Metals and Metalloid Concentrations in Fish, Its Spatial Distribution in PPC, Philippines and the Attributable Risks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling Locations
2.2. Collection, Processing, and Detection of Metals and Metalloids (MMs) in Fish Samples
2.3. Health Risk Assessment of MMs in Fish Samples from PPC
2.3.1. Chronic Daily Intake
2.3.2. Non-Carcinogenic Risk
2.3.3. Carcinogenic Risk
2.3.4. Maximum Allowable Fish Consumption Rates
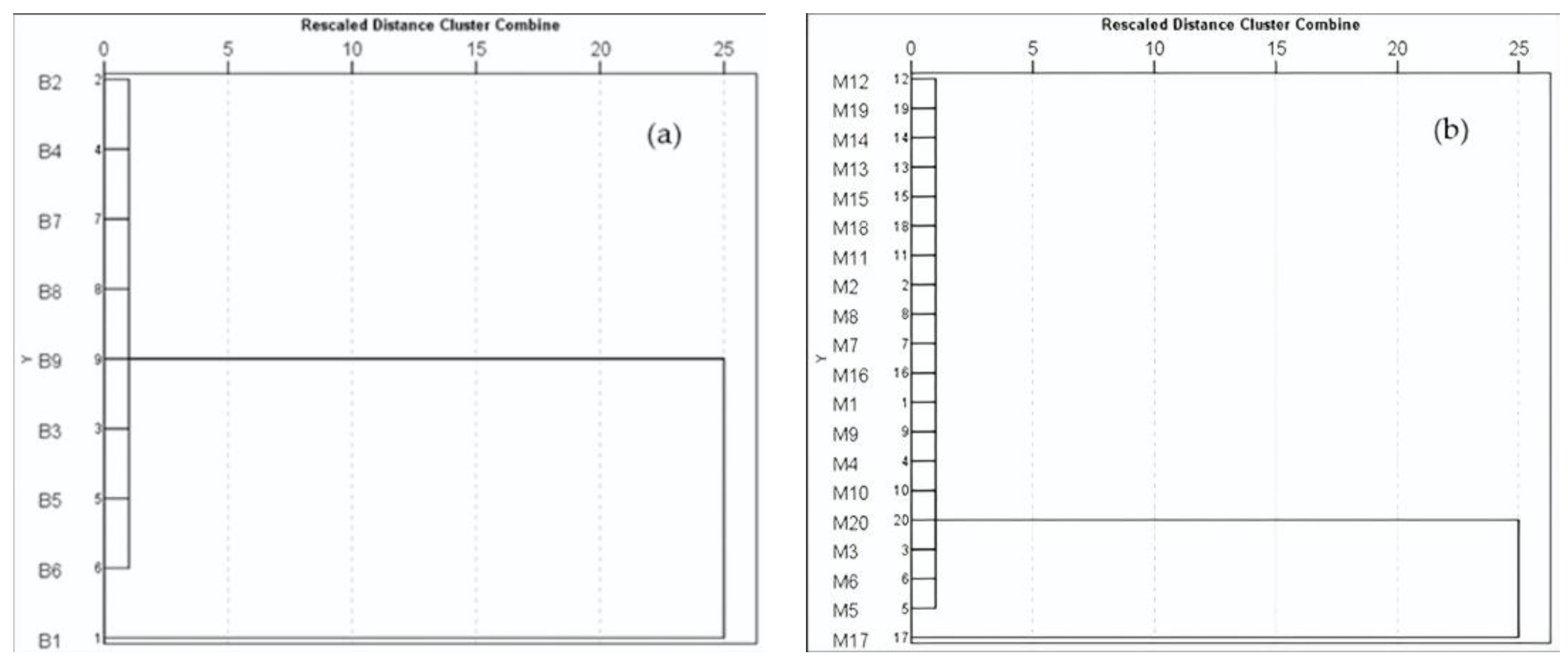
2.4. Statistical Analysis
2.5. Spatial Distribution Maps of MMs in PPC
The Inverse Distance Weighting
3. Results
3.1. Heavy Metals and Metalloids in Fish of PPC
3.2. Spatial Distribution of MMs in the Fish of PPC
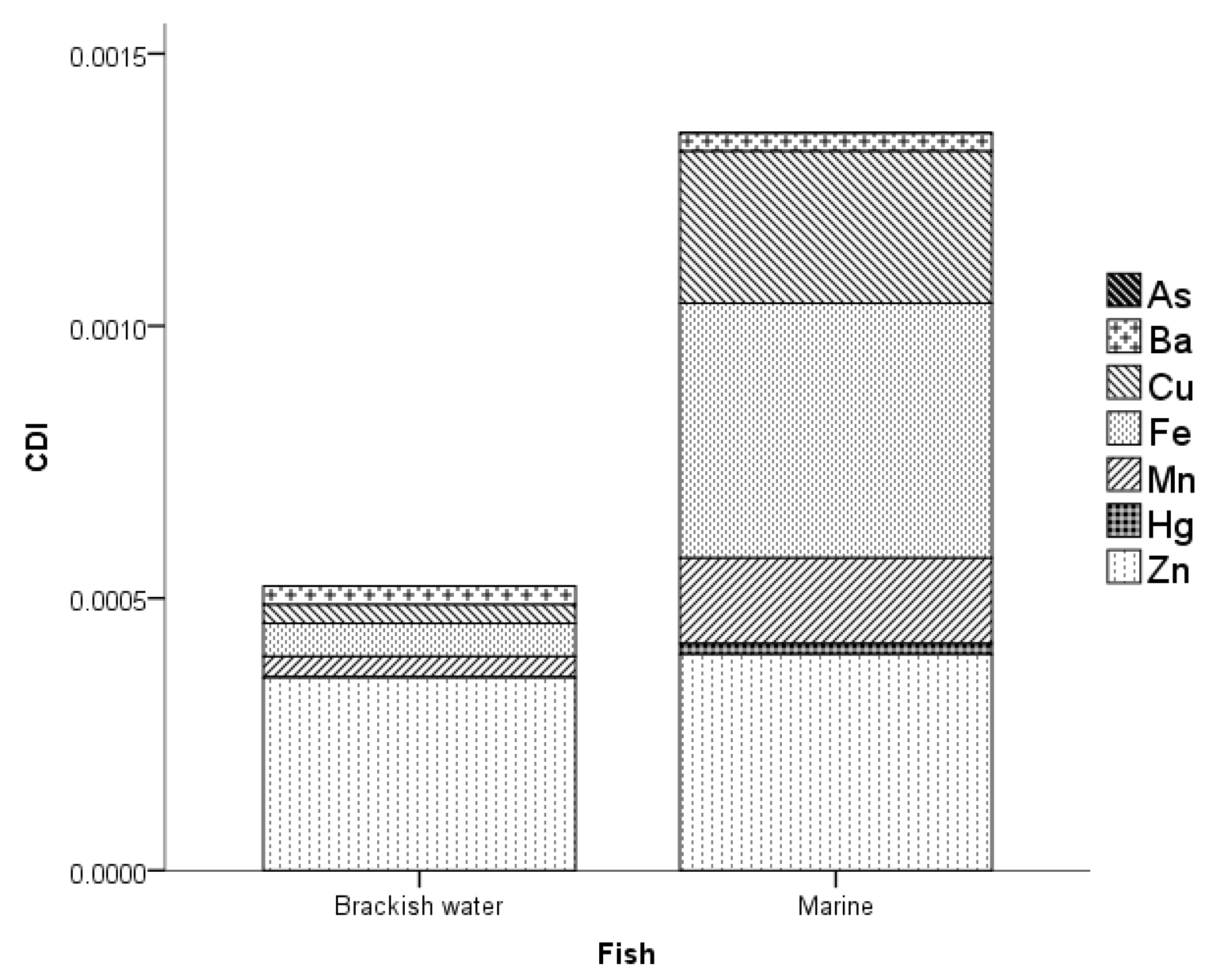
3.3. Health Risk Assessment of MMs in Fish
3.4. Relationship of MMs in Brackish Water and Marine Water Fish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Environment | Code | Barangay | Type of Fish | Scientific Name | English Name | Latitude | Longitude | Weight (g) | Feeding Habit |
|---|---|---|---|---|---|---|---|---|---|
| Brackish Water | B1 | Babuyan | Bangus | Chanos sp. | Milkfish | 9.98029 | 118.91462 | - | Omnivore |
| B2 | Mangingisda | Bangus | Chanos sp. | Milkfish | 9.67527 | 118.73655 | 283.4 | Omnivore | |
| B3 | San Jose | Bangus | Chanos sp. | Milkfish | 9.78193 | 118.74316 | 308.6 | Omnivore | |
| B4 | Tagumpay | Bangus | Chanos sp. | Milkfish | 9.7423 | 118.73625 | 240.6 | Omnivore | |
| B5 | Salvacion | Bangus | Chanos sp. | Milkfish | 9.96691 | 118.78505 | 386.3 | Omnivore | |
| B6 | Tagburos | Bangus | Chanos sp. | Milkfish | 9.84264 | 118.74381 | 351.5 | Omnivore | |
| B7 | Liwanag | Bangus | Chanos sp. | Milkfish | 9.74032 | 118.22863 | 405 | Omnivore | |
| B8 | Masipag | Bangus | Chanos sp. | Milkfish | 9.73514 | 118.73515 | 445.3 | Omnivore | |
| B9 | Sta. Monica | Bangus | Chanos sp. | Milkfish | 9.79418 | 118.73571 | 311 | Omnivore | |
| Marine | M1 | Cabayugan | Buraw | Rastrelliger sp | Mackerel | 10.18221 | 118.89548 | - | Carnivore |
| M2 | Cabayugan | Bisugo | Nemipterus sp. | Threadfin bream | 10.18221 | 118.89548 | - | Carnivore | |
| M3 | Salvacion | Waling | Gazza sp. | slipmouths | 9.94371 | 118.7808 | - | Carnivore | |
| M4 | Salvacion | Black Lapu-lapu | Epinephelus sp. | grouper | 9.94371 | 118.7808 | - | Omnivore | |
| M5 | Inagawan Sub-Colony | Red Lapu-apu | Epinephelus sp. | grouper | 9.61427 | 118.70711 | - | Omnivore | |
| M6 | Babuyan | Black Lapu-lapu | Epinephelus sp. | grouper | 9.98001 | 118.9325 | - | Omnivore | |
| M7 | Tagburos | Lapu-lapu | Epinephelus sp. | grouper | 9.82518 | 118.74225 | - | Omnivore | |
| M8 | Tagburos | Dugso | Letrinus sp. | Emperor fish | 9.82518 | 118.74225 | - | Carnivore | |
| M9 | Napsan | Buraw | Rastrelliger sp | Mackerel | 9.68359 | 118.55226 | 258.3 | Carnivore | |
| M10 | Inagawan | Buraw | Rastrelliger sp | Mackerel | 9.56812 | 118.66583 | 401.8 | Carnivore | |
| M11 | Sta. Monica | Buraw | Rastrelliger sp | Mackerel | 9.79375 | 118.73385 | 212.8 | Carnivore | |
| M12 | San Rafael | Lapu-lapu | Epinephelus sp. | grouper | 9.98571 | 118.96236 | 219.8 | Omnivore | |
| M13 | Cabayugan | Lapu-lapu | Epinephelus sp. | grouper | 10.19408 | 118.89374 | 176.3 | Omnivore | |
| M14 | Bahile | Lapu-lapu | Epinephelus sp. | grouper | 9.99743 | 118.78659 | 456.4 | Omnivore | |
| M15 | Bacungan | Lapu-lapu | Epinephelus sp. | grouper | 9.90981 | 118.70345 | 304.6 | Omnivore | |
| M16 | Tagburos | Lapu-lapu | Epinephelus sp. | grouper | 9.82009 | 118.74429 | 266.1 | Omnivore | |
| M17 | Sicsican | Lapu-lapu | Epinephelus sp. | grouper | 9.79559 | 118.71157 | 323.2 | Omnivore | |
| M18 | Sta. Monica | Lapu-lapu | Epinephelus sp. | grouper | 9.79381 | 118.73435 | 388.3 | Omnivore | |
| M19 | San Miguel | Lapu-lapu | Epinephelus sp. | grouper | 9.7431 | 118.74359 | 452 | Omnivore | |
| M20 | San Manuel | Lapu-lapu | Epinephelus sp. | grouper | 9.76748 | 118.74872 | 411.2 | Omnivore |
| Null Hypothesis | Sig. | Decision |
|---|---|---|
