrossRef]
- Pitchaimani, P.; Lo, K.M.; Elango, K.P. Synthesis, crystal structures, luminescence properties and catalytic application of lanthanide(III) piperidine dithiocarbamate complexes. Polyhedron 2015, 93, 8–16. [Google Scholar] [CrossRef]
- Schwack, W.; Nyanzi, S. Analysis of dithiocarbamate fungicides. Second-derivative UV-spectroscopic determination of CS2, COS, and thiram (TMTD). Z. Lebensm. Unters. Forsch. 1994, 198, 3–7. [Google Scholar] [CrossRef]
- Vale, J.A.; Faustino, W.M.; Menezes, P.H.; De Sá, G.F. Eu(III) dithiocarbamate complex and N-p-tolylsulfonylphenylalanine as a novel chiral catalyst for the asymmetric synthesis of cyanohydrins. Chem. Commun. 2006, 37, 3340–3342. [Google Scholar] [CrossRef]
- Available online: https://www.eurl-pesticides-datapool.eu/ (accessed on 7 August 2023).
- Kaul, L.; Süss, R.; Zannettino, A.; Richter, K. The revival of dithiocarbamates: From pesticides to innovative medical treatments. iScience 2021, 24, 102092. [Google Scholar] [CrossRef] [PubMed]
- Milacic, V.; Chen, D.; Giovagnini, L.; Diez, A.; Fregona, D.; Dou, Q.P. No TitlePyrrolidine dithiocarbamate-zinc(II) and -copper(II) complexes induce apoptosis in tumor cells by inhibiting the proteasomal activity. Toxicol. Appl. Pharmacol. 2008, 231, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Marzano, C.; Zanello, P.; Corsini, M.; Miolo, G.; Macca, C.; Trevisan, A. Gold (III) Dithiocarbamate Derivatives for the Treatment of Cancer: Solution Chemistry, DNA Binding, and Hemolytic Properties. J. Med. Chem. 2006, 49, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Dufour, P.; Lang, J.M.; Giron, C.; Duclos, B.; Haehnel, P.; Jaeck, D.; Jung, J.M.; Oberling, F. Sodium ditiocarb as adjuvant immunotherapy for high risk breast cancer: A randomized study. Biotherapy 1993, 6, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.; Markman, M.; Hakes, T.; Reichman, B.; Rubin, S.; Jones, W.; Lewis, J.L.; Curtin, J.; Barakat, R.; Phillips, M.; et al. Diethyldithiocarbamate chemoprotection of carboplatin-induced hematological toxicity. J. Cancer Res. Clin. Oncol. 1993, 119, 360–362. [Google Scholar] [CrossRef] [PubMed]
- James, P.W.B. Section of Psychiatry. Medicine 1946, XLIII, 37–50. [Google Scholar]
- WHO. Environmental Health Criteria 78 Dithiocarbamate Pesticides, Ethylenethiourea and Propylenethiourea: A General Introduction. International Programme On Chemical Safety; WHO: Geneva, Switzerland, 1988. [Google Scholar]
- Wright, P.; Kraus, J.E. Schizophrenia and related disorders. In Core Psychiatry; Wright, P., Stern, J., Phelan, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 259–286. [Google Scholar]
- Kerfoot, K.E.; Petrakis, I.L. Disulfiram for alcohol and other drug use. In Interventions for Addiction; Academic Press: Cambridge, MA, USA, 2013; pp. 367–374. [Google Scholar] [CrossRef]
- Fuller, R.K.; Gordis, E. Does disulfiram have a role in alcoholism treatment today? Addiction 2004, 99, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Carta, F.; Vullo, D.; Supuran, C.T. Dithiocarbamates strongly inhibit the β-class carbonic anhydrases from Mycobacterium tuberculosis. J. Enzyme Inhib. Med. Chem. 2014, 28, 407–411. [Google Scholar] [CrossRef]
- Aspatwar, A.; Hammaren, M.; Parikka, M.; Parkkila, S.; Carta, F.; Bozdag, M.; Vullo, D.; Supuran, C.T. In vitro inhibition of Mycobacterium tuberculosis β-carbonic anhydrase 3 with Mono- and dithiocarbamates and evaluation of their toxicity using zebrafish developing embryos. J. Enzyme Inhib. Med. Chem. 2020, 35, 65–71. [Google Scholar] [CrossRef]
- Fu, J.; Bao, F.; Gu, M.; Liu, J.; Zhang, Z.; Ding, J.; Xie, S.S.; Ding, J. Design, synthesis and evaluation of quinolinone derivatives containing dithiocarbamate moiety as multifunctional AChE inhibitors for the treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2020, 35, 118–128. [Google Scholar] [CrossRef]
