Effects of Ambient O3 on Respiratory Mortality, Especially the Combined Effects of PM2.5 and O3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data on Mortality Associated with Respiratory Disease
2.3. Data on Environmental Exposure
2.4. Statistical Modeling
2.5. Interaction Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Distribution of Air Pollutants
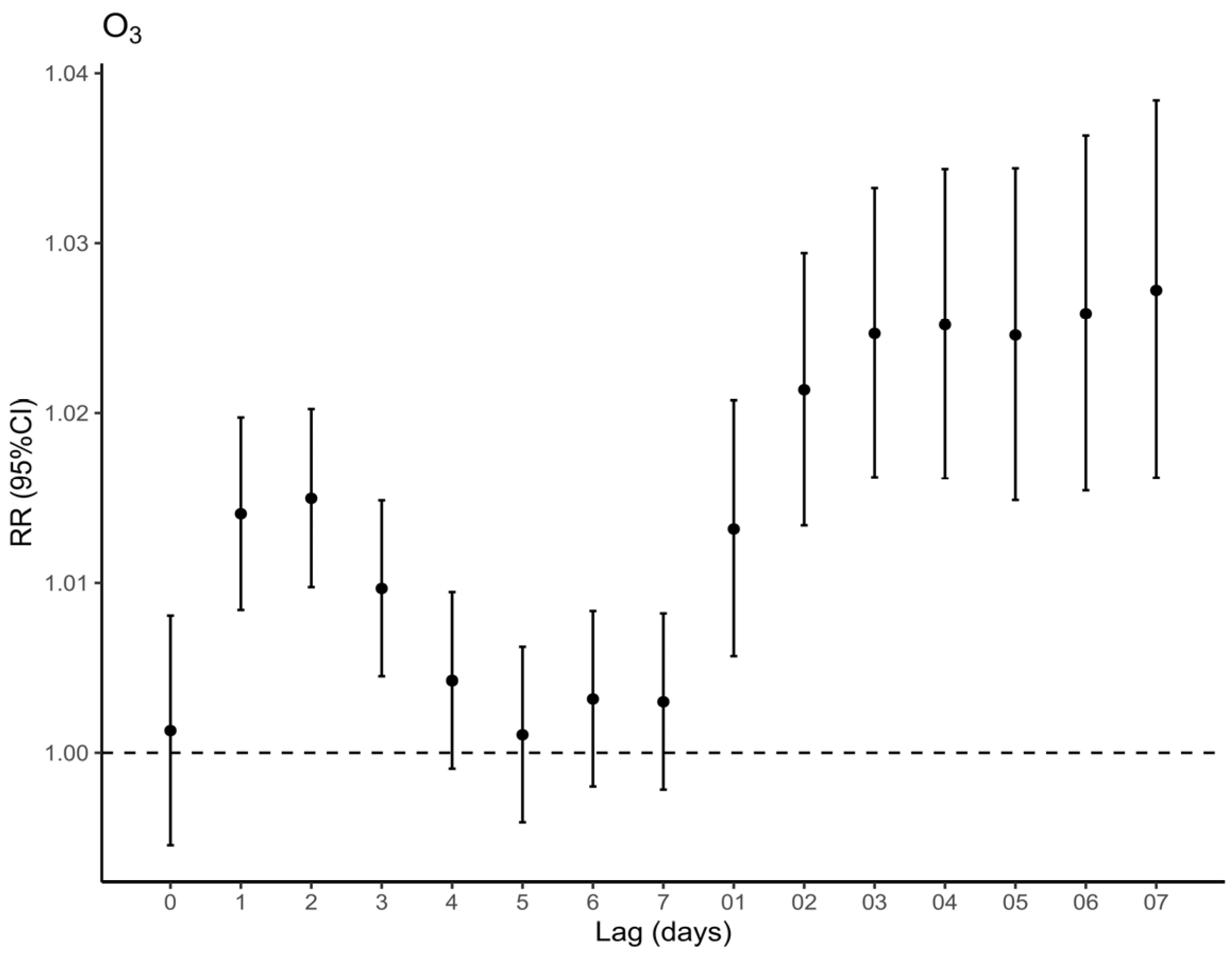
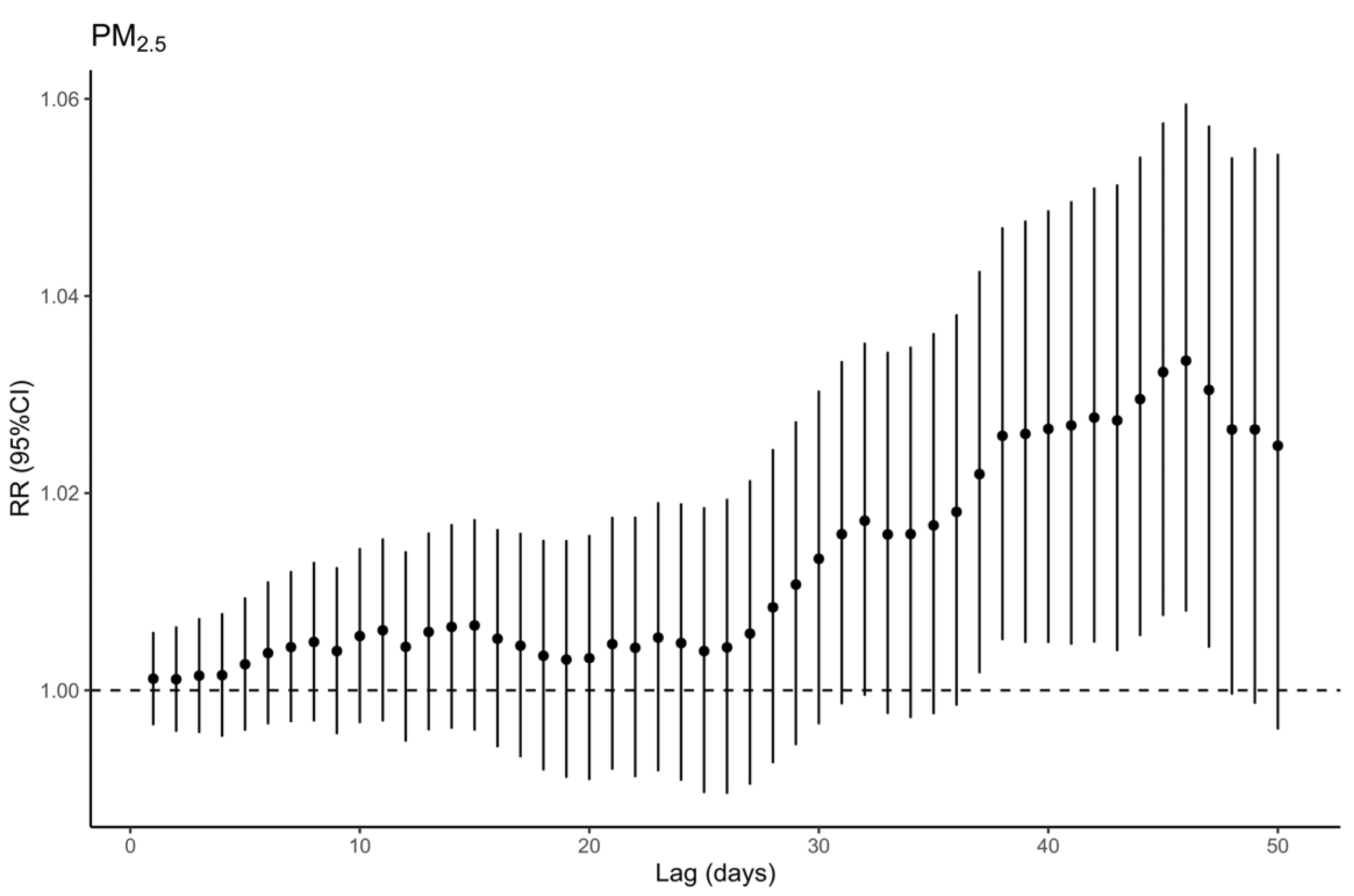
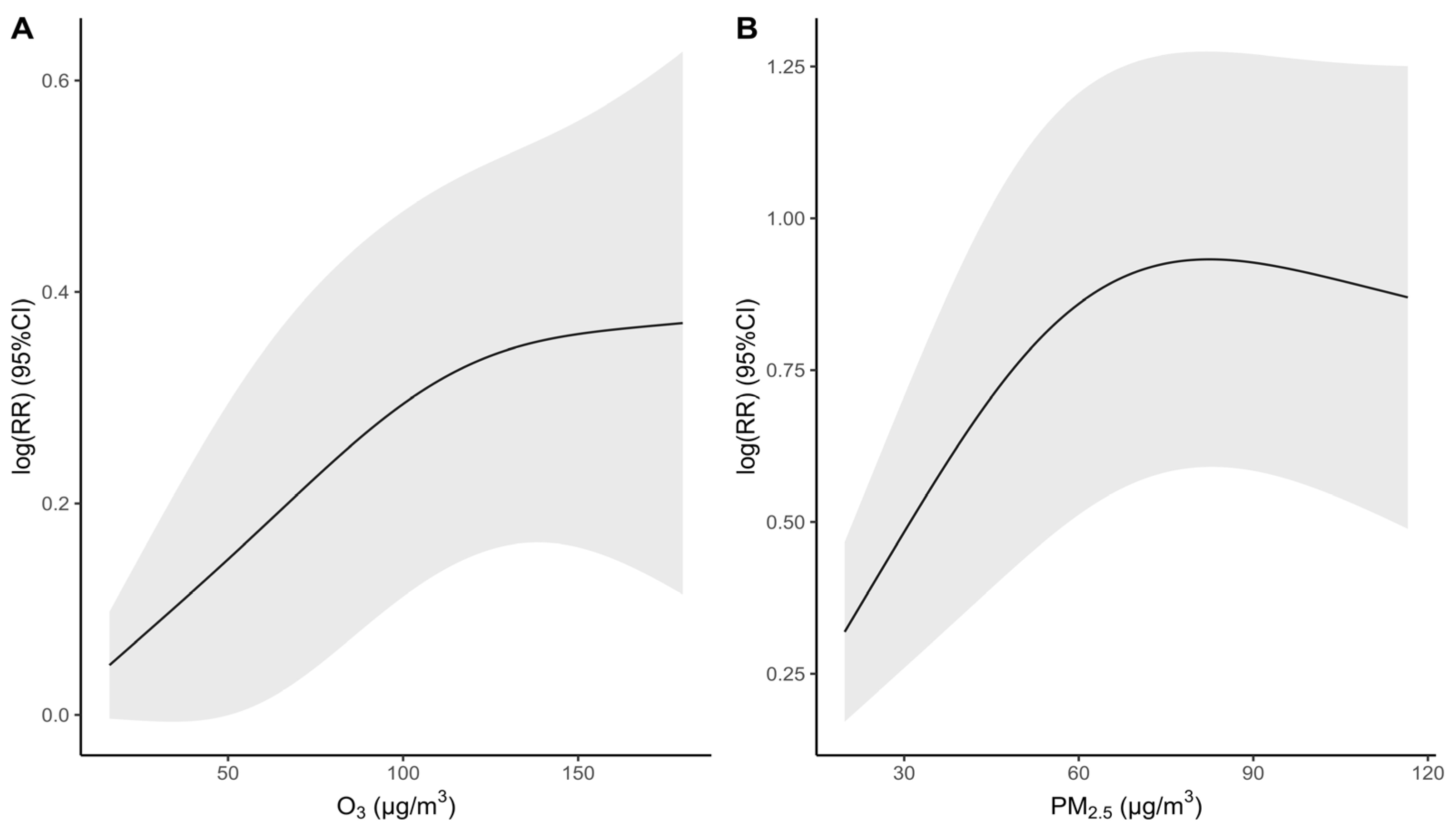
3.3. Air Pollution Exposure and Respiratory Mortality
3.4. Joint Effects of Air Pollutants on Respiratory Mortality