| The distribution of As is the same across the brackish water and marine fish | 0.334 | Retain the null hypothesis |
| The distribution of Ba is the same across the brackish water and marine fish | 0.764 | Retain the null hypothesis |
| The distribution of Cu is the same across the brackish water and marine fish | 0.222 | Retain the null hypothesis |
| The distribution of Fe is the same across the brackish water and marine fish | 0.772 | Retain the null hypothesis |
| The distribution of Mn is the same across the brackish water and marine fish | 0.359 | Retain the null hypothesis |
| The distribution of Hg is the same across the brackish water and marine fish | 0.502 | Retain the null hypothesis |
| The distribution of Zn is the same across the brackish water and marine fish | 0.118 | Retain the null hypothesis |
| Name | Commodity/Mine | Coordinates |
|---|---|---|
| Nickel Mining | ||
| Birong Nickel Occurence | Ni | 9.4173° N 118.1993° E |
| Brookes Point Nickel Occurence | Ni | 8.9173° N 117.8827° E |
| Coral Bay Nickel Corp. | Ni | 8.483828° N,117.4377063° E |
| Palawan Rio Tuba Nickel Laterite deposit | Ni | 8.5007° N 117.3994° E |
| Long Point Nickel Deposit-Palawan Island | Ni | 9.6506° N 118.3326° E |
| Ipilan Nickel Prospect | Ni | 8.8507° N 117.8493° E |
| Rio Tuba Mine | Ni | 8.5868° N 117.4049° E |
| Guintalungan Nickel Deposit | Ni | 8.5549° N 117.3863° E |
| Isabela Nickel Deposit | Ni | 9.1006° N 117.8993° E |
| Zinc Mining | ||
| Balabac Copper mine | Cu, Zn | 7.9841° N 117.0660° E |
| Manganese Mining | ||
| Pina–Balitbitin Manganese Occurence | Mn | 12.0837° N 120.1993° E |
| Binabaan Manganese Occurence | Mn | 11.731° N 120.0276° E |
| Busuanga Island Manganese Mine | Mn | 12.1670° N 119.9993° E |
| Lorraine Orebody–Balabac Island Mine | Al, Fe, Mn, P, SiO2 | 8.0008° N 117.0493° E |
| Busuanga Island: Coron, Borac-East Mine | Al, Fe, Mn, P, SiO2 | 12.1104° N 119.9993° E |
| Iron Mining | ||
| Rio tuba Mine | Fe, Ni | 8.5868° N 117.4049° E |
| Lorraine Orebody–Balabac Island Mine | Al, Fe, Mn, P, SiO2 | 8.0008° N 117.0493° E |
| Busuanga Island: Coron, Borac-East Mine | Al, Fe, Mn, P, SiO2 | 12.1104° N 119.9993° E |
| Copper Mining | ||
| Balabac Copper Mine in Palawan, Philippines | Cu, Zn | 7.9841° N 117.0660° E |
| Atlas Copper Mine in Palawan | Ni, FeCr2O4, and other associated mineral deposits | Espanola-9.106383° N, 118.0779211° E, Narra—9.106383° N,118.0779211° E |

References
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2016; Volume 50, ISBN 9789251091852. [Google Scholar]
- Han, J.L.; Pan, X.D.; Chen, Q.; Huang, B.F. Health Risk Assessment of Heavy Metals in Marine Fish to the Population in Zhejiang, China. Sci. Rep. 2021, 11, 11079. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Cowx, I.G.; Oleksiak, M.F.; Griffiths, A.M.; Grahn, M.; Stevens, J.R.; Carvalho, G.R.; Nicol, E.; Tyler, C.R. Population-Level Consequences for Wild Fish Exposed to Sublethal Concentrations of Chemicals—A Critical Review. Fish Fish. 2016, 17, 545–566. [Google Scholar] [CrossRef]
- Beeler, B.; Immig, J. Chemical Pollution Causes Fish Declines Escalating: Chemical Production Threatens Aquatic Food Chain; IPEN: Gothenburg, Sweden, 2021. [Google Scholar]
- Yousif, R.A.; Choudhary, M.I.; Ahmed, S.; Ahmed, Q. Review: Bioaccumulation of Heavy Metals in Fish and Other Aquatic Organisms from Karachi Coast, Pakistan. Nusant. Biosci. 2021, 13, 73–84. [Google Scholar] [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy Metals in Marine Fish Meat and Consumer Health: A Review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Donati, E. Heavy Metals in the Environment: Microorganisms and Bioremediation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9780367781576. [Google Scholar]
