Evaluation of Gold Complexes to Address Bacterial Resistance, Quorum Sensing, Biofilm Formation, and Their Antiviral Properties against Bacteriophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gold Compounds
2.2. Bacteria and Culture Conditions
2.3. Bacteriophages
2.4. Antibacterial Activity
Determination of the Minimum Inhibitory Concentration (MIC)
2.5. Anti-Quorum Sensing Activity
2.6. Antibiofilm Activity
2.7. Antiviral Activity
2.8. Statistical Analysis
3. Results
3.1. Antibacterial Activity
3.2. Anti-Quorum Sensing Activity
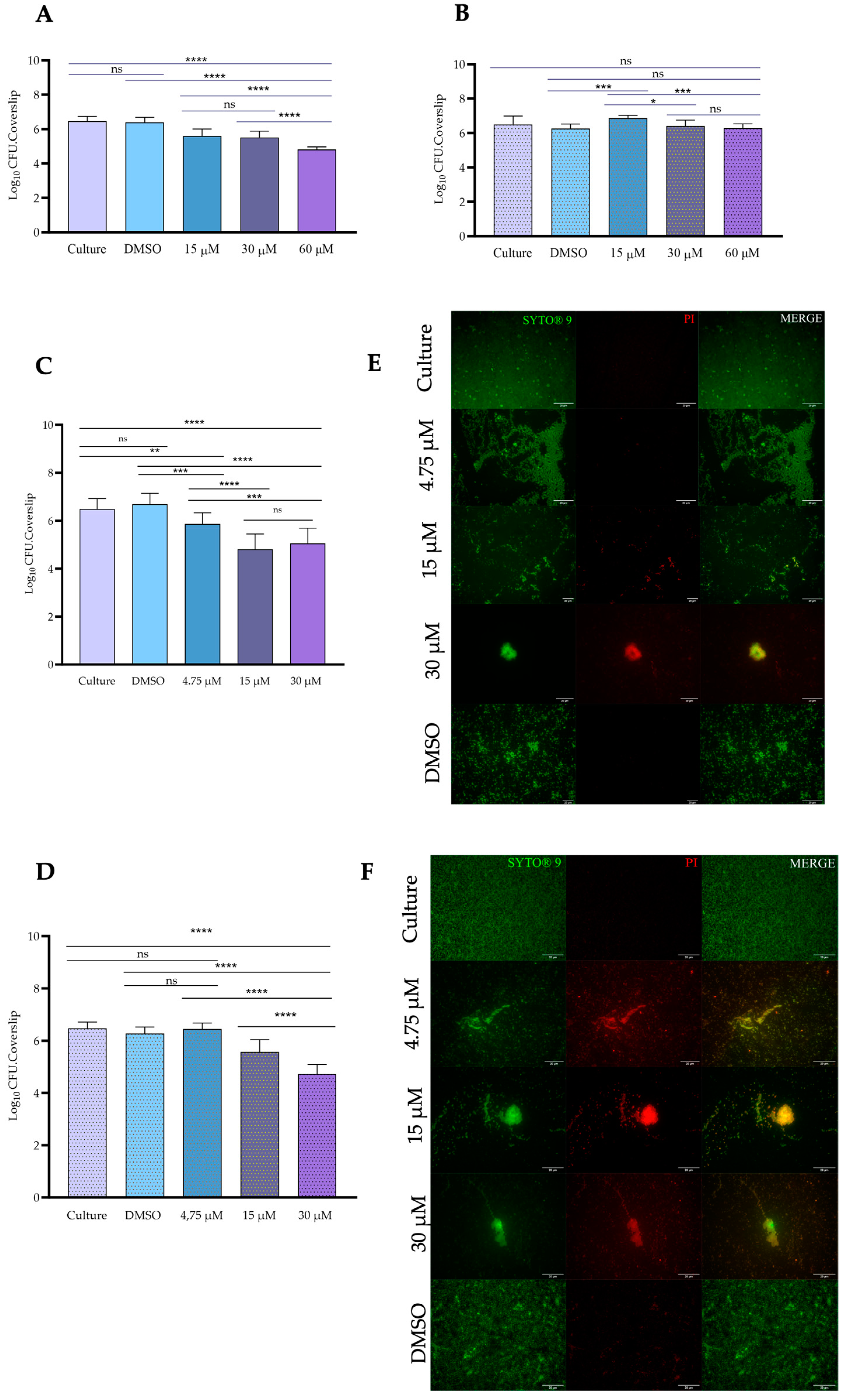
3.3. Antibiofilm Activity
3.4. Antiviral Activity
4. Discussion
4.1. Antibacterial Activity
4.2. Anti-Quorum Sensing and Antibiofilm Activity
4.3. Antiviral Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Apolónio, J.; Faleiro, M.L.; Miguel, M.G.; Neto, L. No induction of antimicrobial resistance in Staphylococcus aureus and Listeria monocytogenes during continuous exposure to eugenol and citral. FEMS Microbiol. Lett. 2014, 354, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Mashayamombe, M.; Carda-Diéguez, M.; Mira, A.; Fitridge, R.; Zilm, P.S.; Kidd, S.P. Subpopulations in Strains of Staphylococcus aureus Provide Antibiotic Tolerance. Antibiotics 2023, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, J.M.; Fu, Y.; Mei, Z.; Schäffer, A.; Dou, Q.; Amelung, W.; Elsner, M.; Adu-Gyamfi, J.; Heng, L.; Virta, M.; et al. Antibiotic resistance genes in food production systems support One Health opinions. Curr. Opin. Environ. Sci. Health 2023, 34, 100492. [Google Scholar] [CrossRef]
- Hegemann, J.D.; Birkelbach, J.; Walesch, S.; Müller, R. Current developments in antibiotic discovery: Global microbial diversity as a source for evolutionary optimized anti-bacterials. EMBO Rep. 2022, 24, e56184. [Google Scholar] [CrossRef] [PubMed]
- Walesch, S.; Birkelbach, J.; Jézéquel, G.; Haeckl, F.P.J.; Hegemann, J.D.; Hesterkamp, T.; Hirsch, A.K.H.; Hammann, P.; Müller, R. Fighting antibiotic resistance—Strategies and (pre)clinical developments to find new antibacterials. EMBO Rep. 2023, 24, e56033. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 2021, 10, 3. [Google Scholar] [CrossRef]
- Caldara, M.; Belgiovine, C.; Secchi, E.; Rusconi, R. Environmental, Microbiological, and Immunological Features of Bacterial Biofilms Associated with Implanted Medical Devices. Clin. Microbiol. Rev. 2022, 35, e0022120. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, T.; Zhang, J.; Jin, X.; Yue, H.; Zhang, X.H.; Du, L.; Bai, F. Indole reverses intrinsic antibiotic resistance by activating a novel dual-function importer. MBio 2019, 10, e00676-19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Z.; Wang, Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl. Microbiol. Biotechnol. 