Biochar as Sustainable Alternative and Green Adsorbent for the Remediation of Noxious Pollutants: A Comprehensive Review
Abstract
:1. Introduction
2. Sources of Biochar
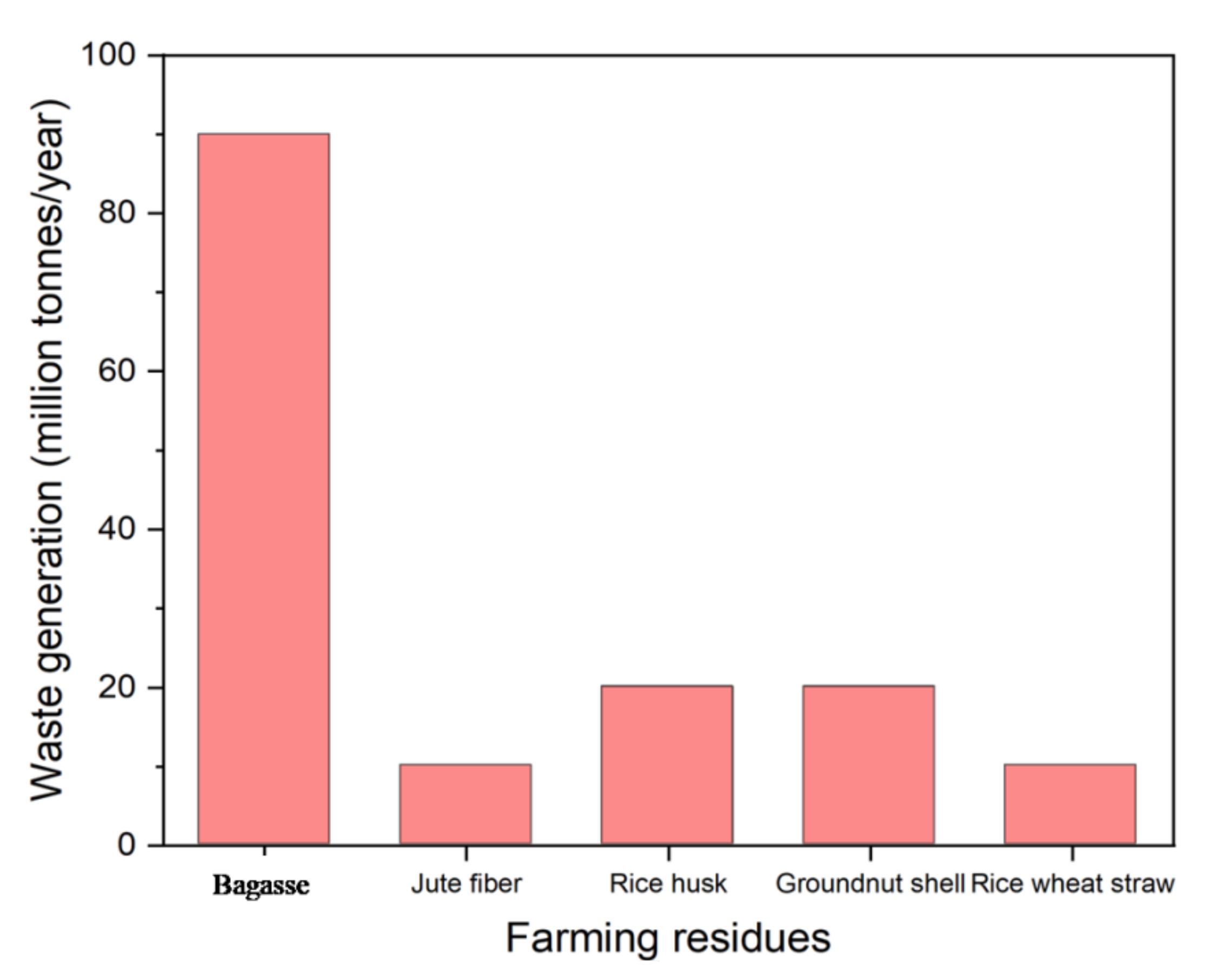
2.1. Farming Residues
2.2. Forestry Residues
2.3. Aquatic Residues
2.4. Industrial Residues
3. Synthetic Routes
3.1. Pyrolysis
3.1.1. Slow Pyrolysis
3.1.2. Fast Pyrolysis
3.1.3. Microwave Pyrolysis
3.2. Hydrothermal Carbonization (HTC)
3.3. Torrefaction
3.4. Gasification
| Method | Temperature (°C) | Conditions | Percent Yield of Products | Advantages | Disadvantages | ||
|---|---|---|---|---|---|---|---|
| Solid (Biochar) | Liquid (Bio-Oil) | Gaseous (Biogas) | |||||
| Slow pyrolysis | 300–500 | Oxygen-free atmosphere | 35 | 30 | 35 | Highest yield of biochar | Further treatment of gases is needed |
| Fast pyrolysis | 500–700 | Oxygen-free atmosphere | 12 | 75 | 13 | Higher yield of bio-oil | Low biochar yield Fine particle biomass is required |
| Microwave pyrolysis | - | - | - | - | - | No size reduction or drying of biomass is required Rapid and uniform heating | Energy requirement is high |
| HTC | <230 | Low pressure | 50–80 | 5–20 | 2–5 | Low operating temperature and residence time | Separation of solid and liquid phase |
| Torrefaction | 200–300 | Inert atmosphere | 60 | 20 | 20 | Zero waste process Upgraded quality of biochar | Feedstock sensitivity High investment cost |
| Gasification | >700 | Oxidizing atmosphere | 10 | 5 | 85 | Reduced emissions High energy efficiency | Complex technology and high operation cost |
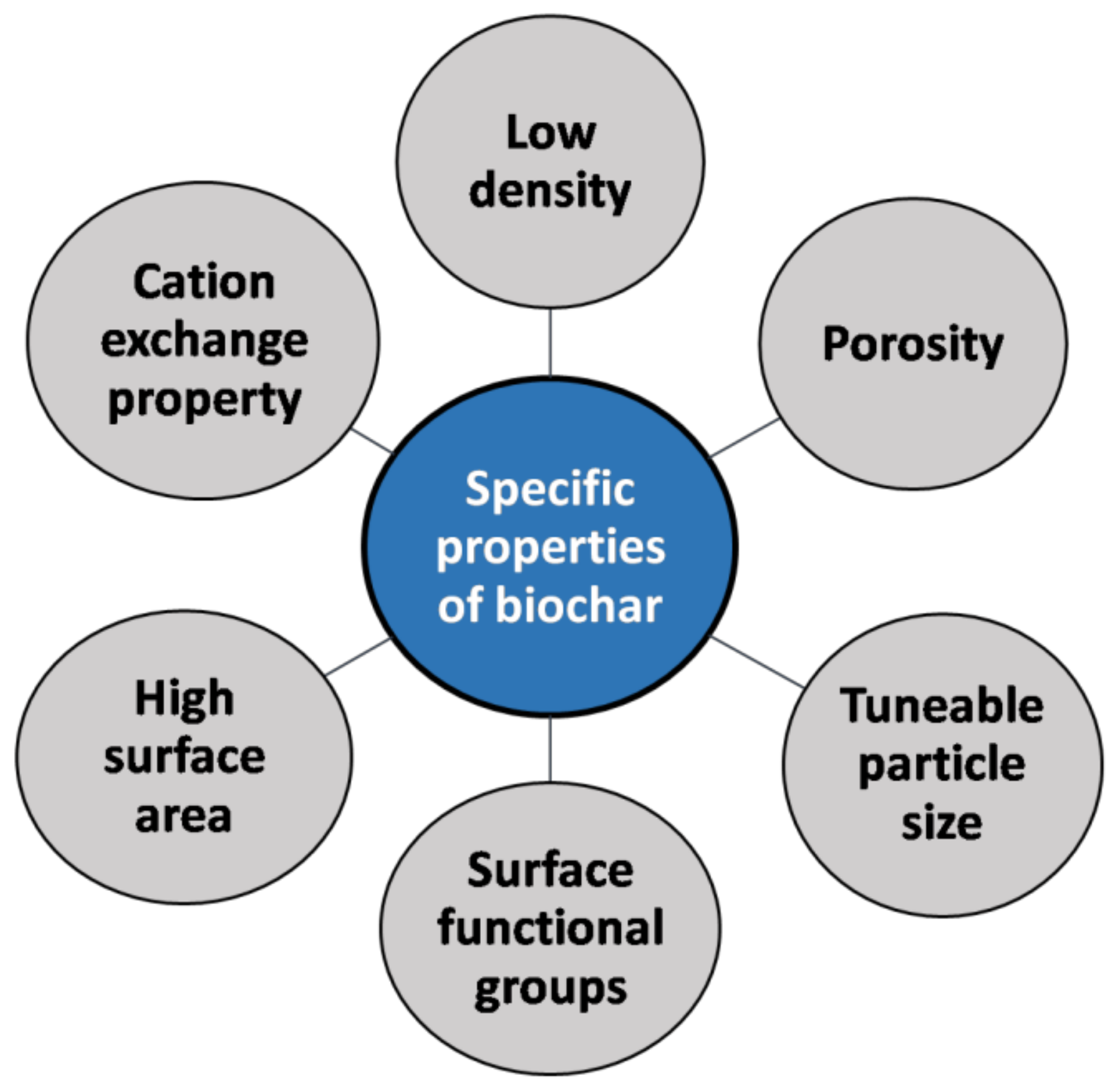
4. Properties of Biochar
5. Adsorption Mechanism
5.1. Complexation
5.2. Precipitation
5.3. Ion Exchange
5.4. Electrostatic Interaction
5.5. Hydrophobic Interaction
5.6. Pore-Filling Interaction
5.7. Hydrogen Bond Formation
6. Applications of Biochar
6.1. Remediation of Organic Pollutant
6.1.1. Remediation of Dyes
| Type of Pollutant | Source of Biochar | Time | Adsorbent Dosage (g/L) | Targeted Molecule | Efficiency | Ref. | |
|---|---|---|---|---|---|---|---|
| Dyes | Cationic | Cattle manure | 24 h | 1.25 | Methylene blue | 241.9 mg/g | [104] |
| Municipal waste | 360 min | 5 | Methylene blue | 7.2 mg/g | [105] | ||
| Rice husk | 2 h | 0.2 | Malachite green | 99.98% | [106] | ||
| Cactus | 210 min | 0.6 | Malachite green | 1341 mg/g | [107] | ||
| Date palm | 24 h | 1 | Crystal violet | 27.4 mg/g | [108] | ||
| Rice straw | 15 min | 0.001 | Methylene blue | 94.45% | [109] | ||
| 20 min | Crystal violet | 92.07% | |||||
| Animal waste | 4 h | 2.5 | Basic red 9 | 52.3 mg/g | [110] | ||
| Litchi peel | 12 h | 1 | Malachite green | 2468 mg/g | [47] | ||
| Anionic | Congo red | 404.4 mg/g | |||||
| Orange peel | 24 h | 3.0 | Congo red | 93% | [111] | ||
| Pine nutshell | 600 min | 0.4 | Acid chrome blue | 27.24 mg/g | [112] | ||
| Oil | Popped rice | 30 min | - | Kerosene | 6.51 g/g | [113] | |
| Goat hair | 120 min | 1.5 | Diesel | 466 mg/L | [114] | ||
| Crude oil | 510 mg/L | ||||||
| Kerosene | 367 mg/L | ||||||
| Petrol | 344, mg/L | ||||||
| Tyres | - | - | Crude oil | 8.89 g/g | [115] | ||
| Mango shell | 75 min | 50 | Crude oil | 95% | [116] | ||
| Crab shell | 240 min | 0.2 | Diesel oil | 93.9 mg/g | [42] | ||
| Textile sludge | - | - | Cooking oil | 120.1 mg/g | [117] | ||
| Coconut coir | 100 min | 12 | Crude oil | 99.9% | [118] | ||
| Water hyacinth | 60 min | 1 | Fuel oil | 80% | [119] | ||
| Commercially available | 60 min | 10 | Crude oil | 11 g/g | [120] | ||
| Other organic molecules | Wood chips | Naphthalene | 76% | [121] | |||
| Astragalusmongholicus | 12 h | 2 | Ciprofloxacin | 40.11 mg/g | [122] | ||
| Bull manure | 180 days | - | Lincomycin | 99% | [123] | ||
| Pine wood | 48 h | 0.5 | Tetracycline | 163 mg/g | [124] | ||
| Pine chips | 7 days | 0.05 | Ibuprofen | 20 µM | [125] | ||
| Pine saw dust | 4 h | 5 | p-nitrophenol | 99.61% | [126] | ||
| Paper sludge | 143 min | 4 | 2,4- dichlorophenol | 99.95% | [127] | ||
| Rice straw | 120 min | 1.2 | Tetracycline | 98.33 mg/g | [128] | ||
| Cotton gin waste | 1500 min | 5 | Sulphapyridine | 70%mg/g | [129] | ||
| Docusate | 98%mg/g | ||||||
| Erythromycin | 74%mg/g | ||||||
| Orange peel | 2 days | 6.25 | p-nitrotoluene | 110 mg/g | [130] | ||
| Sludge | 120 min | 0.1 | Sulfamethoxazole | 5.43 × 10−3 µg/g | [97] | ||
| Enteromorphaprolifera | 24 h | 0.1 | Pyrene | 93.5% | [131] | ||
| Oak | 14 days | 2 | Catechol | 59% | [132] | ||
| Wood | 30 min | - | Butylbenzyl phthalate | 105 µg/g | [133] | ||
| Soybean stover | 48 h | 0.3 | Trichloro ethylene | 25.38 mg/g | [134] | ||
| Rape stalk | 48 h | 0.05 | Tetracycline | 35.90 mg/g | [135] | ||
| Hair waste | 30 min | - | Amoxicillin | 90% | [136] | ||
| Diclofenac | 80% | ||||||
| Heavy metals | Corn cob | 24 h | 2 | Nitrate | 32.33 mg/g | [137] | |
| Sewage sludge | - | 10 | Cr | 3.0 mg/g | [138] | ||
| Pine bark | 5 h | - | Pb | 4.25 mg/g | [139] | ||
| Oak wood | - | - | Pb | 75.8 % | [140] | ||