- Ferrante, M.; Napoli, S.; Grasso, A.; Zuccarello, P.; Cristaldi, A.; Copat, C. Systematic Review of Arsenic in Fresh Seafood from the Mediterranean Sea and European Atlantic Coasts: A Health Risk Assessment. Food Chem. Toxicol. 2019, 126, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Agarin, C.J.M.; Mascareñas, D.R.; Nolos, R.; Chan, E.; Senoro, D.B. Transition Metals in Freshwater Crustaceans, Tilapia, and Inland Water: Hazardous to the Population of the Small Island Province. Toxics 2021, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Weerasinghe, S.; Kaumal, M. Determination of Heavy Metals in Tilapia Using Various Digestion Methods Determination of Heavy Metals in Tilapia Using Various Digestion Methods Department of Chemistry, Faculty of Science, University of Colombo, Sri Lanka Faculty of Applied Sciences. Int. J. Sci. Res. Innov. Technol. 2016, 3, 38–48. [Google Scholar]
- Adei, D.; Braimah, I.; Mensah, J.V.; Mensah, A.A.; Agyemang-Duah, W. Improving upon the Working Environment of Informal Sector Workers in Ghana: The Role of Planning. Cogent Med. 2021, 8, 1911441. [Google Scholar] [CrossRef]
- Zeitoun, M.M.; Mehana, E.S.E. Impact of Water Pollution with Heavy Metals on Fish Health: Overview and Updates. Glob. Vet. 2014, 12, 219–231. [Google Scholar] [CrossRef]
- Ateş, A.; Türkmen, M.; Tepe, Y. Assessment of Heavy Metals in Fourteen Marine Fish Species of Four Turkish Seas. Indian J. Geo-Mar. Sci. 2015, 44, 49–55. [Google Scholar]
- Yi, Y.J.; Zhang, S.H. The Relationships between Fish Heavy Metal Concentrations and Fish Size in the Upper and Middle Reach of Yangtze River. Procedia Environ. Sci. 2012, 13, 1699–1707. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.; Baki, M.A.; Kundu, G.K.; Saiful Islam, M.; Monirul Islam, M.; Muzammel Hossain, M. Human Health Risks from Heavy Metals in Fish of Buriganga River, Bangladesh. Springerplus 2016, 5, 1697. [Google Scholar] [CrossRef]
- Lee, K.G.; Kweon, H.Y.; Yeo, J.H.; Woo, S.O.; Han, S.M.; Kim, J.H. Characterization of Tyrosine-Rich Antheraea Pernyi Silk Fibroin Hydrolysate. Int. J. Biol. Macromol. 2011, 48, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef] [Green Version]
- Tongesayi, T.; Fedick, P.; Lechner, L.; Brock, C.; Le Beau, A.; Bray, C. Daily Bioaccessible Levels of Selected Essential but Toxic Heavy Metals from the Consumption of Non-Dietary Food Sources. Food Chem. Toxicol. 2013, 62, 142–147. [Google Scholar] [CrossRef]
- Nolos, R.C.; Zamroni, A.; Evina, K.F.P. Drivers Of Deforestation And Forest Degradation In Palawan, Philippines: An Analysis Using Social-Ecological Systems (SES) And Institutional Analysis And Development (IAD) Approaches. Geogr. Environ. Sustain. 2023, 15, 44–56. [Google Scholar] [CrossRef]
- Cadag, J.R.; Timbancaya, E.; De la Cruz, E.; Matillano, D.; De la Cruz, L.; Ocampo, D.; Caringal, J.C. Puerto Princesa City Local Disaster Risk Reduction and Management FY 2020–2022; City Disaster Risks Reduction and Management Office: Puerto Princesa City, Philippines, 2022. [Google Scholar]
- PPC LGU. City Government of Puerto Princesa Annual Report of City Government of Puerto Princesa. In Accomplishments of Economic Sector; Puerto Princesa City Local Government: Palawan, Philippines, 2020. [Google Scholar]
- Peralta-milan, S.; Baba, O.; Salmo, S. Linking Marketing of Reef-Sourced Seafood with Tourism: Potential for Improving Fisheries Management. Philipp. Sci. Lett. 2020, 13, 113–123. [Google Scholar]
- Samaniego, J.; Gibaga, C.R.; Tanciongco, A.; Rastrullo, R. Assessment of Trace Elements in Soils and Sediments in the Abandoned Mercury Mine Site in Puerto Princesa City, Philippines. ASEAN J. Sci. Technol. Dev. 2021, 38, 43–49. [Google Scholar] [CrossRef]
- Samaniego, J.; Gibaga, C.R.; Tanciongco, A.; Rastrullo, R. Total Mercury in Soils and Sediments in the Vicinity of Abandoned Mercury Mine Area in Puerto Princesa City, Philippines. Appl. Sci. 2020, 10, 4599. [Google Scholar] [CrossRef]
- Ureta, J.U.; Florece, L.; Pulhin, J. Social Vulnerability and Adaptation to Climate Variability and Extremes of Farming and Fishing Households in Puerto Princesa City, Palawan, Philippines. J. Econ. Manag. Agric. Dev. 2015, 3, 73–88. [Google Scholar]
- City Government of Puerto Princesa Climate and Weather Palawan. Available online: https://palawanperfection.com/climate-weather/563 (accessed on 17 November 2022).
