Zinc Phosphide Poisoning: From A to Z
Abstract
:1. Introduction
2. Case Description
3. Discussion
3.1. Decontamination Measures and Absorption Reduction
3.2. Initial Approach of Poisoned Patient
3.3. Support Measures
3.4. Antioxidants
3.5. Magnesium
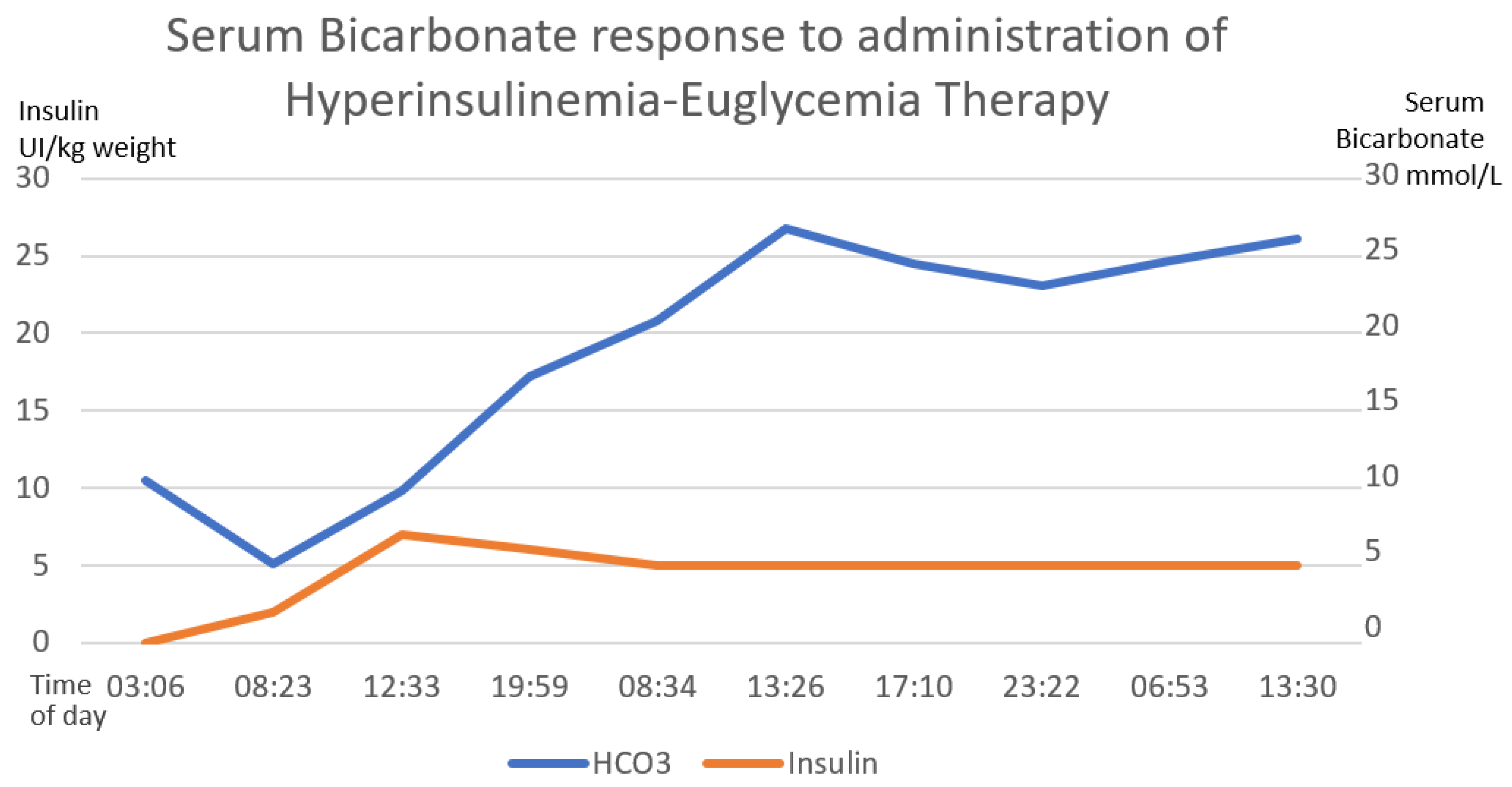
3.6. Hyperinsulinemia–Euglycemia Therapy