2022, 106, 6365–6381. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Li, L.; Nokhodchi, A. Metal, metal oxide and polymeric nanoformulations for the inhibition of bacterial quorum sensing. Drug Discov. Today 2023, 28, 103392. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Marzo, T.; Cirri, D.; Pollini, S.; Prato, M.; Fallani, S.; Cassetta, M.I.; Novelli, A.; Rossolini, G.M.; Messori, L. Auranofin and its Analogues Show Potent Antimicrobial Activity against Multidrug-Resistant Pathogens: Structure–Activity Relationships. ChemMedChem 2018, 13, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Büssing, R.; Karge, B.; Lippmann, P.; Jones, P.G.; Brönstrup, M.; Ott, I. Gold(I) and Gold(III) N-Heterocyclic Carbene Complexes as Antibacterial Agents and Inhibitors of Bacterial Thioredoxin Reductase. ChemMedChem 2021, 16, 3402–3409. [Google Scholar] [CrossRef]
- Chakraborty, P.; Oosterhuis, D.; Bonsignore, R.; Casini, A.; Olinga, P.; Scheffers, D.J. An Organogold Compound as Potential Antimicrobial Agent against Drug-Resistant Bacteria: Initial Mechanistic Insights. ChemMedChem 2021, 16, 3060–3070. [Google Scholar] [CrossRef]
- Ratia, C.; Cepas, V.; Soengas, R.; Navarro, Y.; Velasco-de Andrés, M.; Iglesias, M.J.; Lozano, F.; López-Ortiz, F.; Soto, S.M. A C∧S-Cyclometallated Gold(III) Complex as a Novel Antibacterial Candidate Against Drug-Resistant Bacteria. Front. Microbiol. 2022, 13, 815622. [Google Scholar] [CrossRef]
- Chen, X.; Sun, S.; Huang, S.; Yang, H.; Ye, Q.; Lv, L.; Liang, Y.; Shan, J.; Xu, J.; Liu, W.; et al. Gold(I) selenium N-heterocyclic carbene complexes as potent antibacterial agents against multidrug-resistant gram-negative bacteria via inhibiting thioredoxin reductase. Redox Biol. 2023, 60, 102621. [Google Scholar] [CrossRef] [PubMed]
- Radzig, M.; Koksharova, O.; Khmel, I.; Ivanov, V.; Yorov, K.; Kiwi, J.; Rtimi, S.; Tastekova, E.; Aybush, A.; Nadtochenko, V. Femtosecond spectroscopy of au hot-electron injection into tio 2: Evidence for au/tio 2 plasmon photocatalysis by bactericidal au ions and related phenomena. Nanomaterials 2019, 9, 217. [Google Scholar] [CrossRef]
- Dominelli, B.; Correia, J.D.G.; Kühn, F.E. Medicinal Applications of Gold(I/III)-Based Complexes Bearing N-Heterocyclic Carbene and Phosphine Ligands. J. Organomet. Chem. 2018, 866, 153–164. [Google Scholar] [CrossRef]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Carn, F.; Gazeau, F.; Murphy, C.J. Gold-based therapy: From past to present. Proc. Natl. Acad. Sci. USA 2020, 117, 22639–22648. [Google Scholar] [CrossRef] [PubMed]
- Forestier, J. The treatment of rheumatoid arthritis with gold salts injections. Lancet 1932, 219, 441–444. [Google Scholar] [CrossRef]