- USEPA. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories Volume 1 Fish Sampling and Analysis, 3rd ed.; United States Environmental Protection Agency: Washington, DC, USA, 2000; Volume 1.
- Mendoza, L.C.; Nolos, R.C.; Villaflores, O.B.; Apostol, E.M.D.; Senoro, D.B. Detection of Heavy Metals, Their Distribution in Tilapia Spp., and Health Risks Assessment. Toxics 2023, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Senoro, D.B.; de Jesus, K.L.M.; Nolos, R.C.; Lamac, M.R.L.; Deseo, K.M.; Tabelin, C.B. In Situ Measurements of Domestic Water Quality and Health Risks by Elevated Concentration of Heavy Metals and Metalloids Using Monte Carlo and MLGI Methods. Toxics 2022, 10, 342. [Google Scholar] [CrossRef]
- Medaković, D.; Dolenec, T.; Karlović, D.; Vrhovnik, P.; Rogan Šmuc, N.; Rončević, S.; Pitarević-Svedružić, L.; Dolenec, M. Trace Metals in Fish Biominerals as Environmental Indicators: Handheld XRF Analyses. Key Eng. Mater. 2016, 672, 328–339. [Google Scholar] [CrossRef]
- Philippine Statistics Authority. Consumption of Selected Agricultural Commodities in the Philippines; Philippine Statistics Authority (PSA): Quezon City, Philippines, 2017; Volume 2, pp. 1689–1699.
- Safiur Rahman, M.; Solaiman Hossain, M.; Ahmed, M.K.; Akther, S.; Jolly, Y.N.; Akhter, S.; Jamiul Kabir, M.; Choudhury, T.R. Assessment of Heavy Metals Contamination in Selected Tropical Marine Fish Species in Bangladesh and Their Impact on Human Health. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100210. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Health Effects Assessment Summary Tables; Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 1995; Volume 93.
- Nolos, R.C.; Agarin, C.J.M.; Domino, M.Y.R.; Bonifacio, P.B.; Chan, E.B.; Mascareñas, D.R.; Senoro, D.B. Health Risks Due to Metal Concentrations in Soil and Vegetables from the Six Municipalities of the Island Province in the Philippines. Int. J. Environ. Res. Public Health 2022, 19, 1587. [Google Scholar] [CrossRef]
- Zaghloul, G.Y.; Ezz El-Din, H.M.; Mohamedein, L.I.; El-Moselhy, K.M. Bio-Accumulation and Health Risk Assessment of Heavy Metals in Different Edible Fish Species from Hurghada City, Red Sea, Egypt. Environ. Toxicol. Pharmacol. 2022, 95, 103969. [Google Scholar] [CrossRef]
- Qu, C.-S.; Ma, Z.-W.; Yang, J.; Liu, Y.; Bi, J.; Huang, L. Human Exposure Pathways of Heavy Metals in a Lead-Zinc Mining Area, Jiangsu Province, China. PLoS ONE 2012, 7, e46793. [Google Scholar] [CrossRef] [Green Version]
- Musarrat, M.; Ullah, A.K.M.A.; Moushumi, N.S.; Akon, S.; Nahar, Q.; Saliheen Sultana, S.S.; Quraishi, S.B. Assessment of Heavy Metal(Loid)s in Selected Small Indigenous Species of Industrial Area Origin Freshwater Fish and Potential Human Health Risk Implications in Bangladesh. LWT 2021, 150, 112041. [Google Scholar] [CrossRef]
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy Metal Contamination in Rice-Producing Soils of Hunan Province, China and Potential Health Risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Toxicological Review of Barium and Compounds. In Information on the Integrated Risk Information System; United States Environmental Protection Agency: Washington, DC, USA, 2010; Volume 39, pp. 759–786. [Google Scholar]
- Muhammad, S.; Shah, M.T.; Khan, S. Health Risk Assessment of Heavy Metals and Their Source Apportionment in Drinking Water of Kohistan Region, Northern Pakistan. Microchem. J. 2011, 98, 334–343. [Google Scholar] [CrossRef]
- Yuswir, N.S.; Praveena, S.M.; Aris, A.Z.; Ismail, S.N.S.; Hashim, Z. Health Risk Assessment of Heavy Metal in Urban Surface Soil (Klang District, Malaysia). Bull. Environ. Contam. Toxicol. 2015, 95, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Means, B. Risk-Assessment Guidance for Superfund. Volume 1. Human Health Evaluation Manual. Part A. Interim Report (Final); Office of Solid Waste and Emergency Response, Environmental Protection Agency: Washington, DC, USA, 1989.