- Jiang, N.; Huang, Q.; Liu, J.; Liang, N.; Li, Q.; Li, Q.; Xie, S.S. Design, synthesis and biological evaluation of new coumarin-dithiocarbamate hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 146, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Nutho, B.; Wilasluck, P.; Deetanya, P.; Wangkanont, K.; Arsakhant, P.; Saeeng, R.; Rungrotmongkol, T. Discovery of C-12 dithiocarbamate andrographolide analogues as inhibitors of SARS-CoV-2 main protease: In vitro and in silico studies. Comput. Struct. Biotechnol. J. 2022, 20, 2784–2797. [Google Scholar] [CrossRef] [PubMed]
- Brier, L.; Hassan, H.; Hanoulle, X.; Landry, V.; Moschidi, D.; Desmarets, L.; Rouillé, Y.; Dumont, J.; Herledan, A.; Warenghem, S.; et al. Novel dithiocarbamates selectively inhibit 3CL protease of SARS-CoV-2 and other coronaviruses. Eur. J. Med. Chem. 2023, 250, 115186. [Google Scholar] [CrossRef] [PubMed]
- Nogara, P.A.; Bright, F.; Gustavo, O.; Bolzan, R.; Pereira, C.; Orian, L.; Batista, J.; Rocha, T. Reactivity and binding mode of disulfiram, its metabolites, and derivatives in SARS-CoV-2—PL pro: Insights from computational chemistry studies. J. Mol. Model. 2022, 28, 354. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Ajiboye, T.T.; Marzouki, R.; Onwudiwe, D.C. The Versatility in the Applications of Dithiocarbamates. Int. J. Mol. Sci. 2022, 23, 1317. [Google Scholar] [CrossRef] [PubMed]
- Monser, L.; Adhoum, N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep. Purif. Technol. 2002, 26, 137–146. [Google Scholar] [CrossRef]
- Kanchi, S.; Singh, P.; Bisetty, K. Dithiocarbamates as hazardous remediation agent: A critical review on progress in environmental chemistry for inorganic species studies of 20th century. Arab. J. Chem. 2014, 7, 11–25. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Fogel, R.; Limson, J.; Purcarea, C.; Vasilescu, A. Advances in the detection of dithiocarbamate fungicides: Opportunities for biosensors. Biosensors 2021, 11, 12. [Google Scholar] [CrossRef]
- Thiocarbamates and Dithiocarbamates. OEC—The Observatory of Economic Complexity. Available online: https://oec.world/en/profile/hs/thiocarbamates-and-dithiocarbamates (accessed on 13 February 2023).
- Marchand, P.A. EU Chemical Plant Protection Products in 2023: Current State and Perspectives. Agrochemicals 2023, 2, 106–117. [Google Scholar] [CrossRef]
- Kaufman, D.D. Degradation of Carbamate Herbicides in Soil Certain A-phenyl-, thio-, dithio-, and methyl-carbamates are finding wide application. J. Agric. Food Chem. 1967, 15, 582–591. [Google Scholar] [CrossRef]
- Malhotra, H.; Kaur, S.; Phale, P.S. Conserved Metabolic and Evolutionary Themes in Microbial Degradation of Carbamate Pesticides. Front. Microbiol. 2021, 12, 1863. [Google Scholar] [CrossRef] [PubMed]
- Weissmahr, K.W.; Sedlak, D.L. Effect of metal complexation on the degradation of dithiocarbamate fungicides. Environ. Toxicol. Chem. 2000, 19, 820–826. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC Trends Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Costa, L.G.; Aschner, M. Toxicology of pesticides. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Bansal, O.P. Health Impacts of the Carbamate and Dithiocarbamate Pesticides: A Review. Artic. Int. J. Sci. Res. Publ. 2022, 12, 366. [Google Scholar] [CrossRef]
- Devipriya, S.; Yesodharan, S. Photocatalytic degradation of pesticide contaminants in water. Sol. Energy Mater. Sol. Cells 2005, 86, 309–348. [Google Scholar] [CrossRef]
- Gupta, B.; Rani, M.; Kumar, R.; Dureja, P. Identification of degradation products of thiram in water, soil and plants using LC-MS technique. J. Environ. Sci. Health B 2012, 47, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Veiga-del-Baño, J.M.; Martínez-López, S.; Pérez-Lucas, G.; Cuenca-Martínez, J.J.; Andreo-Martínez, P. Trends in dithiocarbamates food research: A bibliometric vision. Chemosphere 2023, 313, 137342. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 7 September 2023).