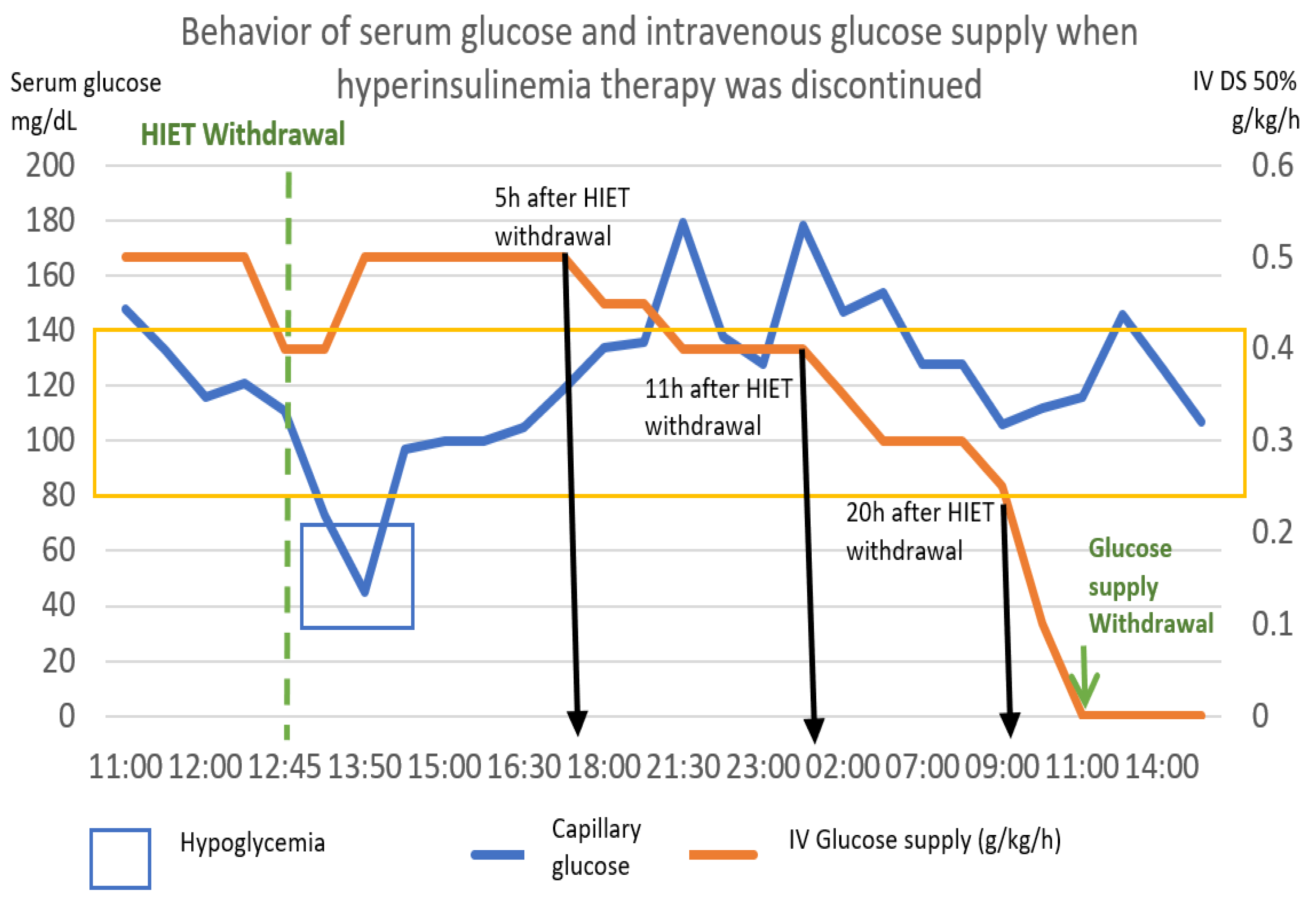
Reduction in Hyperinsulinemia–Euglycemia Therapy
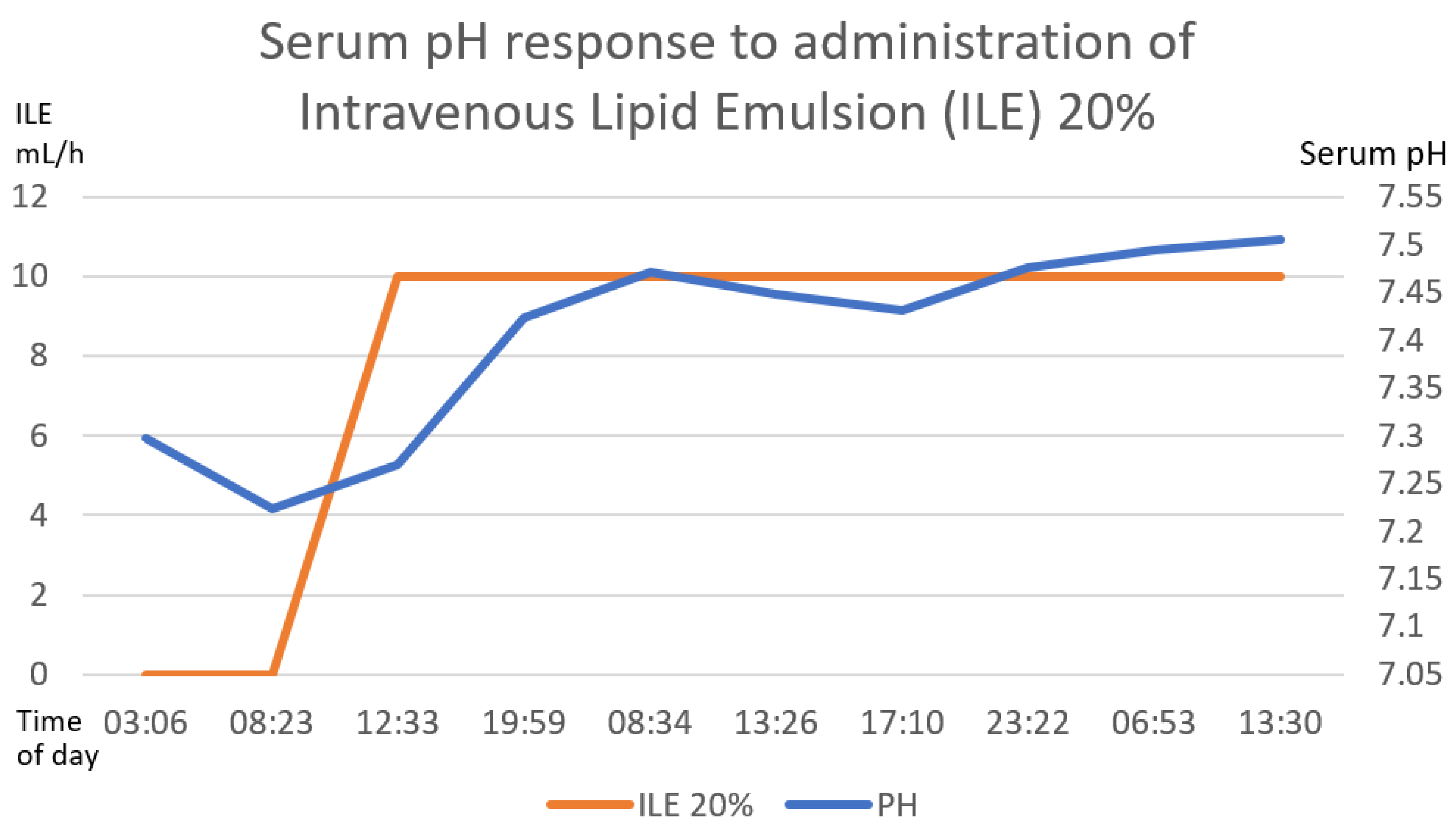
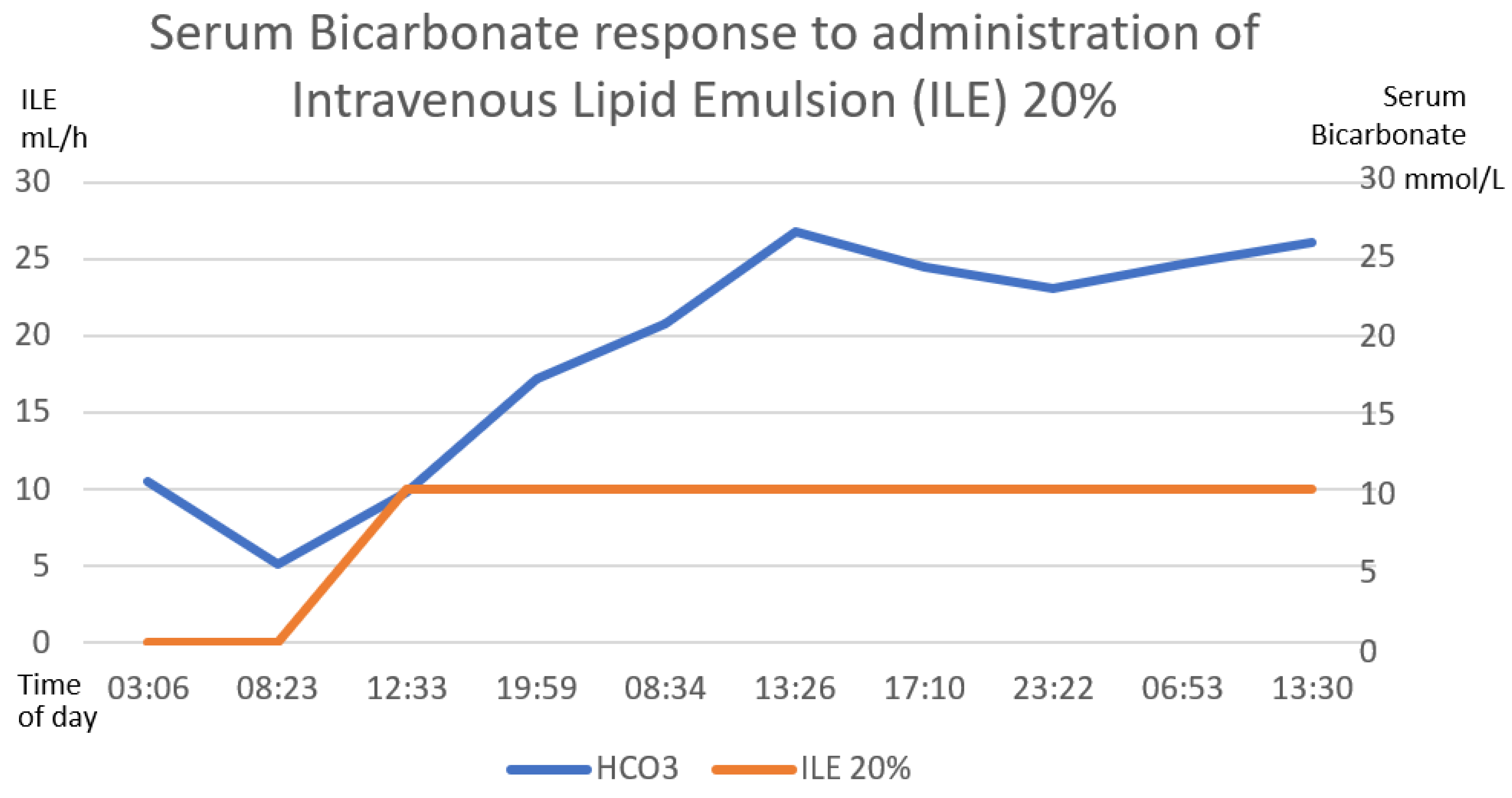
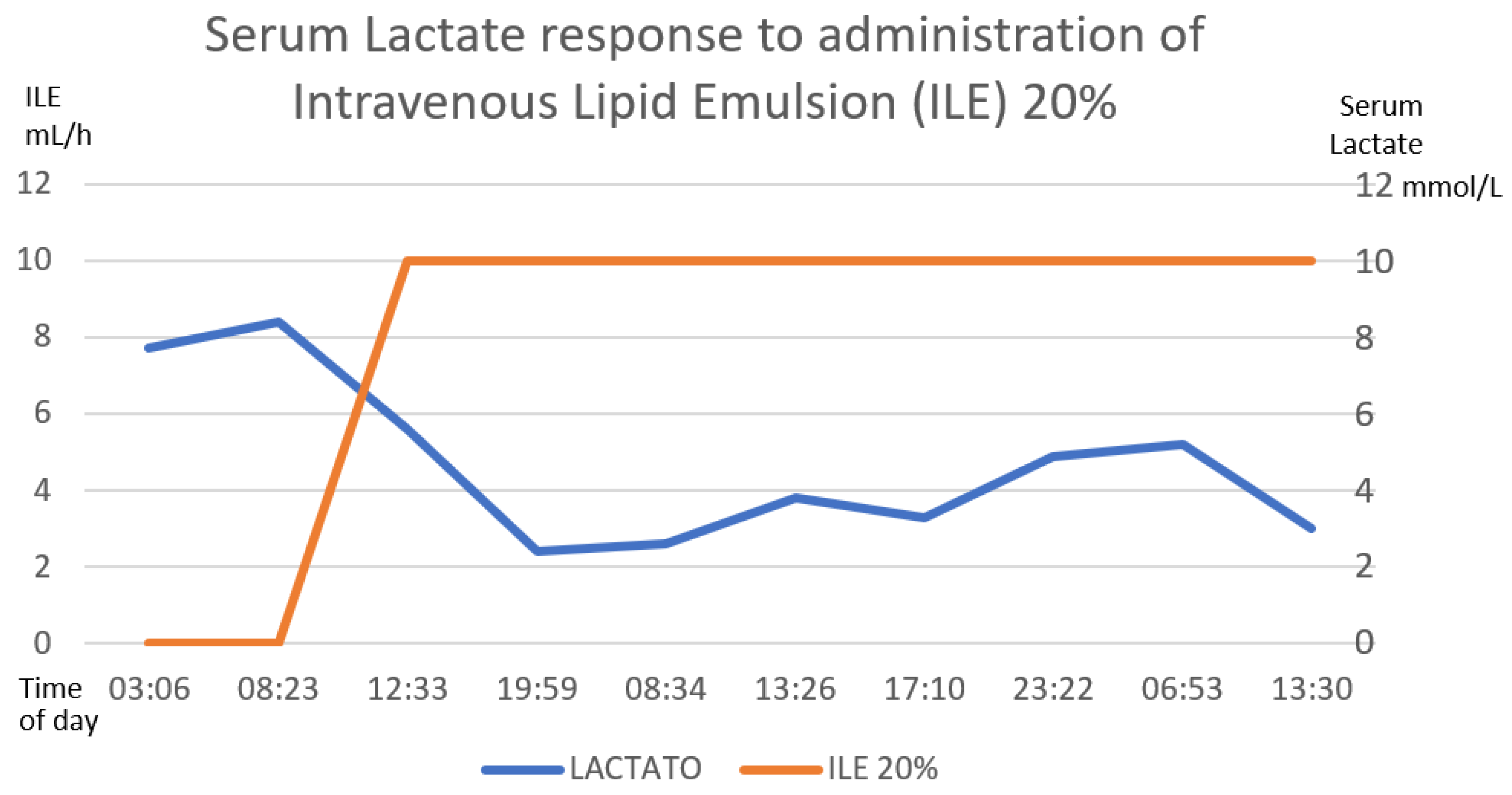
3.7. Intravenous Lipid Emulsion
3.8. Other Therapeutic Strategies
Other Drugs Described in the Literature
4. Analysis of Therapeutic in a Real Clinical Scenario
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çakın, Ö.; Tazegul, G.; Gümüş, A.; Cengiz, M.; Ramazanoğlu, A. Incidental Aluminum Phosphide Poisoning: Case Report and Current Management. Folia Med. 2018, 60, 464–467. [Google Scholar] [CrossRef]
- Moghadamnia, A. An update on toxicology of aluminum phosphide. DARU J. Pharm. Sci. 2012, 20, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catálogo Oficial de Plaguicidas 1991; Comisión intersecretarial para el control del proceso y uso de plaguicidas, fertilizantes y sustancias toxicas: Ciudad de Mexico, Mexico, 1991.