| Cotton stack | - | - | Cd | 77.66% | [141] | ||
| Bamboo | 12 days | - | Cd | 79.6% | [142] | ||
| Yak manure | 48 h | 2 | Pb | 76.41 mg/g | [143] | ||
| Plantain peel | 150 min | 10 | Pb | 4 mg/g | [144] | ||
| Fish scale | 24 h | 2 | Cu | 39.39 mg/g | [44] | ||
| Hard wood | 24 h | 5 | Cu | 6.79 mg/g | [83] | ||
| Zn | 12.52 mg/g | ||||||
| Dairy manure | 24 h | 1.5 | Pb | 175.53 mg/g | [145] | ||
| Sugarcane straw | 24 h | 4 | Cd | 16 mg/g | [146] | ||
| Zn | 6 mg/g | ||||||
| Reed | 2 h | 16 | Cd | 86.8% | [147] | ||
| Pb | 83.5% | ||||||
| Walnut shell | 120 min | 3 | Ni | 13.25 mg/g | [148] | ||
| Rice husk | 24 h | - | As (III) | 85% | [149] | ||
6.1.2. Remediation of Oil
6.1.3. Remediation of Other Organic Contaminants
6.2. Remediation of Inorganic Pollutants
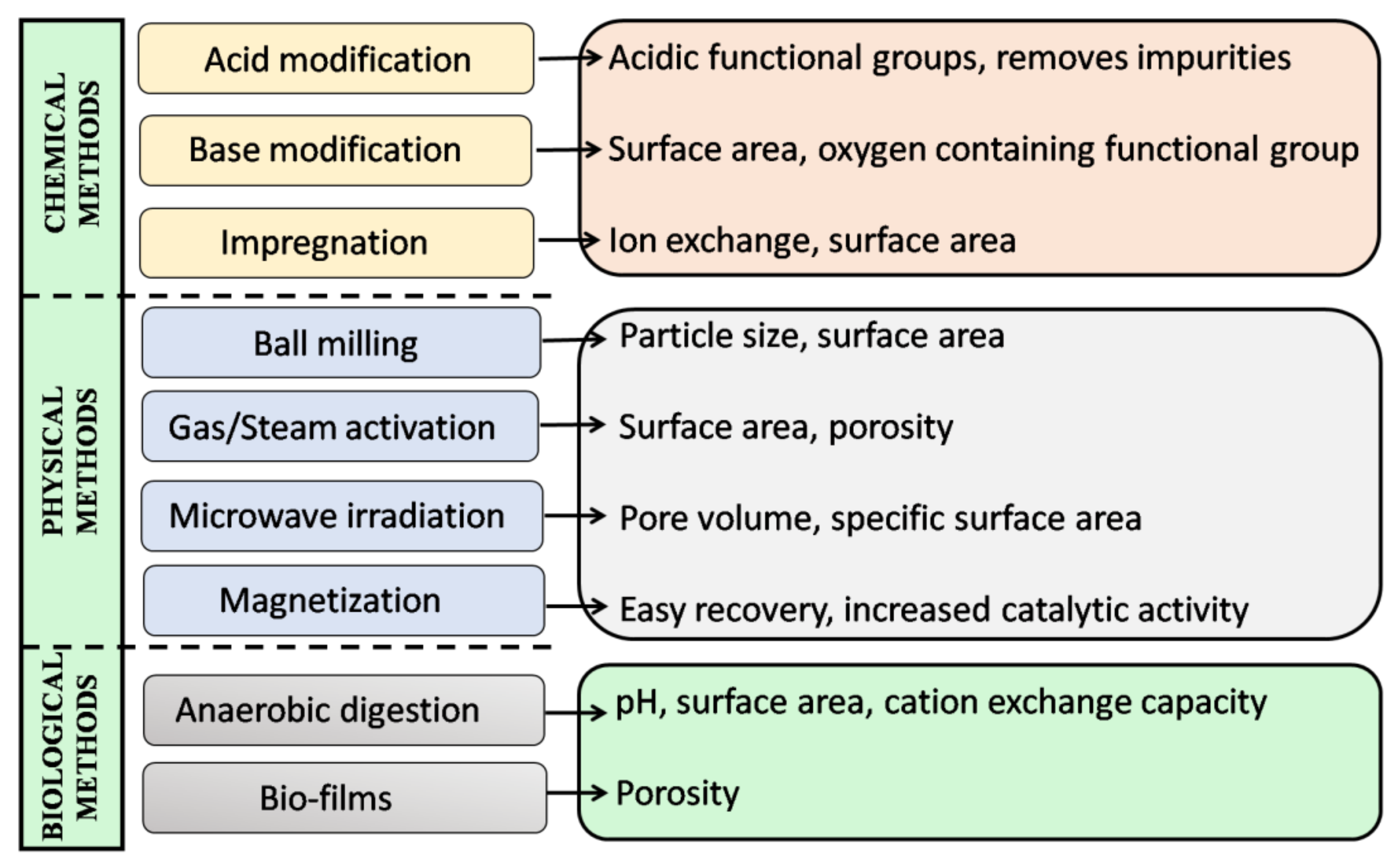
7. Engineered Biochar (EB)
8. Challenges and Future Research Direction with Regard to the Sustainable Development Goals (SDGs)
9. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambaye, T.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S.J. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2020, 18, 3273–3294. [Google Scholar] [CrossRef]
- Möbius, C.H. Adsorption and ion exchange processes for treatment of white water and waste water of paper mills. In Water Pollution Research and Development; Jenkins, S.H., Ed.; Pergamon: Oxford, UK, 1981; pp. 681–695. [Google Scholar]
- Trishitman, D.; Cassano, A.; Basile, A.; Rastogi, N.K. Reverse osmosis for industrial wastewater treatment. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–228. [Google Scholar]
- Fontanier, V.; Farines, V.; Albet, J.; Baig, S.; Molinier, J. Study of catalyzed ozonation for advanced treatment of pulp and paper mill effluents. Water Res. 2006, 40, 303–310. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Brandl, F.; Bertrand, N.; Lima, E.M.; Langer, R. Nanoparticles with photoinduced precipitation for the extraction of pollutants from water and soil. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, L.; Yang, F.; Bai, W.; Zhang, X.; Li, H.; Duan, G.; Xu, Y.; Li, Y. A bioinspired antibacterial and photothermal membrane for stable and durable clean water remediation. Mater. Horiz. 2023, 10, 268–276. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, J.; Zhang, X.; Yuan, D.; Duan, G.; Li, Y. Robust and multifunctional natural polyphenolic composites for water remediation. Mater. Horiz. 2022, 9, 2496–2517. [Google Scholar] [CrossRef]
- Jha, S.; Gaur, R.; Shahabuddin, S.; Ahmad, I.; Sridewi, N. Kinetic and Isothermal Investigations on the Use of Low Cost Coconut Fiber-Polyaniline Composites for the Removal of Chromium from Wastewater. Polymers 2022, 14, 4264. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Adsorption-oriented processes using conventional and non-conventional adsorbents for wastewater treatment. In Green Adsorbents for Pollutant Removal: Fundamentals and Design; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 23–71. [Google Scholar]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. Org. Pollut. Monit. Risk Treat. 2013, 7, 167–194. [Google Scholar]
- Kalita, E.; Baruah, J. Environmental remediation. In Colloidal Metal Oxide Nanoparticles; Thomas, S., Tresa Sunny, A., Velayudhan, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 525–576. [Google Scholar]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef]
- Sodha, V.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Bandyopadhyay, R.; Sridewi, N. Comprehensive Review on Zeolite-Based Nanocomposites for Treatment of Effluents from Wastewater. Nanomaterials 2022, 12, 3199. [Google Scholar] [CrossRef]
- Read, D.J.; Leake, J.R.; Perez-Moreno, J. Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Can. J. Bot. 2004, 82, 1243–1263. [Google Scholar] [CrossRef]
- Mahdi, Z.; El Hanandeh, A.; Yu, Q.J. Preparation, characterization and application of surface modified biochar from date seed for improved lead, copper, and nickel removal from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103379. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Sci. Total Environ. 2021, 765, 142673. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Raja, J.D.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Devi, S.M.; Rathika, B. A complete review on biochar: Production, property, multifaceted applications, interaction mechanism and computational approach. Fuel 2021, 292, 120243. [Google Scholar] [CrossRef]
- Nartey, O.D.; Zhao, B. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview. Adv. Mater. Sci. Eng. 2014, 2014, 715398. [Google Scholar] [CrossRef] [Green Version]
- Shivaram, P.; Leong, Y.K.; Yang, H.; Zhang, D. Flow and yield stress behaviour of ultrafine Mallee biochar slurry fuels: The effect of particle size distribution and additives. Fuel 2013, 104, 326–332. [Google Scholar] [CrossRef]