- Hornos Carneiro, M.F.; Barbosa, F. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J. Toxicol. Environ. Health Part B Crit. Rev. 2016, 19, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Bojic, D.; Liu, M. Applications and safety of gold nanoparticles as therapeutic devices in clinical trials. J. Pharm. Anal. 2023, 13, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C. Synthesis and Applications of Gold Nanoparticles. In Gold Nanoparticles: Synthesis, Optical Properties and Applications for Cancer Treatment; Jarnagin, L.H.A., Ed.; Nova Science Pub Inc.: New York, NY, USA, 2013; pp. 1–38. ISBN 978-1-62257-928-0. [Google Scholar]
- Higby, G. Gold in Medicine-A review of its use in the West before 1900. Gold Bull. 1982, 15, 130–140. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C. Applications of gold nanoparticles in nanomedicine-Recent advances in vaccines. Molecules 2017, 22, 857. [Google Scholar] [CrossRef]
- Berrocal, M.; Cordoba-Granados, J.J.; Carabineiro, S.A.C.; Gutierrez-Merino, C.; Aureliano, M.; Mata, A.M. Gold compounds inhibit the ca2+-atpase activity of brain pmca and human neuroblastoma sh-sy5y cells and decrease cell viability. Metals 2021, 11, 1934. [Google Scholar] [CrossRef]
- Fonseca, C.; Fraqueza, G.; Carabineiro, S.A.C.; Aureliano, M. The ca2+-atpase inhibition potential of gold(I, iii) compounds. Inorganics 2020, 8, 49. [Google Scholar] [CrossRef]
- Aureliano, M.; Marques-da-Silva, D.; Serrano, A.; Martins, J.; Faleiro, L.; Fonseca, C.; Fraqueza, G.; Lagoa, R. Polyoxometalates with anticancer, antibacterial and antiviral activities. In Polyoxometalates: Advances, Properties, and Applications; Rubio, L.R., Vilela, J.L.V., Artetxe, B., Gutiérrez-Zorrilla, J.M., Eds.; Jenny Stanford Publishing Pte. Ltd.: Dubai, United Arab Emirates, 2023; pp. 309–358. ISBN 9781003277446/9789814968140. [Google Scholar]
- Faleiro, L.; Marques, A.; Martins, J.; Jordão, L.; Nogueira, I.; Gumerova, N.I.; Rompel, A.; Aureliano, M. The Preyssler-Type Polyoxotungstate Exhibits Anti-Quorum Sensing, Antibiofilm, and Antiviral Activities. Biology 2022, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Aureliano, M.; De Sousa-Coelho, A.L.; Dolan, C.C.; Roess, D.A.; Crans, D.C. Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. Int. J. Mol. Sci. 2023, 24, 5382. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.A.; Kazakov, A.E.; Zhong, C.; Liu, H.; Kutter, E.; Lui, L.M.; Nielsen, T.N.; Carion, H.; Deutschbauer, A.M.; Mutalik, V.K.; et al. The genetic basis of phage susceptibility, cross-resistance and host-range in Salmonella. Microbiology 2021, 167, 001126. [Google Scholar] [CrossRef] [PubMed]
- Vo, E.; Rengasamy, S.; Shaffer, R. Development of a test system to evaluate procedures for decontamination of respirators containing viral droplets. Appl. Environ. Microbiol. 2009, 75, 7303–7309. [Google Scholar] [CrossRef] [PubMed]