- Fernando Bejarano González. Los Plaguicidas Altamente Peligrosos en México, Primera edición, julio 2017. Red de Acción Sobre Plaguicidas y Alternativas en México, A.C. (RAPAM). Available online: https://www.rapam.org/wp-content/uploads/2017/09/Libro-Plaguicidas-Final-14-agst-2017sin-portada.pdf (accessed on 10 April 2023).
- Comisión Federal Para la Protección Contra Riesgos Sanitarios (COFEPRIS). Plaguicidas 2015. Available online: http://www.cofepris.gob.mx/AZ/Paginas/PlaguicidasyFertilizantes/PlaguicidasYFertilizantes.aspx (accessed on 29 May 2022).
- Karimania, A.; Hooshang, A.; Reza, M.; Rezaee, R.; Megabane, B.; Tsatsakis, A.; Karimi, G. Antidotes for aluminum phosphide poisoning An update. Toxicol. Rep. 2018, 5, 1053–1059. [Google Scholar] [CrossRef]
- Anbalagan, L.C.; Arora, N.; Pannu, A.K. Management of Acute Aluminum Phosphide Poisoning: Has Anything Changed? Drug Metab. Lett. 2021, 14, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Enviromental Health Criteria 73. Phosphine and Selected Metal Phosphides, IPCS International Programme on Chemical Safety; World Health Organization: Geneva, Switzerland, 1988.
- Vij, K. Textbook of Forensic Medicine and Toxicology-Principles and Practice, 4th ed.; Elsevier-A Division of Reed Elsevier India Private Limited: New Delhi, India, 2008. [Google Scholar]
- Gurvinder, S.B. Phosphide poisoning: A review of literature. Forensic Sci. Int. 2012, 214, 1–6. [Google Scholar] [CrossRef]
- Sánchez, M.; Bárcena, A. Intoxicación con fosfuro de zinc en el paciente pediátrico en un centro toxicológico de la Ciudad de México. Rev. Médica Inst. Mex. Seguro Soc. 2017, 55, 44–52. [Google Scholar]
- López Islas, I.; Juan, J.; Nuevo, L. Edema Agudo Pulmonar No Cardiogénico en Pacientes con Intoxicación por Fosfuro de Cinc. Reporte de dos Casos y Revisión Bibliográfica. Med. Intern. Mex. 2008, 24, 424–427. Available online: https://www.medigraphic.com/pdfs/medintmex/mim-2008/mim086i.pdf (accessed on 6 August 2022).