- International Agency for Research on Cancer. Agents Classified by the IARC Monographs; International Agency for Research on Cancer: Lyon, France, 2012; Volume 1–105, pp. 1–5.
- Abdel-Kader, H.H.; Mourad, M.H. Estimation of Cadmium in Muscles of Five Freshwater Fish Species from Manzalah Lake, and Possible Human Risk Assessment of Fish Consumption (Egypt). Biol. Trace Elem. Res. 2023, 201, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Varol, M.; Kaçar, E.; Sünbül, M.R.; Md Towfiqul Islam, A.R. Levels of Metals and Elements in Tissues of Fish Species in the Kızılırmak River (Turkey) and Assessment of Health Risks and Nutritional Benefits. Environ. Res. 2022, 214, 113791. [Google Scholar] [CrossRef]
- Bigler, J. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories: Risk Assessment and Fish Consumption Limits; US EPA Office of Water, Office of Science and Technology: Washington, DC, USA, 1997; Volume 2. [Google Scholar]
- Hossain, M.B.; Tanjin, F.; Rahman, M.S.; Yu, J.; Akhter, S.; Noman, M.A.; Sun, J. Metals Bioaccumulation in 15 Commonly Consumed Fishes from the Lower Meghna River and Adjacent Areas of Bangladesh and Associated Human Health Hazards. Toxics 2022, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lv, L.; An, M.; Wang, T.; Li, M.; Yu, Y. Heavy Metals in Marine Food Web from Laizhou Bay, China: Levels, Trophic Magnification, and Health Risk Assessment. Sci. Total Environ. 2022, 841, 156818. [Google Scholar] [CrossRef] [PubMed]
- Ogunlaja, A.; Ogunlaja, O.O.; Okewole, D.M.; Morenikeji, O.A. Risk Assessment and Source Identification of Heavy Metal Contamination by Multivariate and Hazard Index Analyses of a Pipeline Vandalised Area in Lagos State, Nigeria. Sci. Total Environ. 2019, 651, 2943–2952. [Google Scholar] [CrossRef]
- Geographic Information System Software, ESRI ArcGIS Desktop 10.8.1, ARCPro 2.8; Esri: Redlands, CA, USA.
- Inverse Distance Weighting (IDW) Interpolation—GIS Geography. Available online: https://gisgeography.com/inverse-distance-weighting-idw-interpolation/ (accessed on 16 September 2022).
- Karimi, H.A. Handbook of Research on Geoinformatics. In IGI Global 2009; University of Pittsburgh: Pittsburgh, PA, USA, 2009; pp. 129–136. [Google Scholar]
- Gimond, M. Introduction to GIS and Spatial Analysis. Available online: https://mgimond.github.io/Spatial/ (accessed on 16 September 2022).
- European Commission Commission Regulation (EC). No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- World Health Organization. Summary and Conclusions of the Sixty-First Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO: Roma, Italy, 2003.
- Joint FAO/WHO Expert Committee on Food Additives. Toxicological Evaluation of Certain Food Additives and Contaminants. In Proceedings of the Meeting of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, Switzerland, 21–30 March 1989, International Program on Chemical Safety, 33rd ed.; Geneva, S., Ed.; Cambridge University Press: Cambridge, UK, 1989; p. 362. [Google Scholar]
- Botwe, B.O. Heavy Metal Concentrations in Five Fish Species from the Gulf of Guinea and Their Human Health Implications. Reg. Stud. Mar. Sci. 2021, 44, 101763. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Arsenic, Inorganic; CASRN 7440-38-2; Integrated Risk Information System, (IRIS): Washington, DC, USA; U.S. Chemical Assessment Summary National Center for Environmental Assessment: Washington, DC, USA, 1988; p. 27. [Google Scholar]
- Felix, C.S.A.; Pereira Junior, J.B.; da Silva Junior, J.B.; Cruz, A.S.; Dantas, K.G.F.; Ferreira, S.L.C. Determination and Human Health Risk Assessment of Mercury in Fish Samples. Talanta 2022, 247, 123557. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, B.; Wang, L.; Pan, X. Mercury Concentration in the Muscle of Seven Fish Species from Chagan Lake, Northeast China. Environ. Monit. Assess. 2012, 184, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Melake, B.A.; Nkuba, B.; Groffen, T.; De Boeck, G.; Bervoets, L. Distribution of Metals in Water, Sediment and Fish Tissue. Consequences for Human Health Risks Due to Fish Consumption in Lake Hawassa, Ethiopia. Sci. Total Environ. 2022, 843, 156968. [Google Scholar] [CrossRef] [PubMed]
- Egbueri, J.C. Groundwater Quality Assessment Using Pollution Index of Groundwater (PIG), Ecological Risk Index (ERI) and Hierarchical Cluster Analysis (HCA): A Case Study. Groundw. Sustain. Dev. 2020, 10, 100292. [Google Scholar] [CrossRef]
- Karayakar, F.; Işık, U.; Cicik, B.; Canli, M. Heavy Metal Levels in Economically Important Fish Species Sold by Fishermen in Karatas (Adana/TURKEY). J. Food Compos. Anal. 2022, 106, 104348. [Google Scholar] [CrossRef]
- Steinhausen, S.L.; Agyeman, N.; Turrero, P.; Ardura, A.; Garcia-Vazquez, E. Heavy Metals in Fish Nearby Electronic Waste May Threaten Consumer’s Health. Examples from Accra, Ghana. Mar. Pollut. Bull. 2022, 175, 113162. [Google Scholar] [CrossRef]
- Lawal Usman, U.; Muhammad, A.Z.; Banerjee, S.; Musa, N. Bioaccumulation Potential of Heavy Metals in Some Commercially Fish Species from Cika Koshi Reservoir Katsina North-Western Nigeria: Threat to Ecosystem and Public Health. Mater Today Proc. 2022, 49, 3423–3429. [Google Scholar] [CrossRef]
- Elvira, M.V.; Faustino-Eslava, D.V.; de Chavez, E.R.C.; Losloso, J.A.L.; Fukuyama, M. Human Health Risk Associated with Heavy Metals from Consumption of Asiatic Clam, Corbicula Fluminea, from Laguna de Bay, Philippines. Environ. Sci. Pollut. Res. 2021, 28, 36626–36639. [Google Scholar] [CrossRef]
- Töre, Y.; Ustaoğlu, F.; Tepe, Y.; Kalipci, E. Levels of Toxic Metals in Edible Fish Species of the Tigris River (Turkey); Threat to Public Health. Ecol. Indic. 2021, 123, 107361. [Google Scholar] [CrossRef]
- Rakib, M.; Jahan, R.; Jolly, Y.N.; Enyoh, C.E.; Khandaker, M.U.; Hossain, M.B.; Akther, S.; Alsubaie, A.; Almalki, A.S.A.; Bradley, D.A. Levels and Health Risk Assessment of Heavy Metals in Dried Fish Consumed in Bangladesh. Sci. Rep. 2021, 11, 14642. [Google Scholar] [CrossRef]