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009; WHO: Geneva, Switzerland, 2010; ISBN 9789241547963. [Google Scholar]
- US EPA Environmental Protection Agency. The Grouping of a Series of Dithiocarbamate Pesticides Based on a Common Mechanism of Toxicity; United States Environmental Protection Agency: Washington, DC, USA, 2001. [Google Scholar]
- Silberman, J.; Taylor, A. Carbamate Toxicity; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- International Agency for Research on Cancer. Some Thyrotropic Agents; International Agency for Research on Cancer: Geneva, Switzerland, 2001; Volume 79. [Google Scholar]
- National Toxicology Program. 15th Report on Carcinogens; U.S. Department of Health and Human Services: Washington, DC, USA, 2021. [Google Scholar]
- Lindh, C.H.; Littorin, M.; Johannesson, G.; Jönsson, B.A. Analysis of ethylenethiourea as a biomarker in human urine using liquid chromatography/triple quadrupole mass spectrometry. Rapid Commun. Mass. Spectrom. 2008, 22, 1457–1466. [Google Scholar] [CrossRef]
- Kenny, L.; Jones, K.; Cocker, J.; Bader, M.; Brodbeck, T.; Göen, T.; Hartwig, A.; MAK Commission. Ethylenebis(dithiocarbamates) and ethylenethiourea—Determination of ethylenethiourea in urine by LC-MS/MS: Biomonitoring Method—Translation of the German version from 2021. MAK Collect. Occup. Health Saf. 2021, 6, Doc047. [Google Scholar] [CrossRef]
- National Toxicology Program. National Toxicology Program (2011) Report on Carcinogens; NTP: Research Triangle Park, NC, USA, 2011. [Google Scholar]
- Abdourahime, H.; Anastassiadou, M.; Brancato, A.; Brocca, D.; Cabrera, L.; Delentdecker, C.; Ferreira, L.; Greco, L.; Jarrah, S.; Kardassi, D.; et al. Review of the existing maximum residue levels for dazomet according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2019, 17, e05562. [Google Scholar] [CrossRef]
- Stadler, K.; Li, X.; Liu, B.; Bao, W.; Wang, K.; Lehmler, H.-J. Systematic review of human biomonitoring studies of ethylenethiourea, a urinary biomarker for exposure to dithiocarbamate fungicides. Environ. Pollut. 2022, 292, 118419. [Google Scholar] [CrossRef] [PubMed]
- Crnogorac, G.; Schwack, W. Residue analysis of dithiocarbamate fungicides. TrAC-Trends Anal. Chem. 2009, 28, 40–50. [Google Scholar] [CrossRef]
- Keppel, G.E. Collaborative study of the determination of the dithiocarbamate residues by a modified carbon disulfide evolution method. J. Assoc. Off. Anal. Chem. 1971, 54, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Caldas, E.D.; Miranda, M.C.C.; Conceição, M.H.; De Souza, L.C.K.R. Dithiocarbamates residues in Brazilian food and the potential risk for consumers. Food Chem. Toxicol. 2004, 42, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Pizzutti, I.R.; De Kok, A.; Da Silva, R.C.; Rohers, G.N. Comparison between three chromatographic (GC-ECD, GC-PFPD and GC-ITD-MS) methods and a UV-Vis spectrophotometric method for the determination of dithiocarbamates in lettuce. J. Braz. Chem. Soc. 2017, 28, 775–781. [Google Scholar] [CrossRef]
- Vareli, C.S.; Pizzutti, I.R.; Gebler, L.; Cardoso, C.D.; Gai, D.S.H.; Fontana, M.E.Z. Analytical method validation to evaluate dithiocarbamates degradation in biobeds in South of Brazil. Talanta 2018, 184, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Aulakh, J.S.; Malik, A.K. Thiram: Degradation, applications and analytical methods. J. Environ. Monit. 2003, 5, 717–723. [Google Scholar] [CrossRef]
- Commissione Europea Regolamento (UE) 2016/1 della Commissione del 3 Dicembre 2015 che Modifica gli Allegati II e III del Regolamento (CE) n. 396/2005 del Parlamento Europeo e del Consiglio per Quanto Riguarda i Livelli Massimi di Residui di Bifenazato, Boscalid, Ciazofamid; European Commission: Brussels, Belgium, 2016; pp. 1–62. [Google Scholar]