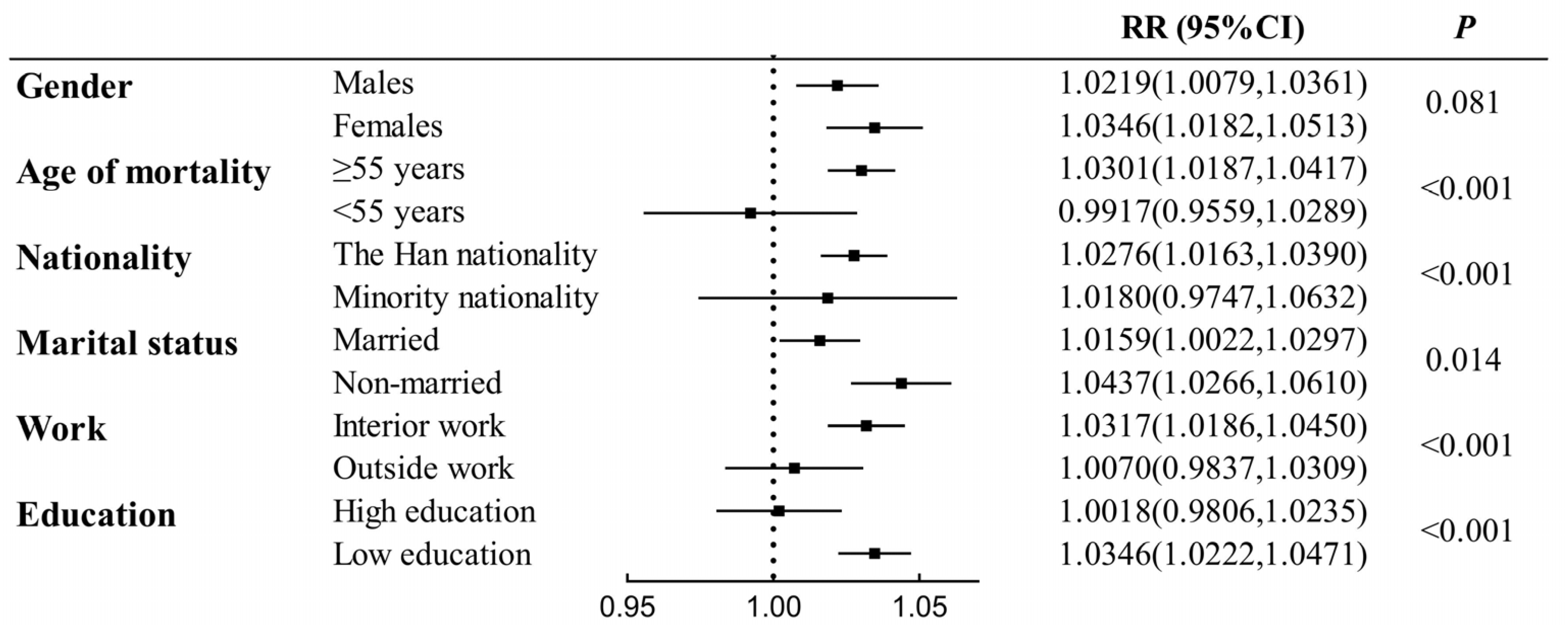
3.5. Subgroup Analysis of O3 Exposure
3.6. Sensitivity Analyses
4. Discussion
4.1. O3 Exposure and Respiratory Diseases
4.2. Subgroups More Sensitive to O3
4.3. Combined Effects between PM2.5 and O3
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 9 February 2022).