- Ali, Z.; Yousafzai, A.M.; Sher, N.; Muhammad, I.; Nayab, G.E.; Aqeel, S.A.M.; Shah, S.T.; Aschner, M.; Khan, I.; Khan, H. Toxicity and Bioaccumulation of Manganese and Chromium in Different Organs of Common Carp (Cyprinus carpio) Fish. Toxicol. Rep. 2021, 8, 343–348. [Google Scholar] [CrossRef]
- Liu, Z.; Kuang, Y.; Lan, S.; Cao, W.; Yan, Z.; Chen, L.; Chen, Q.; Feng, Q.; Zhou, H. Pollution Distribution of Potentially Toxic Elements in a Karstic River Affected by Manganese Mining in Changyang, Western Hubei, Central China. Int. J. Environ. Res. Public Health 2021, 18, 1870. [Google Scholar] [CrossRef]
- Ramprasad, C.; Sona, K.; Afridhi, M.; Kumar, R. Water Quality Assessment of the Cauvery and Vaigai River at Upstream and Downstream Locations: Impact of Domestic and Industrial Effluents. Indian J. Ecol. 2021, 48, 615–619. [Google Scholar]
- Levy, B.S.; Nassetta, W.J. Neurologic Effects of Manganese in Humans: A Review. Int. J. Occup. Environ. Health 2003, 9, 153–163. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Zheng, W. Cardiovascular Toxicities upon Managanese Exposure. Cardiovasc. Toxicol. 2005, 5, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, M.A.; Barst, B.D.; Basu, N. A Review of Mercury Bioavailability in Humans and Fish. Int. J. Environ. Res. Public Health 2017, 14, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Lemes, M.; Khan, M. Metallomics of Mercury: Role of Thiol-and Selenol-Containing Biomolecules. In Environmental Chemistry and Toxicology of Mercury; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 517–544. [Google Scholar]
- Nava, V.; Di Bella, G.; Fazio, E.; Potorti, A.G.; Lo Turco, V.; Licata, P. Hg Content in EU and Non-EU Processed Meat and Fish Foods. Appl. Sci. 2023, 13, 793. [Google Scholar] [CrossRef]
- Langcay, M.L.; Clemente, E.D.; Arranz, C.G. Risk Assessment of Mercury in Soil and Surface Water in Brgy. Santa Lourdes, Puerto Princesa City, Palawan. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Beijing, China, 6–8 December 2020; IOP Publishing: Bristol, UK, 2021; Volume 690, p. 12043. [Google Scholar] [CrossRef]
- Gray, J.E.; Greaves, I.A.; Bustos, D.M.; Krabbenhoft, D.P. Mercury and Methylmercury Contents in Mine-Waste Calcine, Water, and Sediment Collected from the Palawan Quicksilver Mine, Philippines. Environ. Geol. 2003, 43, 298–307. [Google Scholar] [CrossRef]
- World Health Organization. Mercury and Human Health; WHO: Geneva, Switzerland, 2021; ISBN 9789289055888.
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef] [Green Version]
- Caetano, T.; Branco, V.; Cavaco, A.; Carvalho, C. Risk Assessment of Methylmercury in Pregnant Women and Newborns in the Island of Madeira (Portugal) Using Exposure Biomarkers and Food-Frequency Questionnaires. J. Toxicol. Environ. Health A 2019, 82, 833–844. [Google Scholar] [CrossRef]
- Samaniego, J.O.; Gibaga, C.R.L.; Tanciongco, A.M.; Rastrullo, R.M.; Costa, M.A.V. Surface Water Characteristics in the Vicinity of Abandoned Mercury Mine Site in Puerto Princesa City, Philippines. Philipp. J. Sci. 2019, 148, 493–498. [Google Scholar]
- Samaniego, J.O.; Gibaga, C.R.L.; Mendoza, N.D.S.; Racadio, C.D.T.; Tanciongco, A.M.; Rastrullo, R.M. Mercury and Other Heavy Metals in Groundwater in the Abandoned Mercury Mine in Puerto Princesa City, Philippines. Philipp. J. Sci. 2020, 149, 897–901. [Google Scholar] [CrossRef]
- Pugalendhi, T.; Uma Maheswari, G. Concentration of Lead and Cadmium in Some Edible Fishes from Tuticorin. J. Mar. Biol. Ass. India 2007, 49, 254–256. [Google Scholar]
- Mwakalapa, E.B.; Simukoko, C.K.; Mmochi, A.J.; Mdegela, R.H.; Berg, V.; Bjorge Müller, M.H.; Lyche, J.L.; Polder, A. Heavy Metals in Farmed and Wild Milkfish (Chanos chanos) and Wild Mullet (Mugil cephalus) along the Coasts of Tanzania and Associated Health Risk for Humans and Fish. Chemosphere 2019, 224, 176–186. [Google Scholar] [CrossRef]
- Saei-Dehkordi, S.S.; Fallah, A.A.; Nematollahi, A. Arsenic and Mercury in Commercially Valuable Fish Species from the Persian Gulf: Influence of Season and Habitat. Food Chem. Toxicol. 2010, 48, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Mangalagiri, P.; Bikkina, A.; Sundarraj, D.K.; Thatiparthi, B.R. Bioaccumulation of Heavy Metals in Rastrelliger Kanagurta along the Coastal Waters of Visakhapatnam, India. Mar. Pollut. Bull 2020, 160, 111658. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, T.I.; Gashkina, N.A. Distribution and Bioaccumulation of Heavy Metals (Hg, Cd and Pb) in Fish: Influence of the Aquatic Environment and Climate. Environ. Res. Lett. 2020, 15, 115013. [Google Scholar] [CrossRef]
- Moe, S.J.; De Schamphelaere, K.; Clements, W.H.; Sorensen, M.T.; den Brink, P.J.; Liess, M. Combined and Interactive Effects of Global Climate Change and Toxicants on Populations and Communities. Environ. Toxicol. Chem. 2013, 32, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Panebianco, F.; Nava, V.; Giarratana, F.; Gervasi, T.; Cicero, N. Assessment of heavy- and semi-metals contamiantion in edible seaweed and dried fish sold in ethnic food stores on the Italian market. J. Food Compos. Anal. 2021, 104, 104150. [Google Scholar] [CrossRef]
- Senthil Murugan, S.; Karuppasamy, R.; Poongodi, K.; Puvaneswari, S. Bioaccumulation Pattern of Zinc in Freshwater Fish Channa punctatus (Bloch.) after Chronic Exposure. Turk. J. Fish. Aquat. Sci. 2008, 59, 55–59. [Google Scholar]
- Wood, C.M.; Farrell, A.P.; Brauner, C.J. Fish Physiology: Homeostasis and Toxicology of Essential Metals. In Homeostasis and Toxicology of Essential Metals; Academic Press: Waltham, MA, USA, 2011; Volume 31. [Google Scholar]
- Nikinmaa, M. An Introduction to Aquatic Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–240. [Google Scholar] [CrossRef]
- Mensoor, M.; Said, A. Determination of Heavy Metals in Freshwater Fishes of the Tigris River in Baghdad. Fishes 2018, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Memmert, U. Bioaccumulation of Zinc in Two Freshwater Organisms (Daphnia magna, Crustacea and Brachydanio, Rerio, Pisces). Water Res. 1987, 21, 99–106. [Google Scholar] [CrossRef]
- World Health Organization. Daily Iron Supplementation in Adult Women and Adolescent Girls; WHO: Geneva, Switzerland, 2016; p. 44.