- Öter, Ç.; Zorer, Ö.S. Molecularly imprinted polymer synthesis and selective solid phase extraction applications for the detection of ziram, a dithiocarbamate fungicide. Chem. Eng. J. Adv. 2021, 7, 100118. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, C.; Zhang, Z. Graphene oxide embedded sandwich nanostructures for enhanced Raman readout and their applications in pesticide monitoring. Nanoscale 2013, 5, 3773–3779. [Google Scholar] [CrossRef]
- EPA. Environmental Protection Agency Methods for the Determination of Nonconventional Pesticides in Municipal and Industrial Wastewater; United States Environmental Protection Agency: Washington, DC, USA, 1993. [Google Scholar]
- EPA. Environmental Protection Agency Method 630.1: The Determination of Dithiocarbamates Pesticides in Municipal and Industrial Wastewater; United States Environmental Protection Agency: Washington, DC, USA, 1993. [Google Scholar]
- Schwack, W.; Nyanzi, S. Analysis of dithiocarbamate fungicides. Reaction products of the thiuram disulphide fungicide thiram (TMTD) during acid hydrolysis. Z. Lebensm. Unters. Forsch. 1994, 198, 8–10. [Google Scholar] [CrossRef]
- Losacco, D.; Campanale, C.; Tumolo, M.; Ancona, V.; Massarelli, C.; Uricchio, V.F. Evaluating the Influence of Nitrogen Fertilizers and Biochar on Brassica oleracea L. var. botrytis by the Use of Fourier Transform Infrared (FTIR) Spectroscopy. Sustainability 2022, 14, 11985. [Google Scholar] [CrossRef]
- Perz, R.C.; Van Lishaut, H.; Schwack, W. CS2 blinds in Brassica crops: False positive results in the dithiocarbamate residue analysis by the acid digestion method. J. Agric. Food Chem. 2000, 48, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Mert, I.D.; Yiğitkaya, S.; Dagaşan, O.; Sakallı, F.N.; Oztürk, S. The false positive effect of residue of sulphur sources on dithiocarbamate analysis based on CS 2 measurement. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Caldas, E.D.; Conceição, M.H.; Miranda, M.C.C.; De Souza, L.C.K.R.; Lima, J.F. Determination of dithiocarbamate fungicide residues in food by a spectrophotometric method using a vertical disulfide reaction system. J. Agric. Food Chem. 2001, 49, 4521–4525. [Google Scholar] [CrossRef] [PubMed]
- Schwack, W.; Waldner, A.; Nyanzi, S.A. A modified vertical distillation system for the microdetermination of dithiocarbamate fungicides as methyl xanthate in fruits and vegetables. Dtsch. Leb. 2008, 104, 60–65. [Google Scholar]
- Caiel Da Silva, R.; Wickert, C.; Pizzutti, I.R.; De Kok, A. Clean-up Strategy for Dithiocarbamate Fungicide Determination in Soybean by GC-ITD-MS and GC-PFPD: Method Development and Validation. J. Agric. Food Chem. 2021, 69, 11485–11493. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Yano, M.; Makihata, N. Development of a high-sensitivity quantitative analytical method for determining polycarbamate by gas chromatography-mass spectrometry incorporating temperature-programmable inlet on-column injection. J. Chromatogr. A 2005, 1074, 155–161. [Google Scholar] [CrossRef]
- Nakamura, M.; Noda, S.; Kosugi, M.; Ishiduka, N.; Mizukoshi, K.; Taniguchi, M.; Nemoto, S. Determination of dithiocarbamates and milneb residues in foods by gas chromatography-mass spectrometry. J. Food Hyg. Soc. Jpn. 2010, 51, 213–219. [Google Scholar] [CrossRef]
- Jafari, A.; Shoeibi, S.; Amini, M.; Amirahmadi, M.; Rastegar, H.; Ghaffarian, A.; Ghazi-Khansari, M. Monitoring dithiocarbamate fungicide residues in greenhouse and non-greenhouse tomatoes in Iran by HPLC-UV. Food Addit. Contam. Part B Surveill. 2012, 5, 87–92. [Google Scholar] [CrossRef]
- Irth, H.; de Jong, G.J.; Frei, R.W.; Brinkman, U.A.T. Determination of dithiocarbamates in residues by liquid chromatography with selective precolumn or reaction-detection systems. Int. J. Environ. Anal. Chem. 1990, 39, 129–139. [Google Scholar] [CrossRef]