- Angın, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. Chapter 2—A mini review of biochar synthesis, characterization, and related standardization and legislation. In Applications of Biochar for Environmental Safety; Ahmed, A.A., Mohammed, H.H.A., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Filiberto, D.M.; Gaunt, J.L. Practicality of biochar additions to enhance soil and crop productivity. Agriculture 2013, 3, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.; Ranjan, R.; Ali, A.; Goyal, D.; Yadav, A.; Singh, T.B.; Shrivastav, P.; Dantu, P.K. Efficient transformation of agricultural waste in India. In Contaminants in Agriculture; Springer: Cham, Switzerland, 2020; pp. 271–287. [Google Scholar]
- Madurwar, M.V.; Ralegaonkar, R.V.; Mandavgane, S.A. Application of agro-waste for sustainable construction materials: A review. Constr. Build. Mater. 2013, 38, 872–878. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, M. Acid rain and its ecological consequences. J. Environ. Biol. 2007, 29, 15. [Google Scholar]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J. Crop residue burning in India: Policy challenges and potential solutions. Int. J. Environ. Res. Public Health 2019, 16, 832. [Google Scholar] [CrossRef] [Green Version]
- Adejumo, I.O.; Adebiyi, O.A. Chapter 10—Agricultural Solid Wastes: Causes, Effects, and Effective Management. In Strategies of Sustainable Solid Waste Management; Hosam, M.S., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Patil, P.D.; Yadav, G.D. Exploring the untapped potential of solar pretreatment for deconstruction of recalcitrant Kraft lignin in fungal biotransformation. Clean Technol. Environ. Policy 2019, 21, 579–590. [Google Scholar] [CrossRef]
- Hossain, N.; Nizamuddin, S.; Griffin, G.; Selvakannan, P.; Mubarak, N.M.; Mahlia, T.M.I. Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Hameed, K.S.; Sathyaseelan, N.; Rajeela, T.K.; Thomas, G.V. Biochars produced from coconut palm biomass residues can aid regenerative agriculture by improving soil properties and plant yield in humid tropics. Biochar 2020, 2, 211–226. [Google Scholar] [CrossRef] [Green Version]
- Sarfaraz, Q.; da Silva, L.S.; Drescher, G.L.; Zafar, M.; Severo, F.F.; Kokkonen, A.; Dal Molin, G.; Shafi, M.I.; Shafique, Q.; Solaiman, Z.M. Characterization and carbon mineralization of biochars produced from different animal manures and plant residues. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Badgujar, K.C.; Bhanage, B.M. Dedicated and waste feedstocks for biorefinery: An approach to develop a sustainable society. In Waste Biorefinery; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.J., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–38. [Google Scholar]
- Htun, K. Myanmar forestry outlook study. In Asia-Pacific Forestry Sector Outlook Study ΙΙ Working Paper Series; Working Paper No. APFSOS II/WP/2009/07; Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific: Bangkok, Thailand, 2009; pp. 10–23. [Google Scholar]
- Biswas, R.; Teller, P.J.; Ahring, B.K. Pretreatment of forest residues of Douglas fir by wet explosion for enhanced enzymatic saccharification. Bioresour. Technol. 2015, 192, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Lai, C.; Ke, G.; Chung, R.; Chen, C.; Cheng, C.; Pai, C.; Chen, S.; Chen, C. The effects of woodchip biochar application on crop yield, carbon sequestration and greenhouse gas emissions from soils planted with rice or leaf beet. J. Taiwan Inst. Chem. Eng. 2013, 44, 1039–1044. [Google Scholar] [CrossRef]
- Kanouo, B.M.D.; Allaire, S.E.; Munson, A.D. Quality of biochars made from Eucalyptus tree bark and corncob using a pilot-scale retort kiln. Waste Biomass Valorization 2018, 9, 899–909. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, M.; Sachdeva, S.; Puri, S. Aquatic weeds as the next generation feedstock for sustainable bioenergy production. Bioresour. Technol. 2018, 251, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gabhane, J.W.; Bhange, V.P.; Patil, P.D.; Bankar, S.T.; Kumar, S. Recent trends in biochar production methods and its application as a soil health conditioner: A review. SN Appl. Sci. 2020, 2, 1–21. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, Y.; Zhou, Y.; Zhang, X.; Ji, L.; Song, W.; Zhang, H.; Liu, J. Effective adsorption of diesel oil by crab-shell-derived biochar nanomaterials. Materials 2019, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.A.; Paul, N.A.; Dworjanyn, S.A.; Bird, M.I.; de Nys, R. Biochar from commercially cultivated seaweed for soil amelioration. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achieng, G.; Shikuku, V. Adsorption of copper ions from water onto fish scales derived biochar: Isothermal perspectives. J. Mater. Environ. Sci. 2020, 11, 1816–1827. [Google Scholar]
- Xing, R.; Qi, W.; Huber, G.W. Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the pulp and paper and cellulosic ethanol industries. Energy Environ. Sci. 2011, 4, 2193–2205. [Google Scholar] [CrossRef] [Green Version]