- Williams, T.M.; Weeks, J.M.; Apostol, A.N., Jr.; Miranda, C.R. Assessment of Mercury Contamination and Human Exposure Associated with Coastal Disposal of Waste from a Cinnabar Mining Operation, Palawan, Philippines. Environ. Geol. 1999, 39, 51–60. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Y.; Wu, Z.; Yang, R.; Chen, X.; Yang, J.; Zhu, L. Health Risk Assessment of Heavy Metals in Freshwater Fish in the Central and Eastern North China. Ecotoxicol. Environ. Saf. 2018, 157, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Łuczyńska, J.; Paszczyk, B.; Łuczyński, M.J. Fish as a Bioindicator of Heavy Metals Pollution in Aquatic Ecosystem of Pluszne Lake, Poland, and Risk Assessment for Consumer’s Health. Ecotoxicol. Environ. Saf. 2018, 153, 60–67. [Google Scholar] [CrossRef]
- Chandrapalan, T.; Kwong, R.W.M. Functional Significance and Physiological Regulation of Essential Trace Metals in Fish. J. Exp. Biol. 2021, 224, jeb238790. [Google Scholar] [CrossRef]
- Ali, M.M.; Ali, M.L.; Jahan Rakib, M.R.; Islam, M.S.; Bhuyan, M.S.; Senapathi, V.; Chung, S.Y.; Roy, P.D.; Sekar, S.; Md Towfiqul Islam, A.R.; et al. Seasonal Behavior and Accumulation of Some Toxic Metals in Commercial Fishes from Kirtankhola Tidal River of Bangladesh—A Health Risk Taxation. Chemosphere 2022, 301, 134660. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Y.S.; Marie, M.-A.S. Assessment of Metal Contamination in Water, Sediment, and Tissues of Arius Thalassinus Fish from the Red Sea Coast of Yemen and the Potential Human Risk Assessment. Environ. Sci. Pollut. Res. 2015, 22, 5481–5490. [Google Scholar] [CrossRef]
- Jiang, H.; Qin, D.; Chen, Z.; Tang, S.; Bai, S.; Mou, Z. Heavy Metal Levels in Fish from Heilongjiang River and Potential Health Risk Assessment. Bull. Environ. Contam. Toxicol. 2016, 97, 536–542. [Google Scholar] [CrossRef]
- Cai, S.; Ni, Z.; Li, Y.; Shen, Z.; Xiong, Z.; Zhang, Y.; Zhou, Y. Metals in the Tissues of Two Fish Species from the Rare and Endemic Fish Nature Reserve in the Upper Reaches of the Yangtze River, China. Bull. Environ. Contam. Toxicol. 2012, 88, 922–927. [Google Scholar] [CrossRef]