- Atienza, J.; Jimenez, J.J.; Alvarez, J.; Martin, M.T.; Toribio, L. Extraction With Edta/Methanol And Supercritical Carbon Dioxide For The Analysis Of Ziram Residues On Spinach. Toxicol. Environ. Chem. 1994, 45, 179–187. [Google Scholar] [CrossRef]
- Van Lishaut, H.; Schwack, W. Selective trace determination of dithiocarbamate fungicides in fruits and vegetables by reversed-phase ion-pair liquid chromatography with ultraviolet and electrochemical detection. J. AOAC Int. 2000, 83, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Perz, R.; Schwach, W. High performance ion pair chromatography as a routine-compliant tool for surveilling residues of dithiocarbamate fungicides in fruits and vegetables. Dtsch. Leb. 2003, 99, 137–142. [Google Scholar]
- Raina-Fulton, R. A review of methods for the analysis of orphan and difficult pesticides: Glyphosate, glufosinate, quaternary ammonium and phenoxy acid herbicides, and dithiocarbamate and phthalimide fungicides. J. AOAC Int. 2014, 97, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, C.; Yang, Q.; An, W.; Zheng, Z.T.; Jiao, B. Simultaneous Determination of Ethylenebisdithiocarbamate (EBDC) and Propylenebisdithiocarbamate (PBDC) Fungicides in Vegetables, Fruits, and Mushrooms by Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. Food Anal. Methods 2019, 12, 2045–2055. [Google Scholar] [CrossRef]
- Hanada, Y.; Tanizaki, T.; Koga, M.; Shiraishi, H.; Soma, M. LC/MS Studies on Characterization and Determination of N,N′-Ethylenebisdithiocarbamate Fungicides in Environmental Water Samples. Anal. Sci. 2002, 18, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Christensen, H.B.; Petersen, A.; Sloth, J.J.; Poulsen, M.E. Method validation and analysis of nine dithiocarbamates in fruits and vegetables by LC-MS/MS. Food Addit. Contam. Part A 2013, 30, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Patel, H.K.; Kalasariya, R.L.; Shah, P.G. Validation and analysis of thiram, a dithiocarbamate, as CS2 from soybean (Glycine max) samples on GC–MS. Int. J. Environ. Sci. Technol. 2019, 16, 6991–6998. [Google Scholar] [CrossRef]
- Riadi, Y.; El Haddad, M.; Mamouni, R.; Ramli, Y.; Akssira, M.; Fechtali, T.; El Antri, S.; Lazar, S. Determination of kinetics of degradation and mobility of dithiocarbamates fungicides in water and in Moroccan soil. Stud. Cercet. Ştiinţ. 2010, 11, 289–297. [Google Scholar]
- Sayed, R.; Hussein, O.E.; Omran, A.A. Method Optimization and Validation for the Determination of Mancozeb in Chamomile by Modified Quechers and Liquid Chromatography-Tandem Mass Spectrometry. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Kakitani, A.; Yoshioka, T.; Nagatomi, Y.; Harayama, K. A rapid and sensitive analysis of dithiocarbamate fungicides using modified QuEChERS method and liquid chromatography-tandem mass spectrometry. J. Pestic. Sci. 2017, 42, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.R. Determination of Phygon Residues on Food Crops. J. Agric. Food Chem. 1958, 6, 746–747. [Google Scholar] [CrossRef]
- Blasco, C.; Font, G.; Picó, Y. Determination of dithiocarbamates and metabolites in plants by liquid chromatography-mass spectrometry. J. Chromatogr. A 2004, 1028, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hayama, T.; Yada, K.; Onimaru, S.; Yoshida, H.; Todoroki, K.; Nohta, H.; Yamaguchi, M. Simplified method for determination of polycarbamate fungicide in water samples by liquid chromatography with tandem mass spectrometry following derivatization with dimethyl sulfate. J. Chromatogr. A 2007, 1141, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M.; Martin, R.L. Electrochemistry and redox behaviour of transition metal dithiocarbamates. Coord. Chem. Rev. 1984, 54, 23–98. [Google Scholar] [CrossRef]