- Creamer, A.E.; Gao, B.; Zhang, M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Feng, P.; Huang, G.; Xu, C.; Lin, B. High-efficiency removal of dyes from wastewater by fully recycling litchi peel biochar. Chemosphere 2020, 246, 125734. [Google Scholar] [CrossRef]
- Hanoğlu, A.; Çay, A.; Yanık, J. Production of biochars from textile fibres through torrefaction and their characterisation. Energy 2019, 166, 664–673. [Google Scholar] [CrossRef]
- Xu, X.; Hu, X.; Ding, Z.; Chen, Y.; Gao, B. Waste-art-paper biochar as an effective sorbent for recovery of aqueous Pb (II) into value-added PbO nanoparticles. Chem. Eng. J. 2017, 308, 863–871. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Wei, J.; Tu, C.; Yuan, G.; Liu, Y.; Bi, D.; Xiao, L.; Lu, J.; Theng, B.K.; Wang, H.; Zhang, L. Assessing the effect of pyrolysis temperature on the molecular properties and copper sorption capacity of a halophyte biochar. Environ. Pollut. 2019, 251, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Daful, A.; Chandraratne, M. Biochar production from biomass waste-derived material. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Ghani, W.; Mohd, A.; da Silva, G.; Bachmann, R.T.; Taufiq-Yap, Y.H.; Rashid, U.; Ala’a, H. Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: Chemical and physical characterization. Ind. Crops Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Nhuchhen, D.; Afzal, M.; Dreise, T.; Salema, A. Characteristics of biochar and bio-oil produced from wood pellets pyrolysis using a bench scale fixed bed, microwave reactor. Biomass Bioenergy 2018, 119, 293–303. [Google Scholar] [CrossRef]
- Huang, Y.; Chiueh, P.; Lo, S. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Ingole, P.M.; Ranveer, A.C.; Deshmukh, S.M.; Deshmukh, S.K. Microwave assisted pyrolysis of biomass: A review. Int. J. Adv. Technol. Eng. Sci. 2016, 4, 78–84. [Google Scholar]
- Wang, Y.; Zeng, Z.; Tian, X.; Dai, L.; Jiang, L.; Zhang, S.; Wu, Q.; Wen, P.; Fu, G.; Liu, Y.J.B.t. Production of bio-oil from agricultural waste by using a continuous fast microwave pyrolysis system. Bioresour. Technol. 2018, 269, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Wilk, M.; Magdziarz, A. Hydrothermal carbonization, torrefaction and slow pyrolysis of Miscanthus giganteus. Energy 2017, 140, 1292–1304. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Wilske, B.; Buegger, F.; Esperschütz, J.; Kammann, C.I.; Eckhardt, C.; Koestler, M.; Kraft, P.; Bach, M.; Frede, H.G.; et al. Degradation kinetics of biochar from pyrolysis and hydrothermal carbonization in temperate soils. Plant Soil 2013, 372, 375–387. [Google Scholar] [CrossRef]
- Costa, F.F.; Wang, G.; Costa, M. Combustion kinetics and particle fragmentation of raw and torrified pine shells and olive stones in a drop tube furnace. Proc. Combust. Inst. 2015, 35, 3591–3599. [Google Scholar] [CrossRef]
- Pathomrotsakun, J.; Nakason, K.; Kraithong, W.; Khemthong, P.; Panyapinyopol, B.; Pavasant, P. Fuel properties of biochar from torrefaction of ground coffee residue: Effect of process temperature, time, and sweeping gas. Biomass Convers. Biorefinery 2020, 10, 743–753. [Google Scholar] [CrossRef]
- Wannapeera, J.; Worasuwannarak, N. Upgrading of woody biomass by torrefaction under pressure. J. Anal. Appl. Pyrolysis 2012, 96, 173–180. [Google Scholar] [CrossRef]
- Mamvura, T.A.; Danha, G. Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 2020, 6, e03531. [Google Scholar] [CrossRef]
- Wiedner, K.; Rumpel, C.; Steiner, C.; Pozzi, A.; Maas, R.; Glaser, B. Chemical evaluation of chars produced by thermochemical conversion (gasification, pyrolysis and hydrothermal carbonization) of agro-industrial biomass on a commercial scale. Biomass Bioenergy 2013, 59, 264–278. [Google Scholar] [CrossRef]
- Yang, W.; Li, C.; Wang, S.; Zhou, B.; Mao, Y.; Rensing, C.; Xing, S. Influence of biochar and biochar-based fertilizer on yield, quality of tea and microbial community in an acid tea orchard soil. Appl. Soil Ecol. 2021, 166, 104005. [Google Scholar] [CrossRef]
- Oh, T.K.; Shinogi, Y.; Lee, S.J.; Choi, B. Utilization of biochar impregnated with anaerobically digested slurry as slow-release fertilizer. J. Plant Nutr. Soil Sci. 2014, 177, 97–103. [Google Scholar] [CrossRef]
- Hunt, J.; DuPonte, M.; Sato, D.; Kawabata, A. The basics of biochar: A natural soil amendment. Soil Crop Manag. 2010, 30, 1–6. [Google Scholar]
- Krylovaa, A.Y.; Zaitchenkob, V. Hydrothermal carbonization of biomass: A review. Cellulose 2018, 40, 50. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.; Ong, P.; Wong, K.; Bong, C.; Li, C.; Gao, Y. A review on the comparison between slow pyrolysis and fast pyrolysis on the quality of lignocellulosic and lignin-based biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Zhang, H.; Aurangzeb, M.; Bing, W.; Zhang, Y. A Case Study of Bio-char Production from Biomass using Microwave Assisted Pyrolysis and its Utilization. Int. J. Eng. Work. 2018, 5, 87–95. [Google Scholar]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge. A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- Walling, E.; Babin, A.; Vaneeckhaute, C. Nutrient and carbon recovery from organic wastes. In Biorefinery: Integrated Sustainable Processes for Biomass Conversion to Biomaterials, Biofuels, and Fertilizers; Bastidas-Oyanedel, J.R., Schmidt, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 351–373. [Google Scholar]