- Boudaud, N.; Machinal, C.; David, F.; Fréval-Le Bourdonnec, A.; Jossent, J.; Bakanga, F.; Arnal, C.; Jaffrezic, M.P.; Oberti, S.; Gantzer, C. Removal of MS2, Qβ and GA bacteriophages during drinking water treatment at pilot scale. Water Res. 2012, 46, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Brady, T.M.; Strauch, A.L.; Almaguer, C.M.; Niezgoda, G.; Shaffer, R.E.; Yorio, P.L.; Fisher, E.M. Transfer of bacteriophage MS2 and fluorescein from N95 filtering facepiece respirators to hands: Measuring fomite potential. J. Occup. Environ. Hyg. 2017, 14, 898–906. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Bankova, V.; Popova, M.; Neto, L.; Faleiro, M.L.; Da Graça Miguel, M. Moroccan Propolis: A Natural Antioxidant, Antibacterial, and Antibiofilm against Staphylococcus aureus with No Induction of Resistance after Continuous Exposure. Evid. Based Complement. Altern. Med. 2018, 2018, 9759240. [Google Scholar] [CrossRef]
- Walker, J.N.; Horswill, A.R. A coverslip-based technique for evaluating Staphylococcus aureus biofilm formation on human plasma. Front. Cell. Infect. Microbiol. 2012, 2, 39. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.R. Simple colorimetric microplate test of phage lysis in Salmonella enterica. J. Microbiol. Methods 2007, 69, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Faleiro, L.; Antunes, M.D.; Aazza, S.; Duarte, J.; Silvério, A.R. Antimicrobial, antiviral and antioxidant activities of “ água-mel” from Portugal. Food Chem. Toxicol. 2013, 56, 136–144. [Google Scholar] [CrossRef] [PubMed]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, M.I.; Marzo, T.; Fallani, S.; Novelli, A.; Messori, L. Drug repositioning: Auranofin as a prospective antimicrobial agent for the treatment of severe staphylococcal infections. BioMetals 2014, 27, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Karge, B.; Misgeld, R.; Prokop, A.; Franke, R.; Brönstrup, M.; Ott, I. Gold(I) NHC Complexes: Antiproliferative Activity, Cellular Uptake, Inhibition of Mammalian and Bacterial Thioredoxin Reductases, and Gram-Positive Directed Antibacterial Effects. Chem. A Eur. J. 2017, 23, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Ratia, C.; Soengas, R.G.; Soto, S.M. Gold-Derived Molecules as New Antimicrobial Agents. Front. Microbiol. 2022, 13, 846959. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.R.; Slate, A.J.; Ryder, S.F.; Akram, M.; Iruzubieta, C.J.C.; Whitehead, K.A. Ionic gold demonstrates antimicrobial activity against Pseudomonas aeruginosa strains due to cellular ultrastructure damage. Arch. Microbiol. 2021, 203, 3015–3024. [Google Scholar] [CrossRef]
- Samanta, T.; Roymahapatra, G.; Porto, W.F.; Seth, S.; Ghorai, S.; Saha, S.; Sengupta, J.; Franco, O.L.; Dinda, J.; Mandal, S.M. N, N′-Olefin Functionalized Bis-Imidazolium Gold(I) Salt Is an Efficient Candidate to Control Keratitis-Associated Eye Infection. PLoS ONE 2013, 8, e58346. [Google Scholar] [CrossRef]