- Giannakopoulos, E.; Deligiannakis, Y. Thermodynamics of adsorption of dithiocarbamates at the hanging mercury drop. Langmuir 2007, 23, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.M.; Hoppin, J.A.; Córdoba, L.; Cano, J.C.; Soto-Martínez, M.; Eskenazi, B.; Lindh, C.H.; Joode, B. van W. de J. Prenatal pesticide exposure and respiratory health outcomes in the first year of life: Results from the infants’ Environmental Health (ISA) study. Int. J. Hyg. Environ. Health 2020, 225, 113474. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.M.B.F.; Fátima Barroso, M.; Morais, S.; Araújo, M.; Freire, C.; de Lima-Neto, P.; Correia, A.N.; Oliveira, M.B.P.P.; Delerue-Matos, C. Laccase-Prussian blue film-graphene doped carbon paste modified electrode for carbamate pesticides quantification. Biosens. Bioelectron. 2013, 47, 292–299. [Google Scholar] [CrossRef]
- Noguer, T.; Marty, J.L. High sensitive bienzymic sensor for the detection of dithiocarbamate fungicides. Anal. Chim. Acta 1997, 347, 63–70. [Google Scholar] [CrossRef]
- Lima, R.S.; Nunes, G.S.; Noguer, T.; Marty, J.L. Biossensor enzimático para detecção de fungicidas ditiocarbamatos. Estudo cinético da enzima aldeído desidrogenase e otimização do biossensor. Quim. Nova 2007, 30, 9–17. [Google Scholar] [CrossRef]
- Noguer, T.; Balasoiu, A.M.; Avramescu, A.; Marty, J.L. Development of a disposable biosensor for the detection of metam-sodium and its metabolite MITC. Anal. Lett. 2001, 34, 513–528. [Google Scholar] [CrossRef]
- Flampouri, K.; Mavrikou, S.; Kintzios, S.; Miliadis, G.; Aplada-Sarlis, P. Development and validation of a cellular biosensor detecting pesticide residues in tomatoes. Talanta 2010, 80, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.M.B.F.; Barroso, M.F.; Morais, S.; Araújo, M.; Freire, C.; de Lima-Neto, P.; Correia, A.N.; Oliveira, M.B.P.P.; Delerue-Matos, C. Sensitive bi-enzymatic biosensor based on polyphenoloxidases-gold nanoparticles-chitosan hybrid film-graphene doped carbon paste electrode for carbamates detection. Bioelectrochemistry 2014, 98, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, A.; Hanot, V.; Van Loco, J. A rapid and environmental friendly determination of the dithiocarbamate metabolites ethylenethiourea and propylenethiourea in fruit and vegetables by ultra high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 4627–4631. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wei, J.; Chen, Z.; Lei, Y.; Zhang, Y.; Deng, C.; Tan, H.; Li, X. Determination of propineb and its metabolites propylenethiourea and propylenediamine in banana and soil using gas chromatography with flame photometric detection and LC–MS/MS analysis. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2018, 53, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, J.S.; Del Rosario, A.R.; Tamplin, B.R. Simultaneous determination and confirmation of sodium n-methyldithiocarbamate (metham sodiumR) and methyl isothiocyanate in water by high performance liquid chromatography with diode array detection. Int. J. Environ. Anal. Chem. 1993, 53, 165–171. [Google Scholar] [CrossRef]
- Nantaphol, S.; Moonla, C.; Promvichai, S.; Tangkuaram, T.; Chailapakul, O.; Siangproh, W. A new alternative assay for sensitive analysis of ethylenethiourea and propylenethiourea in fruit samples after their separation. Anal. Methods 2020, 12, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Wachtler, A.-K.; Kolberg, D.I.; Eichhorn, E.; Marks, H.; Benkenstein, A.; Zechmann, S.; Mack, D.; Wildgrube, C.; Barth, A.; et al. Quick Method for the Analysis of Highly Polar Pesticides in Food Involving Extraction with Acidified Methanol and LC- or IC-MS/MS Measurement—I. Food of Plant Origin (QuPPe-PO-Method)—Version 12; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Elmi, M.; Eskandari, S.A.M. Simultaneous Analysis of Metabolite Residues from Degradation of Dithiocarbamates in Cereals Using Liquid Chromatography Coupled with Tandem Mass Spectrometry. Iran. J. Nutr. Sci. Food Technol. 2023, 18, 81–91. [Google Scholar]