- Evans, M.R.; Jackson, B.E.; Popp, M.; Sadaka, S. Chemical properties of biochar materials manufactured from agricultural products common to the Southeast United States. Horttechnology 2017, 27, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Kathrin, W.; Peter, Q. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Shackley, S.; Sohi, S.; Ibarrola, R.; Hammond, J.; Mašek, O.; Brownsort, P.; Cross, A.; Prendergast-Miller, M.; Haszeldine, S. Biochar, tool for climate change mitigation and soil management. In Geoengineering Responses to Climate Change; Springer: Berlin/Heidelberg, Germany, 2013; pp. 73–140. [Google Scholar]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, S.; Chen, H.; Huang, L.; Qiu, R. Pb (II) and Cr (VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions. Bioresour. Technol. 2013, 147, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. General Properties of Organometallic Complexes. In The Organometallic Chemistry of the Transition Metals; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 29–49. [Google Scholar]
- Harvey, O.R.; Herbert, B.E.; Rhue, R.D.; Kuo, L. Metal interactions at the biochar-water interface: Energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ. Sci. Technol. 2011, 45, 5550–5556. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Krauskoph, K. Introduction to Geochemistry; McGraw-Hill, Inc.: New York, NY, USA, 1967; p. 721. [Google Scholar]
- Puga, A.; Abreu, C.; Melo, L.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Ma, L.Q.; Li, Y. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef]
- Xie, L.; Yang, D.; Lu, Q.; Zhang, H.; Zeng, H. Role of molecular architecture in the modulation of hydrophobic interactions. Curr. Opin. Colloid Interface Sci. 2020, 47, 58–69. [Google Scholar] [CrossRef]
- Murray, C.C.; Vatankhah, H.; McDonough, C.A.; Nickerson, A.; Hedtke, T.T.; Cath, T.Y.; Higgins, C.P.; Bellona, C.L. Removal of per-and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J. Hazard. Mater. 2019, 366, 160–168. [Google Scholar] [CrossRef]
- Kandanelli, R.; Meesala, L.; Kumar, J.; Raju, C.S.K.; Peddy, V.R.; Gandham, S.; Kumar, P. Cost effective and practically viable oil spillage mitigation: Comprehensive study with biochar. Mar. Pollut. Bull. 2018, 128, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Kwon, S.; Lu, Y. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): Attenuation of surface activity by humic and fulvic acids. Environ. Sci. Technol. 2006, 40, 7757–7763. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, L.; Wu, L.; Li, P.; Qi, X.; He, L.; Cui, S.; Ding, Y.; Zhang, Z. Carbon nanotube supported sludge biochar as an efficient adsorbent for low concentrations of sulfamethoxazole removal. Sci. Total Environ. 2020, 718, 137299. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yuan, S.; Hong, M.; Zhang, L.; Huang, Q. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Tomul, F.; Ha, N.T.H.; Nguyen, D.T.; Lima, E.C.; Le, G.T.; Chang, C.T.; Masindi, V.; Woo, S.H. Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J. Hazard. Mater. 2020, 394, 122255. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, W.; Chen, Y.; Li, Y. Nickel aluminum layered double oxides modified magnetic biochar from waste corncob for efficient removal of acridine orange. Bioresour. Technol. 2020, 315, 123834. [Google Scholar] [CrossRef]
- Sewu, D.D.; Jung, H.; Kim, S.S.; Lee, D.S.; Woo, S.H. Decolorization of cationic and anionic dye-laden wastewater by steam-activated biochar produced at an industrial-scale from spent mushroom substrate. Bioresour. Technol. 2019, 277, 77–86. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an eco-friendly and economical adsorbent for the removal of colorants (dyes) from aqueous environment: A review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2020, 392, 123681. [Google Scholar] [CrossRef]
- Zhu, Y.; Yi, B.; Yuan, Q.; Wu, Y.; Wang, M.; Yan, S. Removal of methylene blue from aqueous solution by cattle manure-derived low temperature biochar. RSC Adv. 2018, 8, 19917–19929. [Google Scholar] [CrossRef] [Green Version]
- Hoslett, J.; Ghazal, H.; Mohamad, N.; Jouhara, H. Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef]
- Ganguly, P.; Sarkhel, R.; Das, P. Synthesis of pyrolyzed biochar and its application for dye removal: Batch, kinetic and isotherm with linear and non-linear mathematical analysis. Surf. Interfaces 2020, 20, 100616. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef] [PubMed]