- Torres, N.S.; Montelongo-Jauregui, D.; Abercrombie, J.J.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Leung, K.P. Antimicrobial and antibiofilm activity of synergistic combinations of a commercially available small compound library with colistin against Pseudomonas aeruginosa. Front. Microbiol. 2018, 9, 2541. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Podda, E.; Caria, V.; Carta, S.A.; Cherchi, M.F.; Lippolis, V.; Murgia, S.; Orrù, G.; Pippia, G.; Scano, A.; et al. [AuIII(N^N)Br2](PF6): A Class of Antibacterial and Antibiofilm Complexes (N^N = 2,2′-Bipyridine and 1,10-Phenanthroline Derivatives). Inorg. Chem. 2023, 62, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Ratia, C.; Ballén, V.; Gabasa, Y.; Soengas, R.G.; Velasco-de Andrés, M.; Iglesias, M.J.; Cheng, Q.; Lozano, F.; Arnér, E.S.J.; López-Ortiz, F.; et al. Novel gold(III)-dithiocarbamate complex targeting bacterial thioredoxin reductase: Antimicrobial activity, synergy, toxicity, and mechanistic insights. Front. Microbiol. 2023, 14, 1198473. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.S.; Abercrombie, J.J.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Leung, K.P. Screening a commercial library of pharmacologically active small molecules against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2016, 60, 5663–5672. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Boles, B.R.; Horswill, A.R. agr-Mediated Dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef] [PubMed]
- Pratten, J.; Foster, S.J.; Chan, P.F.; Wilson, M.; Nair, S.P. Staphylococcus aureus accessory regulators: Expression within biofilms and effect on adhesion. Microbes Infect. 2001, 3, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Gil-Moles, M.; Basu, U.; Büssing, R.; Hoffmeister, H.; Türck, S.; Varchmin, A.; Ott, I. Gold Metallodrugs to Target Coronavirus Proteins: Inhibitory Effects on the Spike-ACE2 Interaction and on PLpro Protease Activity by Auranofin and Gold Organometallics. Chem. A Eur. J. 2020, 26, 15140–15144. [Google Scholar] [CrossRef]
- Marzo, T.; Messori, L. A Role for Metal-Based Drugs in Fighting COVID-19 Infection? The Case of Auranofin. ACS Med. Chem. Lett. 2020, 11, 1067–1068. [Google Scholar] [CrossRef]
- Sonzogni-Desautels, K.; Ndao, M. Will Auranofin Become a Golden New Treatment Against COVID-19? Front. Immunol. 2021, 12, 683694. [Google Scholar] [CrossRef]
- Aires, R.L.; Santos, I.A.; Fontes, J.V.; Bergamini, F.R.G.; Jardim, A.C.G.; Abbehausen, C. Triphenylphosphine gold (I) derivatives promote antiviral effects against the Chikungunya virus. Metallomics 2022, 14, mfac056. [Google Scholar] [CrossRef]