- Vaclavik, L.; Shippar, J.J.; Koesukwiwat, U.; Mastovska, K. Method development and validation for low-level propineb and propylenethiourea analysis in baby food, infant formula and related matrices using liquid chromatography-tandem mass spectrometry. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 2387–2399. [Google Scholar] [CrossRef]
- Zhang, Y.; Wade, K.L.; Prestera, T.; Talalay, P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disufide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal. Biochem. 1996, 239, 160–167. [Google Scholar] [CrossRef]
- Pilipczuk, T.; Kusznierewicz, B.; Chmiel, T.; Przychodzeń, W.; Bartoszek, A. Simultaneous determination of individual isothiocyanates in plant samples by HPLC-DAD-MS following SPE and derivatization with N-acetyl-L-cysteine. Food Chem. 2017, 214, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Jardim, A.N.O.; Caldas, E.D. Brazilian monitoring programs for pesticide residues in food—Results from 2001 to 2010. Food Control 2012, 25, 607–616. [Google Scholar] [CrossRef]
- Berrada, H.; Fernández, M.; Ruiz, M.J.; Moltó, J.C.; Mañes, J.; Font, G. Surveillance of pesticide residues in fruits from Valencia during twenty months (2004/05). Food Control 2010, 21, 36–44. [Google Scholar] [CrossRef]
- Valcke, M.; Bourgault, M.H.; Rochette, L.; Normandin, L.; Samuel, O.; Belleville, D.; Blanchet, C.; Phaneuf, D. Human health risk assessment on the consumption of fruits and vegetables containing residual pesticides: A cancer and non-cancer risk/benefit perspective. Environ. Int. 2017, 108, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, P. The 2021 European Union Report on Pesticide Residues in Food; European Food Safety Authority (EFSA): Parma, Italy, 2023; Volume 21. [Google Scholar]
- Carrasco Cabrera, L.; Medina Pastor, P. The 2019 European Union Report on Pesticide Residues in Food; European Food Safety Authority (EFSA): Parma, Italy, 2021; Volume 19. [Google Scholar]
- Carrasco Cabrera, L.; Medina Pastor, P. The 2020 European Union Report on Pesticide Residues in Food; European Food Safety Authority (EFSA): Parma, Italy, 2022; Volume 20. [Google Scholar]
- Medina-Pastor, P.; Triacchini, G. The 2018 european union report on pesticide residues in food. EFSA J. 2020, 18, 1–103. [Google Scholar] [CrossRef]
- Jones, K.; Patel, K.; Cocker, J.; Bevan, R.; Levy, L. Determination of ethylenethiourea in urine by liquid chromatography-atmospheric pressure chemical ionisation-mass spectrometry for monitoring background levels in the general population. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2563–2566. [Google Scholar] [CrossRef] [PubMed]
- EPA, Environmental Protection Agency. Mancozeb Facts; United States Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Panganiban, L.R.; Cortes-Maramba, N.; Dioquino, C.; Suplido, M.L.; Ho, H.; Francisco-Rivera, A.; Manglicmot-Yabes, A. Correlation between blood enthylenethiourea and thyroid gland disorders among banana plantation workers in the Philippines. Environ. Health Perspect. 2004, 112, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Belaid, C.; Sbartai, I.; Djebar, M.-R. Populational effect of a dithiocarbamate (thiram) fungicide on a freshwater cladocerus Daphnia magna. Stud. Univ. Vasile Goldis Arad Ser. Stiint. Viet. 2020, 29, 121–128. [Google Scholar]