- Chahinez, H.; Abdelkader, O.; Leila, Y.; Tran, H.N. One-stage preparation of palm petiole-derived biochar: Characterization and application for adsorption of crystal violet dye in water. Environ. Technol. Innov. 2020, 19, 100872. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.; Emran, M.; El-Sadek, M.; El-Shanshory, A.A.; Soliman, H.; Akl, M.; Rashad, M. Enhanced removal of cationic dye by eco-friendly activated biochar derived from rice straw. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Côrtes, L.; Druzian, S.; Streit, A.; Godinho, M.; Perondi, D.; Collazzo, G.; Oliveira, M.; Cadaval, T., Jr.; Dotto, G. Biochars from animal wastes as alternative materials to treat colored effluents containing basic red 9. J. Environ. Chem. Eng. 2019, 7, 103446. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Peng, W.; Wong, C.C.; Liew, R.K.; Ho, Y.L.; Mahari, W.A.W.; Azwar, E.; Yuan, T.Q.; Tabatabaei, M.; Aghbashlo, M. Engineered biochar via microwave CO2 and steam pyrolysis to treat carcinogenic Congo red dye. J. Hazard. Mater. 2020, 395, 122636. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Gao, Y. Cetyl trimethyl ammonium bromide modified magnetic biochar from pine nut shells for efficient removal of acid chrome blue K. Bioresour. Technol. 2020, 312, 123564. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, Y.; Yu, R. Popped rice biochar and superhydrophobic SiO2/popped rice biochar for oil adsorption. Silicon 2021, 13, 2661–2669. [Google Scholar] [CrossRef]
- Nduka, J.K.; Ezenweke, L.O.; Ezenwa, E.T. Comparison of the mopping ability of chemically modified and unmodified biological wastes on crude oil and its lower fractions. Bioresour. Technol. 2008, 99, 7902–7905. [Google Scholar] [CrossRef]
- Aisien, F.A.; Aisien, E.T. Application of activated recycled rubber from used tyres in oil spill clean up. Turk. J. Eng. Environ. Sci. 2012, 36, 171–177. [Google Scholar]
- Olufemi, B.A.; Otolorin, F. Comparative adsorption of crude oil using mango (Mangnifera indica) shell and mango shell activated carbon. Environ. Eng. Res. 2017, 22, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Sohaimi, K.S.A.; Ngadi, N. Removal of Oil using Activated Carbon from Textile Sludge Biochars. Proc. Appl. Mech. Mater. 2016, 818, 237–241. [Google Scholar] [CrossRef]
- Oboh, I.O.; Ukpong, A. Adsorption Studies of Oil Spill Clean-up Using Coconut Coir Activated Carbon. Am. J. Chem. Eng. 2020, 8, 36–47. [Google Scholar]
- Shokry, H.; Elkady, M.; Salama, E. Eco-friendly magnetic activated carbon nano-hybrid for facile oil spills separation. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Navarathna, C.M.; BombuwalaDewage, N.; Keeton, C.; Pennisson, J.; Henderson, R.; Lashley, B.; Zhang, X.; Hassan, E.B.; Perez, F.; Mohan, D.; et al. Biochar adsorbents with enhanced hydrophobicity for oil spill removal. ACS Appl. Mater. Interfaces 2020, 12, 9248–9260. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Evaluation of biochar as a potential filter media for the removal of mixed contaminants from urban storm water runoff. J. Environ. Eng. 2014, 140, 4014043. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Kong, X.; He, L.; Li, W.; Liao, Q. Low-cost biochar derived from herbal residue: Characterization and application for ciprofloxacin adsorption. Int. J. Environ. Sci. Technol. 2016, 13, 2449–2458. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chuang, Y.; Li, H.; Teppen, B.J.; Boyd, S.A.; Gonzalez, J.M.; Johnston, C.T.; Lehmann, J.; Zhang, W. Sorption of lincomycin by manure-derived biochars from water. J. Environ. Qual. 2016, 45, 519. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhu, X.; He, H.; Fang, Y.; Dong, H.; Lü, J.; Li, J.; Li, Y. Adsorption of two antibiotics on biochar prepared in air-containing atmosphere: Influence of biochar porosity and molecular size of antibiotics. J. Mol. Liq. 2019, 274, 353–361. [Google Scholar] [CrossRef]
- Jung, C.; Park, J.; Lim, K.H.; Park, S.; Heo, J.; Her, N.; Oh, J.; Yun, S.; Yoon, Y. Adsorption of selected endocrine disrupting compounds and pharmaceuticals on activated biochars. J. Hazard. Mater. 2013, 263, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, G.; Shi, X. Adsorption characteristics and mechanism of p-nitrophenol by pine sawdust biochar samples produced at different pyrolysis temperatures. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kalderis, D.; Kayan, B.; Akay, S.; Kulaksız, E.; Gözmen, B. Adsorption of 2, 4-dichlorophenol on paper sludge/wheat husk biochar: Process optimization and comparison with biochars prepared from wood chips, sewage sludge and hog fuel/demolition waste. J. Environ. Chem. Eng. 2017, 5, 2222–2231. [Google Scholar] [CrossRef]
- Dai, J.; Meng, X.; Zhang, Y.; Huang, Y. Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour. Technol. 2020, 311, 123455. [Google Scholar] [CrossRef]