- Levine, S.M.; Marciniuk, D.D. Global Impact of Respiratory Disease: What Can We Do, Together, to Make a Difference? Chest 2022, 161, 1153–1154. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161. [CrossRef] [PubMed]
- Chi, C.; Sheikh, A.; Yao, M.; Cheng, K.K.; Wang, C. Improving the prevention and management of respiratory diseases in China: The crucial role of primary care. Lancet Respir. Med. 2015, 3, s2213–s2600. [Google Scholar] [CrossRef] [PubMed]
- von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef]
- He, M.; Zhong, Y.; Chen, Y.; Zhong, N.; Lai, K. Association of short-term exposure to air pollution with emergency visits for respiratory diseases in children. iScience 2022, 25, 104879. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Kingdon, C. Air pollution is the largest environmental risk to public health and children are especially vulnerable. BMJ 2023, 381, 1037. [Google Scholar] [CrossRef]
- Linou, N.; Beagley, J.; Huikuri, S.; Renshaw, N. Air pollution moves up the global health agenda. BMJ 2018, 363, k4933. [Google Scholar] [CrossRef]
- Zou, B.; You, J.; Lin, Y.; Duan, X.; Zhao, X.; Fang, X.; Campen, M.J.; Li, S. Air pollution intervention and life-saving effect in China. Environ. Int. 2019, 125, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.S. Air pollution and China’s ageing society. Lancet Public Health 2018, 3, e457–e458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wild, O.; Chen, X.; Wu, Q.; Gao, M.; Chen, H.; Qi, Y.; Wang, Z. Health impacts of long-term ozone exposure in China over 2013–2017. Environ. Int. 2020, 144, 106030. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, T.; Sun, Q.; Shi, W.; He, M.Z.; Wang, J.; Liu, J.; Zhang, M.; Jiang, Q.; Wang, M.; et al. Short-term exposure to ozone and cause-specific mortality risks and thresholds in China: Evidence from nationally representative data, 2013–2018. Environ. Int. 2023, 171, 107666. [Google Scholar] [CrossRef] [PubMed]
- Adar, S.D.; Filigrana, P.A.; Clements, N.; Peel, J.L. Ambient Coarse Particulate Matter and Human Health: A Systematic Review and Meta-Analysis. Curr. Environ. Health Rep. 2014, 1, 258–274. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Yue, W.; Tong, L.; Liu, X.; Weng, X.; Chen, X.; Wang, D.; Dudley, S.C.; Weir, E.K.; Ding, W.; Lu, Z.; et al. Short term PM2.5 exposure caused a robust lung inflammation, vascular remodeling, and exacerbated transition from left ventricular failure to right ventricular hypertrophy. Redox Biol. 2019, 22, 101161. [Google Scholar] [CrossRef]
- Yue, L.; Qidian, L.; Jiawei, W.; Rou, X.; Miao, H. Acute iron oxide nanoparticles exposure induced murine eosinophilic airway inflammation via TLR2 and TLR4 signaling. Environ. Toxicol. 2022, 37, 925–935. [Google Scholar] [CrossRef]
- Fan, X.; Dong, T.; Yan, K.; Ci, X.; Peng, L. PM2.5 increases susceptibility to acute exacerbation of COPD via NOX4/Nrf2 redox imbalance-mediated mitophagy. Redox Biol. 2023, 59, 102587. [Google Scholar] [CrossRef]
- Alexis, N.E.; Lay, J.C.; Zeman, K.; Bennett, W.E.; Peden, D.B.; Soukup, J.M.; Devlin, R.B.; Becker, S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J. Allergy Clin. Immunol. 2006, 117, 1396–1403. [Google Scholar] [CrossRef]
- Malashock, D.A.; Delang, M.N.; Becker, J.S.; Serre, M.L.; West, J.J.; Chang, K.L.; Cooper, O.R.; Anenberg, S.C. Global trends in ozone concentration and attributable mortality for urban, peri-urban, and rural areas between 2000 and 2019: A modelling study. Lancet Planet. Health 2022, 6, e958–e967. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Cao, R.; Yin, P.; Wang, L.; Liu, Y.; Pan, X.; Li, G.; Zhou, M. The association between ozone and years of life lost from stroke, 2013–2017: A retrospective regression analysis in 48 major Chinese cities. J. Hazard. Mater. 2021, 405, 124220. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, J.; Li, G.; Wang, W.; Wang, K.; Wang, J.; Wei, C.; Li, Y.; Deng, F.; Baccarelli, A.A.; et al. Ozone pollution and hospital admissions for cardiovascular events. Eur. Heart J. 2023, 44, 1622–1632. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, Y.; Chen, R.; Yin, P.; Meng, X.; Wang, W.; Liu, C.; Ji, J.S.; Qiu, Y.; Kan, H.; et al. Long-term exposure to ozone and cardiovascular mortality in China: A nationwide cohort study. Lancet Planet. Health 2022, 6, e496–e503. [Google Scholar] [CrossRef]