- Kwon, D.; Chung, H.K.; Shin, W.S.; Park, Y.S.; Kwon, S.C.; Song, J.S.; Park, B.G. Toxicological evaluation of dithiocarbamate fungicide mancozeb on the endocrine functions in male rats. Mol. Cell. Toxicol. 2018, 14, 105–112. [Google Scholar] [CrossRef]
- Santoriello, C.; Zon, L.I. Hooked! modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef]
- Truong, L.; Reif, D.M.; Mary, L.S.; Geier, M.C.; Truong, H.D.; Tanguay, R.L. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 2014, 137, 212–233. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2013; pp. 1–22. [Google Scholar]
- Park, H.; You, H.H.; Song, G. Multiple toxicity of propineb in developing zebrafish embryos: Neurotoxicity, vascular toxicity, and notochord defects in normal vertebrate development. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2021, 243, 108993. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, R.E.; Frear, D.S. Behavior and fate of ethylenethiourea in plants. J. Agric. Food Chem. 1976, 24, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Downing, E. Environmental Fate of Maneb; Environmental Monitoring and Pest Management, Department of Pesticide Regulation: Sacramento, CA, USA, 2000; Volume 2. [Google Scholar]
- Goldoni, A.; Klauck, C.R.; Da Silva, S.T.; Da Silva, M.D.; Ardenghi, P.G.; Da Silva, L.B. DNA damage in Wistar rats exposed to dithiocarbamate pesticide mancozeb. Folia Biol. 2014, 60, 202–204. [Google Scholar]
- Official Journal of the European Union Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC; European Union: Brussels, Belgium, 2009.
- Official Journal of the European Union Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 Implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as Regards the List of Approved Active Substances; European Union: Brussels, Belgium, 2011.
- Pesticide Action Network Europe. FACTSHEET Mancozeb. 1. 2018, 1–4. Available online: https://pan-international.org/europe/ (accessed on 7 September 2023).
- Pe, G.; Hering, D.; Kachler, J.; Bruelheide, H.; Wittmer, H.; Bonn, A. Scientists Support the EU’s Green Deal and Reject the Unjustified Argumentation against the Sustainable Use Regulation and the Nature Restoration Law; University of Helsinki: Helsinki, Finland, 2021; pp. 1–5. [Google Scholar]
- Bongaarts, J. IPBES, 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Popul. Dev. Rev. 2019, 45, 680–681. [Google Scholar] [CrossRef]
- FAO. Protecting Pollinators from Pesticides—Urgent Need for Action; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- FAO. Scientific Review of the Impact of Climate Change on Plant Pests; FAO: Rome, Italy, 2021; ISBN 9789251344354. [Google Scholar]
- Petit, S.; Landis, D.A. Landscape-scale management for biodiversity and ecosystem services. Agric. Ecosyst. Environ. 2023, 347, 108370. [Google Scholar] [CrossRef]
- Binetti, M.S.; Campanale, C.; Uricchio, V.F.; Massarelli, C. In-Depth Monitoring of Anthropic Activities in the Puglia Region: What Is the Acceptable Compromise between Economic Activities and Environmental Protection ? Sustainability 2023, 15, 8875. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanale, C.; Triozzi, M.; Ragonese, A.; Losacco, D.; Massarelli, C. Dithiocarbamates: Properties, Methodological Approaches and Challenges to Their Control. Toxics 2023, 11, 851. https://doi.org/10.3390/toxics11100851
Campanale C, Triozzi M, Ragonese A, Losacco D, Massarelli C. Dithiocarbamates: Properties, Methodological Approaches and Challenges to Their Control. Toxics. 2023; 11(10):851. https://doi.org/10.3390/toxics11100851
Chicago/Turabian StyleCampanale, Claudia, Mariangela Triozzi, Annamaria Ragonese, Daniela Losacco, and Carmine Massarelli. 2023. "Dithiocarbamates: Properties, Methodological Approaches and Challenges to Their Control" Toxics 11, no. 10: 851. https://doi.org/10.3390/toxics11100851