- Ndoun, M.C.; Elliott, H.A.; Preisendanz, H.E.; Williams, C.F.; Knopf, A.; Watson, J.E. Adsorption of pharmaceuticals from aqueous solutions using biochar derived from cotton gin waste and guayule bagasse. Biochar 2021, 3, 89–104. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S.J.B.T. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Tian, W.; Bai, J.; Dong, J.; Zhao, J.; Gong, X.; Liu, S. Preparation of biochar from Enteromorpha prolifera and its use for the removal of polycyclic aromatic hydrocarbons (PAHs) from aqueous solution. Ecotoxicol. Environ. Saf. 2018, 149, 80–87. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef]
- Sun, K.; Jin, J.; Keiluweit, M.; Kleber, M.; Wang, Z.; Pan, Z.; Xing, B. Polar and aliphatic domains regulate sorption of phthalic acid esters (PAEs) to biochars. Bioresour. Technol. 2012, 118, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover-and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, X.; Wang, L.; Gao, B.; Luo, J.; Fang, R.; Zou, W.; Meng, N. Sorption of tetracycline on H2O2-modified biochar derived from rape stalk. Environ. Pollut. Bioavailab. 2019, 31, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, F.; Montoya-Ruiz, C.; Estiati, I.; Saldarriaga, J.F. Removal of drugs in polluted waters with char obtained by pyrolysis of hair waste from the tannery process. ACS Omega 2020, 5, 24389–24402. [Google Scholar] [CrossRef]
- Long, L.; Xue, Y.; Hu, X.; Zhu, Y. Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar. Environ. Sci. Pollut. Res. 2019, 26, 3065–3074. [Google Scholar] [CrossRef]
- Otero, M.; Rozada, F.; Morán, A.; Calvo, L.; García, A. Removal of heavy metals from aqueous solution by sewage sludge based sorbents: Competitive effects. Desalination 2009, 239, 46–57. [Google Scholar] [CrossRef]
- Patra, J.; Panda, S.; Dhal, N. Biochar as a low-cost adsorbent for heavy metal removal: A review. Int. J. Res. Biosci. 2017, 6, 1–7. [Google Scholar]
- Ahmad, M.; Lee, S.S.; Yang, J.E.; Ro, H.-M.; Lee, Y.H.; Ok, Y.S. Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol. Environ. Saf. 2012, 79, 225–231. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, C.; Chen, J.; Zhang, Q. Remediation effects of cotton stalk carbon on cadmium (Cd) contaminated soil. Ecol. Environ. 2008, 17, 1857–1860. [Google Scholar]
- Ma, J.W.; Wang, H.; Luo, Q.S. Movement-adsorption and its mechanism of Cd in soil under combining effects of electrokinetics and a new type of bamboo charcoal. Huan Jing Ke Xue Huanjing Kexue 2007, 28, 1829–1834. [Google Scholar]
- Wang, Y.; Liu, R. H2O2 treatment enhanced the heavy metals removal by manure biochar in aqueous solutions. Sci. Total Environ. 2018, 628, 1139–1148. [Google Scholar] [CrossRef]
- Nworie, F.; Oroke, E.; Ikelle, I.; Nworu, J. Equilibrium and Kinetic Studies for the Adsorptive Removal of Lead (II) Ions from Aqueous Solution Using Activated Plantain Peel Biochar. Acta Chem. Malays. 2020, 4, 9–16. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Huang, L.; Yuan, Z.; Li, Z.; Liu, M. Removal of Cd and Pb with biochar made from dairy manure at low temperature. J. Integr. Agric. 2019, 18, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Melo, L.C.; Coscione, A.R.; Abreu, C.A.; Puga, A.P.; Camargo, O.A. Influence of pyrolysis temperature on cadmium and zinc sorption capacity of sugar cane straw–derived biochar. Bioresources 2013, 8, 4992–5004. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Chen, T.; Yin, C.; Yan, J.; Ippolito, J.A.; Hussain, Q. Mechanism of adsorption of cadmium and lead ions by iron-activated biochar. BioResources 2019, 14, 842–857. [Google Scholar] [CrossRef]
- Georgieva, V.G.; Gonsalvesh, L.; Tavlieva, M.P. Thermodynamics and kinetics of the removal of nickel (II) ions from aqueous solutions by biochar adsorbent made from agro-waste walnut shells. J. Mol. Liq. 2020, 312, 112788. [Google Scholar] [CrossRef]
- Singh, P.; Sarswat, A.; Pittman, C.U., Jr.; Mlsna, T.; Mohan, D. Sustainable low-concentration arsenite [As (III)] removal in single and multicomponent systems using hybrid iron oxide–biochar nanocomposite adsorbents—A mechanistic study. Acs Omega 2020, 5, 2575–2593. [Google Scholar] [CrossRef]
- AlAmeri, K.; Giwa, A.; Yousef, L.; Alraeesi, A.; Taher, H. Sorption and removal of crude oil spills from seawater using peat-derived biochar: An optimization study. J. Environ. Manag. 2019, 250, 109465. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.; Chen, Y.; Gao, J. Biochar produced from cotton husks and its application for the adsorption of oil products. IOP Conf. Ser. Earth Environ. Sci. 2020, 545, 12022. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Choi, Y.K.; Kim, H.J.; Song, H.-S.; Park, S.L.; Lee, H.S.; Lee, S.M.; Choi, K.Y. Adsorptive removal of crude petroleum oil from water using floating pinewood biochar decorated with coconut oil-derived fatty acids. Sci. Total Environ. 2021, 781, 146636. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Khare, P.; Dilshad, U.; Rout, P.; Yadav, V.; Jain, S. Plant refuses driven biochar: Application as metal adsorbent from acidic solutions. Arab. J. Chem. 2017, 10, S3054–S3063. [Google Scholar] [CrossRef] [Green Version]