- Watson, W.A.; Litovitz, T.L.; Klein-Schwartz, W.; Rodgers, G.C.; Youniss, J.; Reid, N.; Youniss, J.; Flanagan, A.; Wruk, K.M. Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am. J. Emerg. Med. 2004, 22, 335–404. [Google Scholar] [CrossRef]
- Mehrpour, O.; Jafarzadeh, M.; Abdollahi, M. A Systematic Review of Aluminium Phosphide Poisoning. Arch. Ind. Hyg. Toxicol. 2012, 63, 61–73. [Google Scholar] [CrossRef]
- Pérez Navero, J.L.; Ibarra de la Rosa, I.; Frías Pérez, M.A.; Arroyo Marín, M.J.; Pérez Jorge, P. Intoxicación letal por inhalación accidental de fosfuro alumínico. An. Pediatría 2009, 71, 427–431. [Google Scholar] [CrossRef]
- Mehrpour, O.; Asadi, S.; Yaghoubi, M.A.; Azdaki, N.; Mahmoodabadi, N.; Javadmoosavi, S. Cardiogenic Shock Due to Aluminum Phosphide Poisoning Treated with Intra-aortic Balloon Pump: A Report of Two Cases. Cardiovasc. Toxicol. 2019, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Pannu, A.; Bhalla, A.; Gantala, N.; Shafrma, S.; Kumar, S.; Dhibar, D. Glucose-insulin-potassium infusion for the treatment of acute aluminum phosphide poisoning: An open-label pilot study. Clin. Toxicol. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hassanian-Moghaddam, H.; Zamani, N. Therapeutic role of hyperinsulinemia/euglycemia in aluminum phosphide poisoning. Clin. Trial/Exp. Study Med. 2016, 95, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shadnia, S.; Rahimi, M.; Pajoumand, A.; Rasouli, M.-H.; Abdollahi, M. Successful treatment of acute aluminium phosphide poisoning:Possible benefit of coconut oil. Hum. Exp. Toxicol. 2005, 24, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Sasser, S.M. Rodenticides. In Emergency Toxicology, 11th ed.; Viccellio, P., Bania, T., Brent, J., Hoffman, R.S., Kulig, K.W., Mofenson, H.C., Osborn, H.H., Wang, R.Y., Wax, P.M., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 2019; Volume 1528. [Google Scholar]
- Chugh, S.N.; Kolley, T.; Kakkar, R.; Chugh, K.; Sharma, A. A critical evaluation of anti-peroxidant effect of intravenous magnesium in acute aluminium phosphide poisoning. Magnes. Res. 1997, 10, 225–230. Available online: https://europepmc.org/article/med/94834831 (accessed on 16 October 2022).
- Chugh, S.N.; Arora, V.; Sharma, A.; Chugh, K. Free Radical Scavengers & Lipid Peroxidation in Acute Aluminium Phosphide Poisoning. Indian J. Med. Res. 1996, 104, 190–193. [Google Scholar]
- Petrovic, M.; Otero, D.; Leigh, A.; Singh, V. Acute Hearth Failure Due to Aluminium Phosphide Poisoning. Methidist DeBakey Cardiovasc. J. 2021, 17, 6–12. [Google Scholar] [CrossRef]
- Sudakin, D.L. Occupational exposure to aluminium phosphide and phosphine gas? A suspected case report and review of the literature. Hum. Exp. Toxicol. 2005, 24, 27–33. [Google Scholar] [CrossRef]
- Bogle, R.G. Aluminium phosphide poisoning. Emerg. Med. J. 2006, 23, 1–2. [Google Scholar] [CrossRef]
- Udismita, B.; Ameeta, S.; Harish, S. Successful management of aluminium phosphide poisoning using intravenous lipid emulsion: Report of two cases. Indian J. Crit. Care Med. 2015, 19, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, S.S.; Bajwa Kaur, S.; Kaur, J.; Singh, K.; Panda, A. Management of celphos poisoning with a novel intervention: A ray of hope in the darkest of clouds. Anesth. Essays Res. 2010, 4, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Chyka, P.A.; Seger, D.; Krenzelok, E.P.; Vale, J.A. Position Paper: Single-Dose Activated Charcoal. American Academy of Clinical Toxicology & European Association of Poisons Centers and Clinical Toxicologists. Clin. Toxicol. 2005, 43, 61–87. [Google Scholar] [CrossRef]
- Corcoran, J.N.; Jacoby, K.J.; Olives, T.D.; Bangh, S.A.; Cole, J.B. In Reply: “On Insulin Kinetics Following High-Dose Insulin Therapy, and When to Stop Therapy”. J. Med. Toxicol. 2021, 17, 235–236. [Google Scholar] [CrossRef]
- Saraswat, P.K.; Gupta, B.P.; Malhotra, V.K.; Goyal, V.K. Prevalence of fatalities due to aluminium phosphide poisoning in Southern Rajasthan (an epidemiological study). J. Forensic Med. Toxicol 1985, 11, 1–7. [Google Scholar]
- Dorado García, R.; Soto Estrada, M.; Ontiveros Holguín, A. 10 Errores Graves en el Manejo del Paciente Intoxicado. Crit. Care Emerg. Med. 2022, 1, 1–7. [Google Scholar] [CrossRef]
- Burket, G.; Zane, B.; Hendrickson, R.; Beauchamp, G. Endotraqueal Intubation in the Pharmaceutical-Poisoned Patient: A Narrative Review of the Literatura. J. Med. Toxicol. 2020, 17, 61–69. [Google Scholar] [CrossRef]
- Stephen, V.; Pluymers, N.; Gauton, S. Emergency management of calcium channel blocker overdose. Afr. Med. 2019, 109, 635–638. [Google Scholar] [CrossRef] [Green Version]
- Marashi, S.; Nasri, Z. Can sodium bicarbonate really help in treating metabolic acidosis caused by aluminium phosphide poisoning? Arh. Hig. Rada Toksikol. 2015, 66, 83–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoog, C.; Engebretsen, K. Are vasopressors useful in toxin-induced cardiogenic shock? Clin. Toxicol. 2017, 55, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Karami-Mohajeri, S. A comprehensive review on experimental and clinical findings in intermediate syndrome caused by organophosphate poisoning. Toxicol. Appl. Pharmacol. 2012, 258, 309–314. [Google Scholar] [CrossRef]
- Azad, A.; Lall, S.B.; Mittra, S. Effect of N-acetylcysteine and L-NAME on aluminium phosphide induced cardiovascular toxicity in rats. [Internet]. Acta Pharmacol. Sin. 2001, 22, 298–304. Available online: https://pubmed.ncbi.nlm.nih.gov/11742581/ (accessed on 12 December 2022).