- Zhao, N.; Pinault, L.; Toyib, O.; Vanos, J.; Tjepkema, M.; Cakmak, S. Long-term ozone exposure and mortality from neurological diseases in Canada. Environ. Int. 2021, 157, 106817. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, Y.; Liu, H.; Si, Y.; Wu, Y.; Wang, X.; Wang, M.; Wu, J.; Chen, L.; Wei, C.; et al. The impact of ambient ozone pollution on pneumonia: A nationwide time-series analysis. Environ. Int. 2020, 136, 105498. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, J.; Zhang, Y.; Wang, H.; Li, H.; Cheng, Y.; Guo, Y. Short-term effect of ambient ozone pollution on respiratory diseases in western China. Environ. Geochem. Health 2022, 44, 4129–4140. [Google Scholar] [CrossRef]
- Lavigne, E.; Burnett, R.T.; Weichenthal, S. Association of short-term exposure to fine particulate air pollution and mortality: Effect modification by oxidant gases. Sci. Rep. 2018, 8, 16097. [Google Scholar] [CrossRef]
- Cakmak, S.; Hebbern, C.; Vanos, J.; Crouse, D.L.; Burnett, R. Ozone exposure and cardiovascular-related mortality in the Canadian Census Health and Environment Cohort (CANCHEC) by spatial synoptic classification zone. Environ. Pollut. 2016, 214, 589–599. [Google Scholar] [CrossRef]
- Ravindra, K.; Rattan, P.; Mor, S.; Aggarwal, A.N. Generalized additive models: Building evidence of air pollution, climate change and human health. Environ. Int. 2019, 132, 104987. [Google Scholar] [CrossRef]
- Peng, R.D.; Dominici, F.; Louis, T.A. Model Choice in Time Series Studies of Air Pollution and Mortality. J. R. Stat. Soc. Ser. A Stat. Soc. 2006, 169, 179–203. [Google Scholar] [CrossRef]
- Li, M.; Dong, H.; Wang, B.; Zhao, W.; Zare Sakhvidi, M.J.; Li, L.; Lin, G.; Yang, J. Association between ambient ozone pollution and mortality from a spectrum of causes in Guangzhou, China. Sci. Total Environ. 2021, 754, 142110. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Gasparrini, A.; Hajat, S.; Smeeth, L.; Armstrong, B. Time series regression studies in environmental epidemiology. Int. J. Epidemiol. 2013, 42, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, K.; Fu, J. A Time-Series Study for Effects of Ozone on Respiratory Mortality and Cardiovascular Mortality in Nanchang, Jiangxi Province, China. Front. Public Health 2022, 10, 864537. [Google Scholar] [CrossRef] [PubMed]
- Goodman, P.G.; Dockery, D.W.; Clancy, L. Cause-specific mortality and the extended effects of particulate pollution and temperature exposure. Environ. Health Perspect. 2004, 112, 179–185. [Google Scholar] [CrossRef]
- Qiu, H.; Pun, V.C.; Tian, L. Short-term effects of fine and coarse particles on deaths in Hong Kong elderly population: An analysis of mortality displacement. Environ. Pollut. 2018, 241, 148–154. [Google Scholar] [CrossRef]
- Wu, C.; Yan, Y.; Chen, X.; Gong, J.; Guo, Y.; Zhao, Y.; Yang, N.; Dai, J.; Zhang, F.; Xiang, H. Short-term exposure to ambient air pollution and type 2 diabetes mortality: A population-based time series study. Environ. Pollut. 2021, 289, 117886. [Google Scholar] [CrossRef]
- Xia, S.Y.; Huang, D.S.; Jia, H.; Zhao, Y.; Li, N.; Mao, M.Q.; Lin, H.; Li, Y.X.; He, W.; Zhao, L. Relationship between atmospheric pollutants and risk of death caused by cardiovascular and respiratory diseases and malignant tumors in Shenyang, China, from 2013 to 2016: An ecological research. Chin. Med. J. 2019, 132, 2269–2277. [Google Scholar] [CrossRef]