- Widianarko, B.; Van Gestel, C.A.M.; Verweij, R.A.; Van Straalen, N.M. Associations between Trace Metals in Sediment, Water, and Guppy, Poecilia Reticulata (Peters), from Urban Streams of Semarang, Indonesia. Ecotoxicol. Environ. Saf. 2000, 46, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Senoro, D.B.; de Jesus, K.L.M.; Mendoza, L.C.; Apostol, E.M.D.; Escalona, K.S.; Chan, E.B. Groundwater Quality Monitoring Using In-Situ Measurements and Hybrid Machine Learning with Empirical Bayesian Kriging Interpolation Method. Appl. Sci. 2021, 12, 132. [Google Scholar] [CrossRef]

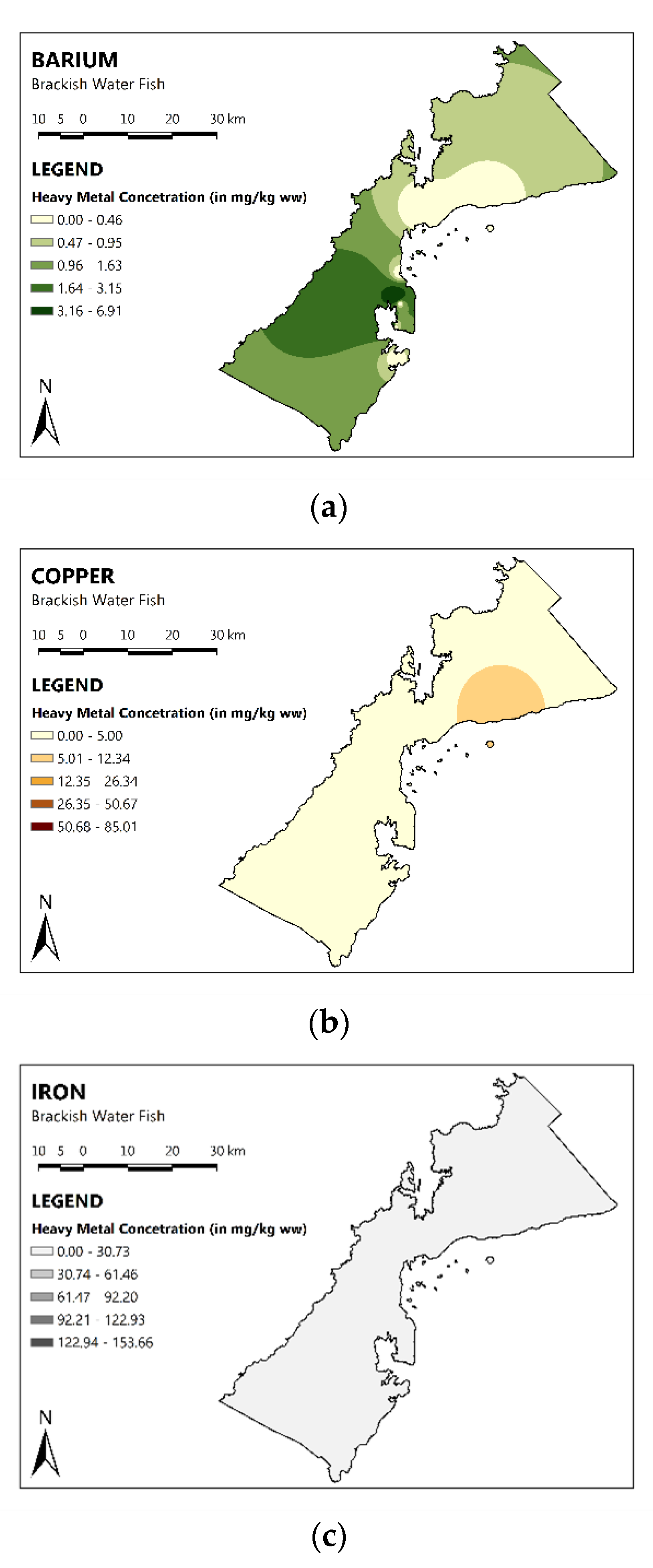


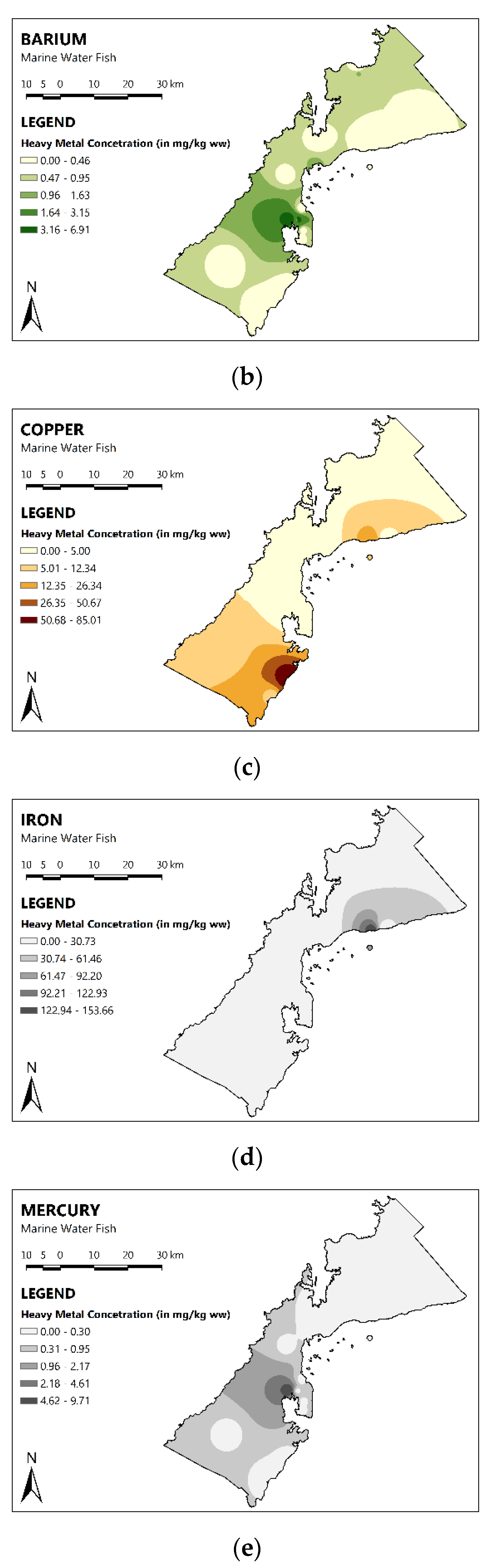



| Fish | As | Ba | Cu | Fe | Mn | Hg | Zn |
|---|---|---|---|---|---|---|---|
| Brackish | <LOD | <LOD–6.91 | <LOD–9.60 | <LOD–11.21 | <LOD–12.35 | <LOD | 3.83–26.18 |
| Marine | <LOD | <LOD–6.68 | <LOD–85.01 | <LOD–153.66 | 0–12.35 | <LOD–9.71 | 0–30.01 |
| FAO/WHO [9,56] | - | - | 30 | 100 | 1 | - | 100 |
| JECFA [55] | 0.002 | - | - | - | - | - | - |
| EC [54] | - | - | - | - | - | 0.5 | - |
| Fish | CR | Risk [44,56] |
|---|---|---|
| Brackish Water | 0 | negligible |
| Marine | negligible |
| Fish | MMs | CRlim (Non-Carcinogenic) | CRlim (Carcinogenic) |
|---|---|---|---|
| Brackish Water Fish | As | - | - |
| Ba | 8862.21 | - | |
| Cu | 1619.06 | - | |
| Fe | 17,219.82 | - | |
| Mn | 5423.41 | - | |
| Hg | - | - | |
| Zn | 1274.99 | - | |
| Marine Water Fish | As | 78,750.00 | 1750.00 |
| Ba | 14,629.68 | - | |
| Cu | 320.61 | - | |
| Fe | 3611.47 | - | |
| Mn | 2152.15 | - | |
| Hg | 19.77 | - | |
| Zn | 1824.69 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senoro, D.B.; Plasus, M.M.G.; Gorospe, A.F.B.; Nolos, R.C.; Baaco, A.T.; Lin, C. Metals and Metalloid Concentrations in Fish, Its Spatial Distribution in PPC, Philippines and the Attributable Risks. Toxics 2023, 11, 621. https://doi.org/10.3390/toxics11070621
Senoro DB, Plasus MMG, Gorospe AFB, Nolos RC, Baaco AT, Lin C. Metals and Metalloid Concentrations in Fish, Its Spatial Distribution in PPC, Philippines and the Attributable Risks. Toxics. 2023; 11(7):621. https://doi.org/10.3390/toxics11070621
Chicago/Turabian StyleSenoro, Delia B., Maria Mojena G. Plasus, Alejandro Felipe B. Gorospe, Ronnel C. Nolos, Allaine T. Baaco, and Chitsan Lin. 2023. "Metals and Metalloid Concentrations in Fish, Its Spatial Distribution in PPC, Philippines and the Attributable Risks" Toxics 11, no. 7: 621. https://doi.org/10.3390/toxics11070621