- Aliasghar, M.; Ahangar, R.M.; Pedram, B.; Samaneh, N.; Omid, M. A case of successful treatment of heart failure due to simultaneous poisoning with aluminum phosphide and zinc phosphide, Iran. Red Crescent Med. J. 2018, in press. [Google Scholar] [CrossRef]
- Tehrani, H.; Halvaie, Z.; Shadnia, S.; Soltaninejad, K.; Abdollahi, M. Protective effects of N-acetylcysteine on aluminum phosphide-induced oxidative stress in acute human poisoning. Clin. Toxicol. 2012, 51, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Siwach, S.B.; Singh, P.; Ahlawat, S.; Dua, A.; Sharma, D. Serum & tissue magnesium content in patients of aluminium phosphide poisoning and critical evaluation of high dose magnesium sulphate therapy in reducing mortality. [Internet]. J. Assoc. Physicians India 1994, 42, 107–110. Available online: https://pubmed.ncbi.nlm.nih.gov/7860467/ (accessed on 8 December 2022).
- Chugh, S.N.; Kamar, P.; Sharma, A.; Chugh, K.; Mittal, A.; Arora, B. Magnesium status and parenteral magnesium sulphate therapy in acute aluminum phosphide intoxication. [Internet]. Magnes. Res. 1994, 7, 289–294. Available online: https://pubmed.ncbi.nlm.nih.gov/7786693 (accessed on 8 December 2022). [PubMed]
- Stellpflug, S.; Kerns, W. High-Dose Insulin. Goldfrank’s Toxicologic Emergencies, 8th ed.; Flomenbaum, N.E., Goldfrank, L.R., Eds.; McGraw-Hill: New York, NY, USA, 2006; Volume 41, pp. 953–958. [Google Scholar]
- Dua, R.; Gill, K.D. Aluminium Phosphide Exposure: Implications on Rat Brain Lipid Peroxidation and Antioxidant Defense System. Pharmacol. Toxicol. 2001, 89, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.N.; Kumar, P.; Aggarwal, H.K.; Sharma, A.; Mahajan, S.K.; Malhotra, K.C. Efficacy of magnesium sulphate in aluminium phosphide poisoning--comparison of two different dose schedules. J. Assoc. Physicians India 1994, 42, 373–375. [Google Scholar]
- Krenz, J.; Kaakeh, Y. An Overview of Hyperinsulinemic-Euglycemic Therapy in Calcium Channel Blocker and β-blocker Overdose. Pharmacotherapy 2018, 38, 1130–1142. [Google Scholar] [CrossRef]
- Hassanian-Moghaddam, H. NACCT Abstracts of the 2008 North American Congress of Clinical Toxicology Annual Meeting, Toronto, ON, Canada, 11–16 September 2008; ClinTox: Philadelphia, PA, USA, 2008; Volume 46, pp. 591–645. [Google Scholar]
- Hamzic, J.; Raos, D.; Radulovic, B. High-Dose Insulin euglucemic therapy. Acta Clínica Croac. 2022, 61 (Suppl. S1), 73–77. [Google Scholar] [CrossRef]
- Engebretsen, K.M.; Kaczmarek, K.M.; Morgan, J.; Holger, J.S. High-dose insulin therapy in beta-blocker and calcium channel-blocker poisoning. [Internet]. Clin. Toxicol. 2011, 49, 277–283. Available online: http://crashingpatient.com/wp-content/uploads/2011/07/hiet-review.pdf (accessed on 22 February 2019). [CrossRef]
- Gawedzki, P.; Paloucek, F.P. Additional Considerations for Persistent Hyperinsulinemia. J. Med. Toxicol. 2021, 17, 233–244. [Google Scholar] [CrossRef]
- Corcoran, J.N.; Jacoby, K.J.; Olives, T.D.; Bangh, S.A.; Cole, J.B. Persistent Hyperinsulinemia Following High-Dose Insulin Therapy: A Case Report. J. Med. Toxicol. 2020, 16, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Eisenkraft, A.; Falk, A. The possible role of intravenous lipid emulsion in the treatment of chemical warfare agent poisoning. Toxicol. Rep. 2016, 3, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Heard, K.; Foran, M.; Koyfan, A. Intravenous Lipid Emulsion in the Emergency Department: A systemic review of recent literature. J. Emerg. Med. 2015, 48, 387–397. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhan, C.; Li, Y.; Zhong, Q.; Pan, H.; Yang, G. Intravenous lipid emulsions combine extracorporeal blood purification: A novel therapeutic strategy for severe organophosphate poisoning. Med. Hypotheses 2010, 74, 309–311. [Google Scholar] [CrossRef]