- Chen, G.H.; Song, G.X.; Jiang, L.L.; Zhang, Y.H.; Zhao, N.Q.; Chen, B.H.; Kan, H.D. Interaction between ambient particles and ozone and its effect on daily mortality. Biomed. Environ. Sci. BES 2007, 20, 502–505. [Google Scholar]
- Källberg, H.; Ahlbom, A.; Alfredsson, L. Calculating measures of biological interaction using R. Eur. J. Epidemiol. 2006, 21, 571–573. [Google Scholar] [CrossRef]
- Sun, N.; Niu, Y.; Zhang, R.; Huang, Y.; Wang, J.; Qiu, W.; Zhang, X.; Han, Z.; Bao, J.; Zhu, H.; et al. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and Th2-skew immune response in a rat model of AR. Biomed. Pharmacother. 2021, 137, 111261. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, D.I.; Wilkinson, J.E.; Cho, Y.; Cardoso, A.P.; Huttenhower, C.; Shore, S.A. The interleukin-33 receptor contributes to pulmonary responses to ozone in male mice: Role of the microbiome. Respir. Res. 2019, 20, 197. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, Z.; Wei, Y. Advances in understanding mechanisms underlying mitochondrial structure and function damage by ozone. Sci. Total Environ. 2023, 861, 160589. [Google Scholar] [CrossRef] [PubMed]
- Hodge, M.X.; Henriquez, A.R.; Kodavanti, U.P. Adrenergic and Glucocorticoid Receptors in the Pulmonary Health Effects of Air Pollution. Toxics 2021, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Niu, Y.; Wang, C.; Wang, W.; Liu, C.; Meng, X.; Chu, C.; Chen, R.; Kan, H. Ozone exposure and blood transcriptome: A randomized, controlled, crossover trial among healthy adults. Environ. Int. 2022, 163, 107242. [Google Scholar] [CrossRef] [PubMed]
- Faridi, S.; Shamsipour, M.; Krzyzanowski, M.; Künzli, N.; Amini, H.; Azimi, F.; Malkawi, M.; Momeniha, F.; Gholampour, A.; Hassanvand, M.S.; et al. Long-term trends and health impact of PM2.5 and O3 in Tehran, Iran, 2006–2015. Environ. Int. 2018, 114, 37–49. [Google Scholar] [CrossRef]
- WHO. Air quality guidelines. WHO Regional Office for Europe. In Global Update 2021; WHO: Copenhagen, Denmark, 2021. [Google Scholar]
- Powell, H.; Lee, D.; Bowman, A. Estimating constrained concentration-response functions between air pollution and health. Environmetrics 2012, 23, 228–237. [Google Scholar] [CrossRef]
- Wang, B.; Eum, K.D.; Kazemiparkouhi, F.; Li, C.; Manjourides, J.; Pavlu, V.; Suh, H. The impact of long-term PM2.5 exposure on specific causes of death: Exposure-response curves and effect modification among 53 million U.S. Medicare beneficiaries. Environ. Health 2020, 19, 20. [Google Scholar] [CrossRef]
- Hazari, M.S.; Stratford, K.M.; Krantz, Q.T.; King, C.; Krug, J.; Farraj, A.K.; Gilmour, M.I. Comparative Cardiopulmonary Effects of Particulate Matter- And Ozone-Enhanced Smog Atmospheres in Mice. Environ. Sci. Technol. 2018, 52, 3071–3080. [Google Scholar] [CrossRef]
- Peavy, D.L.; Fairchild, E.J., 2nd. Toxicologic interactions between ozone and bacterial endotoxin. Environ. Res. 1987, 42, 63–71. [Google Scholar] [CrossRef]
- Kesic, M.J.; Meyer, M.; Bauer, R.; Jaspers, I. Exposure to ozone modulates human airway protease/antiprotease balance contributing to increased influenza A infection. PLoS ONE 2012, 7, e35108. [Google Scholar] [CrossRef] [PubMed]
- Strosnider, H.M.; Chang, H.H.; Darrow, L.A.; Liu, Y.; Vaidyanathan, A.; Strickland, M.J. Age-Specific Associations of Ozone and Fine Particulate Matter with Respiratory Emergency Department Visits in the United States. Am. J. Respir. Crit. Care Med. 2019, 199, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. Off. J. Eur. Soc. Organ Transpl. 2009, 22, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Arjomandi, M.; Balmes, J.R.; Frampton, M.W.; Bromberg, P.; Rich, D.Q.; Stark, P.; Alexis, N.E.; Costantini, M.; Hollenbeck-Pringle, D.; Dagincourt, N.; et al. Respiratory Responses to Ozone Exposure. MOSES (The Multicenter Ozone Study in Older Subjects). Am. J. Respir. Crit. Care Med. 2018, 197, 1319–1327. [Google Scholar] [CrossRef]