| Formula | Abbreviation | Net Charge | MW (g/mol) | CAS Number | Purity |
|---|---|---|---|---|---|
| C3H9PAuCl | 1 | +1 | 308.50 | 15278-97-4 | 99% |
| C18H15PAuCl | 2 | +1 | 494.71 | 14243-64-2 | ≥99.9% |
| C6H4NAuCl2O2 | 3 | +3 | 389.97 | 88215-41-2 | 99% |
| C27H36AuClN2 | 4 | +1 | 621.01 | 852445-83-1 | 99% |
| Bacteria | Origin and Characteristics | Source |
|---|---|---|
| Escherichia coli DSM 1077 | K12 galR arg nad | German Collection of Microorganisms |
| Escherichia coli DSM 5210 | Hfr 3000 U 432. Host of phage Qß (DSM 5696) | German Collection of Microorganisms |
| Escherichia coli DSM 498 | K12 wildtype. Host of phage Ffm (DSM 18264) | German Collection of Microorganisms |
| Escherichia coli I731940778-1 | Multi-resistant. Isolated from urine | Laboratory of Microbiology, ABC-RI, UAlg 1 |
| Staphylococcus aureus ATCC 6538 | Wound | American Type Culture Collection |
| Staphylococcus aureus methicillin-resistant 15 (MRSA 15) | Clinic | Laboratory of Microbiology, ABC-RI, UAlg 1 |
| Staphylococcus aureus methicillin-resistant 16 (MRSA 16) | Clinic | Laboratory of Microbiology, ABC-RI, UAlg 1 |
| Chromobacterium violaceum (CV026) | Biosensor strain of the production of homoserine lactone (HgR, cvil::Tn5 xylE, KanR, higher spontaneous resistance StrR) | Gift of Professor Mondher El Jaziri of the University Libre of Brussels |
| Bacteriophages | Family | Host |
| Escherichia coli phage Ffm DSM 18264 | Autographiviridae | For propagation, E. coli DSM 498, rough-LPS Salmonella mutants are also receptors |
| Escherichia coli phage Qbeta (Enterobacteria phage Qbeta) DSM 13768 | Leviviridae | For propagation E. coli DSM 5210 |
| Bacteria | MIC (μg/mL) 1 | MBC (μg/mL) 1 |
|---|---|---|
| S. aureus ATCC 6538 | 0.59 | 4.63 |
| MRSA12 | 1.16 | 18.5 |
| MRSA15 | 1.16 | 18.5 |
| E. coli DSM 1077 | 4.63 | 9.25 |
| Multi-resistant E. coli I731940778-1 | 9.25 | 37.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.; Carabineiro, S.A.C.; Aureliano, M.; Faleiro, L. Evaluation of Gold Complexes to Address Bacterial Resistance, Quorum Sensing, Biofilm Formation, and Their Antiviral Properties against Bacteriophages. Toxics 2023, 11, 879. https://doi.org/10.3390/toxics11110879
Marques A, Carabineiro SAC, Aureliano M, Faleiro L. Evaluation of Gold Complexes to Address Bacterial Resistance, Quorum Sensing, Biofilm Formation, and Their Antiviral Properties against Bacteriophages. Toxics. 2023; 11(11):879. https://doi.org/10.3390/toxics11110879
Chicago/Turabian StyleMarques, Ana, Sónia A. C. Carabineiro, Manuel Aureliano, and Leonor Faleiro. 2023. "Evaluation of Gold Complexes to Address Bacterial Resistance, Quorum Sensing, Biofilm Formation, and Their Antiviral Properties against Bacteriophages" Toxics 11, no. 11: 879. https://doi.org/10.3390/toxics11110879