- French, D.; Smollin, C.; Ruan, W.; Wong, A.; Drasner, K.; Wu, A.H. Partition constant and volume of distribution as predictors of clinical efficacy of lipid rescue for toxicological emergencies. Clin. Toxicol. 2011, 49, 801–809. [Google Scholar] [CrossRef]
- Chaudhary, S. An Epidemiological Study of Fatal Aluminium Phosphide Poisoning at Rajkot. IOSR J. Pharm. 2013, 3, 17–23. [Google Scholar] [CrossRef]
- Kordrostami, R.; Akhgari, M.; Ameri, M.; Ghadipasha, M.; Aghakhani, K. Forensic toxicology analysis of self-poisoning suicidal deaths in Tehran, Iran; trends between 2011–2015. DARU J. Pharm. Sci. 2017, 25, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadnia, S.; Sasanian, G.; Allami, P.; Hosseini, A.; Ranjbar, A.; Amini-Shirazi, N.; Abdollahi, M. A retrospective 7-years study of aluminum phosphide poisoning in Tehran: Opportunities for prevention. Hum. Exp. Toxicol. 2009, 28, 209–213. [Google Scholar] [CrossRef]
- Moghadamnia, A.A.; Abdollahi, M. An epidemiological study of poisoning in northern Islamic Republic of Iran. East. Mediterr. Health J. 2002, 8, 88–94. [Google Scholar] [CrossRef]
- Mehrpour, O.; Singh, S. Rice tablet poisoning: A major concern in Iranian population. Hum. Exp. Toxicol. 2010, 29, 701–702. [Google Scholar] [CrossRef]
- Hosseinian, A.; Pakravan, N.; Rafiei, A.; Feyzbakhsh, S. Aluminum phosphide poisoning known as rice tablet: A common toxicity in North Iran. Indian J. Med. Sci. 2011, 65, 143–150. [Google Scholar] [CrossRef]
- Hassanian, H.M.; Pajoumand, A. Two years epidemiological survey of aluminum phosphide poisoning in Tehran. Iran J. Toxicol. 2007, 1, 35–39. [Google Scholar]
- Nosrati, A.; Karami, M.; Esmaeilnia, M. Aluminum phosphide poisoning: A case series in north Iran. Asia Pac. J. Med. Toxicol. 2013, 2, 111–113. [Google Scholar] [CrossRef]
- Gosselin, S.; Hoegberg, L.C.G.; Hoffman, R.S.; Graudins, A.; Stork, C.M.; Thomas, S.H.L.; Stellpflug, S.J.; Hayes, B.D.; Levine, M.; Morris, M.; et al. Evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning. Clin. Toxicol. 2016, 54, 899–923. [Google Scholar] [CrossRef] [Green Version]
- Hayes, B.; Gosselin, S.; Calello, D.; Nacca, N.; Rollins, C.; Abourbih, D.; Morris, M.; Nesbitt, A.; Morais, J.; Lavergne, V. Lipid Emulsion Workgroup. Systematic review of clinical adverse events reported after acute intravenous lipid emulsion administration. Clin. Toxicol. 2016, 18, 365–404. [Google Scholar] [CrossRef] [PubMed]
- Changal, K.H.; Latief, M.; Parry, M.; Abbas, F. Aluminium phosphide poisoning with severe cardiac dysfunction and the role of digoxin. BMJ Case Rep. 2017, 2017, 1–4. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juárez-Martínez, A.; Madrigal-Anaya, J.d.C.; Rodríguez-Torres, Y.P.; Dorado-García, R.; Montes-Ventura, D.M.; Jiménez-Ruiz, A. Zinc Phosphide Poisoning: From A to Z. Toxics 2023, 11, 555. https://doi.org/10.3390/toxics11070555
Juárez-Martínez A, Madrigal-Anaya JdC, Rodríguez-Torres YP, Dorado-García R, Montes-Ventura DM, Jiménez-Ruiz A. Zinc Phosphide Poisoning: From A to Z. Toxics. 2023; 11(7):555. https://doi.org/10.3390/toxics11070555
Chicago/Turabian StyleJuárez-Martínez, Anabell, Jesús del Carmen Madrigal-Anaya, Yessika Paola Rodríguez-Torres, Ramsés Dorado-García, Daphne Marisol Montes-Ventura, and Ahgiel Jiménez-Ruiz. 2023. "Zinc Phosphide Poisoning: From A to Z" Toxics 11, no. 7: 555. https://doi.org/10.3390/toxics11070555