- Zafirah, Y.; Lin, Y.K.; Andhikaputra, G.; Deng, L.W.; Sung, F.C.; Wang, Y.C. Mortality and morbidity of asthma and chronic obstructive pulmonary disease associated with ambient environment in metropolitans in Taiwan. PLoS ONE 2021, 16, e0253814. [Google Scholar] [CrossRef]
- Lu, J.; Yao, L. Observational evidence for detrimental impact of inhaled ozone on human respiratory system. BMC Public Health 2023, 23, 929. [Google Scholar] [CrossRef]
- Fuentes, N.; Roy, A.; Mishra, V.; Cabello, N.; Silveyra, P. Sex-specific microRNA expression networks in an acute mouse model of ozone-induced lung inflammation. Biol. Sex Differ. 2018, 9, 18. [Google Scholar] [CrossRef]
- Henriquez, A.R.; Snow, S.J.; Jackson, T.W.; House, J.S.; Alewel, D.I.; Schladweiler, M.C.; Valdez, M.C.; Freeborn, D.L.; Miller, C.N.; Grindstaff, R.; et al. Social isolation exacerbates acute ozone inhalation induced pulmonary and systemic health outcomes. Toxicol. Appl. Pharmacol. 2022, 457, 116295. [Google Scholar] [CrossRef]
- Zhao, T.; Markevych, I.; Romanos, M.; Nowak, D.; Heinrich, J. Ambient ozone exposure and mental health: A systematic review of epidemiological studies. Environ. Res. 2018, 165, 459–472. [Google Scholar] [CrossRef]
- Kabel, J.R.; Ben-Jebria, A.; Ultman, J.S. Longitudinal distribution of ozone absorption in the lung: Comparison of nasal and oral quiet breathing. J. Appl. Physiol. 1994, 77, 2584–2592. [Google Scholar] [CrossRef]
- Nazaroff, W.W.; Weschler, C.J. Indoor ozone: Concentrations and influencing factors. Indoor Air 2022, 32, e12942. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zheng, H.; Xia, P.; Cheng, X.; Wu, W.; Li, Y.; Ma, C.; Zhu, G.; Xu, T.; Zheng, Y.; et al. Long-term ambient PM2.5 exposure associated with cardiovascular risk factors in Chinese less educated population. BMC Public Health 2021, 21, 2241. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, P.; Wang, L.; Zhang, X.; Liu, J.; Liu, Y.; Zhou, M. Ambient ozone pollution and years of life lost: Association, effect modification, and additional life gain from a nationwide analysis in China. Environ. Int. 2020, 141, 105771. [Google Scholar] [CrossRef] [PubMed]
- Yanosky, J.D.; Schwartz, J.; Suh, H.H. Associations between measures of socioeconomic position and chronic nitrogen dioxide exposure in Worcester, Massachusetts. J. Toxicol. Environ. Health A 2008, 71, 1593–1602. [Google Scholar] [CrossRef]
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr. Environ. Health Rep. 2015, 2, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, R.B. Interaction of gaseous and particulate pollutants in the respiratory tract: Mechanisms and modulators. Toxicology 1995, 105, 315–325. [Google Scholar] [CrossRef]
- Majumder, N.; Goldsmith, W.T.; Kodali, V.K.; Velayutham, M.; Friend, S.A.; Khramtsov, V.V.; Nurkiewicz, T.R.; Erdely, A.; Zeidler-Erdely, P.C.; Castranova, V.; et al. Oxidant-induced epithelial alarmin pathway mediates lung inflammation and functional decline following ultrafine carbon and ozone inhalation co-exposure. Redox Biol. 2021, 46, 102092. [Google Scholar] [CrossRef]
- Majumder, N.; Kodali, V.; Velayutham, M.; Goldsmith, T.; Amedro, J.; Khramtsov, V.V.; Erdely, A.; Nurkiewicz, T.R.; Harkema, J.R.; Kelley, E.E.; et al. Aerosol physicochemical determinants of carbon black and ozone inhalation co-exposure induced pulmonary toxicity. Toxicol. Sci. 2023, 191, 61–78. [Google Scholar] [CrossRef]
- Lin, H.; Guo, Y.; Ruan, Z.; Yang, Y.; Chen, Y.; Zheng, Y.; Cummings-Vaughn, L.A.; Rigdon, S.E.; Vaughn, M.G.; Sun, S.; et al. Ambient PM2.5 and O3 and their combined effects on prevalence of presbyopia among the elderly: A cross-sectional study in six low- and middle-income countries. Sci. Total Environ. 2019, 655, 168–173. [Google Scholar] [CrossRef]