- Mohan, D.; Pittman, C.U., Jr.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H.; et al. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; ur Rehman, M.Z.; Al-Wabel, M.I. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 1–23. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent advances in engineered biochar productions and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Qiu, Y.; Zheng, Z.; Zhou, Z.; Sheng, G.D. Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour. Technol. 2009, 100, 5348–5351. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, T.; Naushad, M.; Alshehri, S.M. Ultra-fast spill oil recovery using a mesoporous lignin based nanocomposite prepared from date palm pits (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2019, 130, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Nazifa, T.; Hadibarata, T.; Yuniarto, A.; Elshikh, M.; Syafiuddin, A. Equilibrium, kinetic and thermodynamic analysis petroleum oil adsorption from aqueous solution by magnetic activated carbon. IOP Conf. Ser. Mater. Sci. Eng. 2019, 495, 12060. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, P.; Bogusz, A.; Dobrzyńska, J.; Dobrowolski, R.; Oleszczuk, P.J. Engineered biochar modified with iron as a new adsorbent for treatment of water contaminated by selenium. J. Saudi Chem. Soc. 2020, 24, 824–834. [Google Scholar] [CrossRef]
- Qin, Y.; Zhu, X.; Su, Q.; Anumah, A.; Gao, B.; Lyu, W.; Zhou, X.; Xing, Y.; Wang, B. Enhanced removal of ammonium from water by ball-milled biochar. Environ. Geochem. Health 2020, 42, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Luyen, N.T.; Linh, H.X.; Huy, T.Q. Preparation of rice husk biochar-based magnetic nanocomposite for effective removal of crystal violet. J. Electron. Mater. 2020, 49, 1142–1149. [Google Scholar] [CrossRef]
- Cho, D.W.; Yoon, K.; Kwon, E.E.; Biswas, J.K.; Song, H. Fabrication of magnetic biochar as a treatment medium for As (V) via pyrolysis of FeCl3-pretreated spent coffee ground. Environ. Pollut. 2017, 229, 942–949. [Google Scholar] [CrossRef]
- Lee, J.; Cho, W.C.; Poo, K.M.; Choi, S.; Kim, T.N.; Son, E.B.; Choi, Y.J.; Kim, Y.M.; Chae, K. Refractory oil wastewater treatment by dissolved air flotation, electrochemical advanced oxidation process, and magnetic biochar integrated system. J. Water Process Eng. 2020, 36, 101358. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Li, K.; Mo, Y.; Li, H.; Gu, Z. Combined performance of biochar sorption and magnetic separation processes for treatment of chromium-contained electroplating wastewater. Bioresour. Technol. 2014, 174, 67–73. [Google Scholar] [CrossRef]
- Li, S.; Chan, C.Y.; Sharbatmaleki, M.; Trejo, H.; Delagah, S. Engineered biochar production and its potential benefits in a closed-loop water-reuse agriculture system. Water 2020, 12, 2847. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2019, 35, 777–815. [Google Scholar] [CrossRef]
- Hu, X.; Xue, Y.; Long, L.; Zhang, K. Characteristics and batch experiments of acid-and alkali-modified corncob biomass for nitrate removal from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 19932–19940. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wei, C.; Zhang, S.; Wang, Y.; Kuzyakov, Y.; Ding, X. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J. Clean. Prod. 2019, 235, 901–909. [Google Scholar] [CrossRef]
- Premarathna, K.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Dalahmeh, S.; Ahrens, L.; Gros, M.; Wiberg, K.; Pell, M. Potential of biochar filters for onsite sewage treatment: Adsorption and biological degradation of pharmaceuticals in laboratory filters with active, inactive and no biofilm. Sci. Total Environ. 2018, 612, 192–201. [Google Scholar] [CrossRef]
- Youngwilai, A.; Kidkhunthod, P.; Jearanaikoon, N.; Chaiprapa, J.; Supanchaiyamat, N.; Hunt, A.J.; Ngernyen, Y.; Ratpukdi, T.; Khan, E.; Siripattanakul-Ratpukdi, S. Simultaneous manganese adsorption and biotransformation by Streptomyces violarus strain SBP1 cell-immobilized biochar. Sci. Total Environ. 2020, 713, 136708. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, S.; Gaur, R.; Shahabuddin, S.; Tyagi, I. Biochar as Sustainable Alternative and Green Adsorbent for the Remediation of Noxious Pollutants: A Comprehensive Review. Toxics 2023, 11, 117. https://doi.org/10.3390/toxics11020117
Jha S, Gaur R, Shahabuddin S, Tyagi I. Biochar as Sustainable Alternative and Green Adsorbent for the Remediation of Noxious Pollutants: A Comprehensive Review. Toxics. 2023; 11(2):117. https://doi.org/10.3390/toxics11020117
Chicago/Turabian StyleJha, Stuti, Rama Gaur, Syed Shahabuddin, and Inderjeet Tyagi. 2023. "Biochar as Sustainable Alternative and Green Adsorbent for the Remediation of Noxious Pollutants: A Comprehensive Review" Toxics 11, no. 2: 117. https://doi.org/10.3390/toxics11020117