| Characteristic | n (%) |
|---|---|
| Total | 35,385 (100.00) |
| Gender | |
| Males | 20,572 (58.14) |
| Females | 14,813 (41.86) |
| Age of mortality | |
| ≥55 years | 32,780 (92.64) |
| <55 years | 2605 (7.36) |
| Nationality | |
| The Han nationality | 33,465 (94.57) |
| Minority nationality | 1911 (5.40) |
| unknown | 9 (0.03) |
| Marital status | |
| Married | 20,932 (59.16) |
| Non-married | 14,196 (40.12) |
| unknown | 257 (0.73) |
| Work | |
| Interior work | 24,374 (68.89) |
| Outside work | 6532 (18.46) |
| unknown | 4479 (12.66) |
| Education | |
| High education | 8148 (23.03) |
| Low education | 27,237 (76.97) |
| Cause of deaths | |
| Influenza and pneumonia (J09–J18) | 13,466 (38.06) |
| Lower respiratory tract infection disease (J12–J18, J20–J22) | 13,578 (38.37) |
| Chronic lower respiratory disease (J40–J47) | 2799 (7.91) |
| Respiratory failure (J96) | 17,081 (48.27) |
| Mean | Min | P25 | P50 | P75 | Max | |
|---|---|---|---|---|---|---|
| T (℃) | 9.1 | −22.8 | −2.8 | 11.0 | 21.2 | 32.4 |
| RH (%) | 59.9 | 15.1 | 47.8 | 61.3 | 72.0 | 98.0 |
| O3 (μg/m3) | 66.7 | 9.0 | 35.0 | 59.0 | 88.0 | 250.0 |
| PM2.5 (μg/m3) | 56.1 | 4.0 | 28.0 | 43.0 | 72.0 | 291.0 |
| PM10 (μg/m3) | 94.6 | 8.0 | 56.0 | 81.0 | 119.0 | 396.0 |
| SO2 (μg/m3) | 47.5 | 3.0 | 15.0 | 26.0 | 57.0 | 332.0 |
| NO2 (μg/m3) | 41.8 | 12.0 | 29.0 | 39.0 | 51.0 | 125.0 |
| CO (mg/m3) | 1.0 | 0.3 | 0.7 | 0.9 | 1.2 | 3.3 |
| O3 | PM2.5 | PM10 | SO2 | NO2 | CO | T | RH | |
|---|---|---|---|---|---|---|---|---|
| O3 (μg/m3) | 1.00 | |||||||
| PM2.5 (μg/m3) | −0.17 * | 1.00 | ||||||
| PM10 (μg/m3) | −0.09 * | 0.90 * | 1.00 | |||||
| SO2 (μg/m3) | −0.39 * | 0.70 * | 0.67 * | 1.00 | ||||
| NO2 (μg/m3) | −0.34 * | 0.74 * | 0.68 * | 0.71 * | 1.00 | |||
| CO (mg/m3) | −0.19 * | 0.80 * | 0.70 * | 0.63 * | 0.72 * | 1.00 | ||
| T (℃) | 0.70 * | −0.37 * | −0.35 * | −0.67 * | −0.40 * | −0.21 * | 1.00 | |
| RH (%) | −0.07 * | −0.01 | −0.20 * | −0.21 * | −0.02 | 0.18 * | 0.32 * | 1.00 |
| Category | Value & 95% CI |
|---|---|
| OR | |
| low O3 + low PM2.5 | 1 |
| high O3 + low PM2.5 | 1.065 (1.017, 1.116) |
| low O3 + high PM2.5 | 0.978 (0.945, 1.013) |
| high O3 + high PM2.5 | 1.102 (1.015, 1.196) |
| SI | 2.372 (1.127, 3.617) |
| RERI | 0.059 (−0.021, 0.139) |
| AP | 0.054 (−0.015, 0.123) |
| Pollutant(s) | Lag 2 | Lag 07 |
|---|---|---|
| O3 | 1.0150 (1.0098, 1.0202) | 1.0272 (1.0162, 1.0384) |
| O3 + PM2.5 | 1.0150 (1.0098, 1.0203) | 1.0273 (1.0162, 1.0385) |
| O3 + PM10 | 1.0149 (1.0097, 1.0201) | 1.0269 (1.0159, 1.0381) |
| O3 + SO2 | 1.0150 (1.0098, 1.0203) | 1.0276 (1.0165, 1.0388) |
| O3 + NO2 | 1.0152 (1.0099, 1.0205) | 1.0278 (1.0166, 1.0391) |
| O3 + PM 2.5 + SO2 | 1.0150 (1.0098, 1.0203) | 1.0275 (1.0164, 1.0388) |
| O3 + PM 2.5 + NO2 | 1.0152 (1.0100, 1.0205) | 1.0279 (1.0167, 1.0392) |
| O3 + SO2 + NO2 | 1.0152 (1.0099, 1.0205) | 1.0278 (1.0166, 1.0391) |
| O3 + PM 2.5 + SO2 + NO2 | 1.0152 (1.0100, 1.0205) | 1.0279 (1.0167, 1.0392) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Wang, J.; Sun, L.; Wang, Y.; Chen, J.; Zhao, Z.; Wang, T.; Xiang, Y.; Wang, Y.; Chen, J.; et al. Effects of Ambient O3 on Respiratory Mortality, Especially the Combined Effects of PM2.5 and O3. Toxics 2023, 11, 892. https://doi.org/10.3390/toxics11110892
Deng Y, Wang J, Sun L, Wang Y, Chen J, Zhao Z, Wang T, Xiang Y, Wang Y, Chen J, et al. Effects of Ambient O3 on Respiratory Mortality, Especially the Combined Effects of PM2.5 and O3. Toxics. 2023; 11(11):892. https://doi.org/10.3390/toxics11110892
Chicago/Turabian StyleDeng, Ye, Junlong Wang, Li Sun, Yue Wang, Jiaoyang Chen, Zhixin Zhao, Tianyun Wang, Yuting Xiang, Yuting Wang, Jiamei Chen, and et al. 2023. "Effects of Ambient O3 on Respiratory Mortality, Especially the Combined Effects of PM2.5 and O3" Toxics 11, no. 11: 892. https://doi.org/10.3390/toxics11110892