Synthesis and Biodegradation Test of a New Polyether Polyurethane Foam Produced from PEG 400, L-Lysine Ethyl Ester Diisocyanate (L-LDI) and Bis-hydroxymethyl Furan (BHMF)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of HMF from d-Fructose
2.2.2. Synthesis of BHMF from HMF
2.2.3. Prepolymer Synthesis
2.2.4. Polyurethane Synthesis Using BHMF for Chain Extension
2.2.5. Hydrolytic Degradation
2.2.6. Enzymatic Degradation
2.2.7. Soil Burial Degradation Test in the Garden
2.2.8. Characterization
3. Results and Discussion
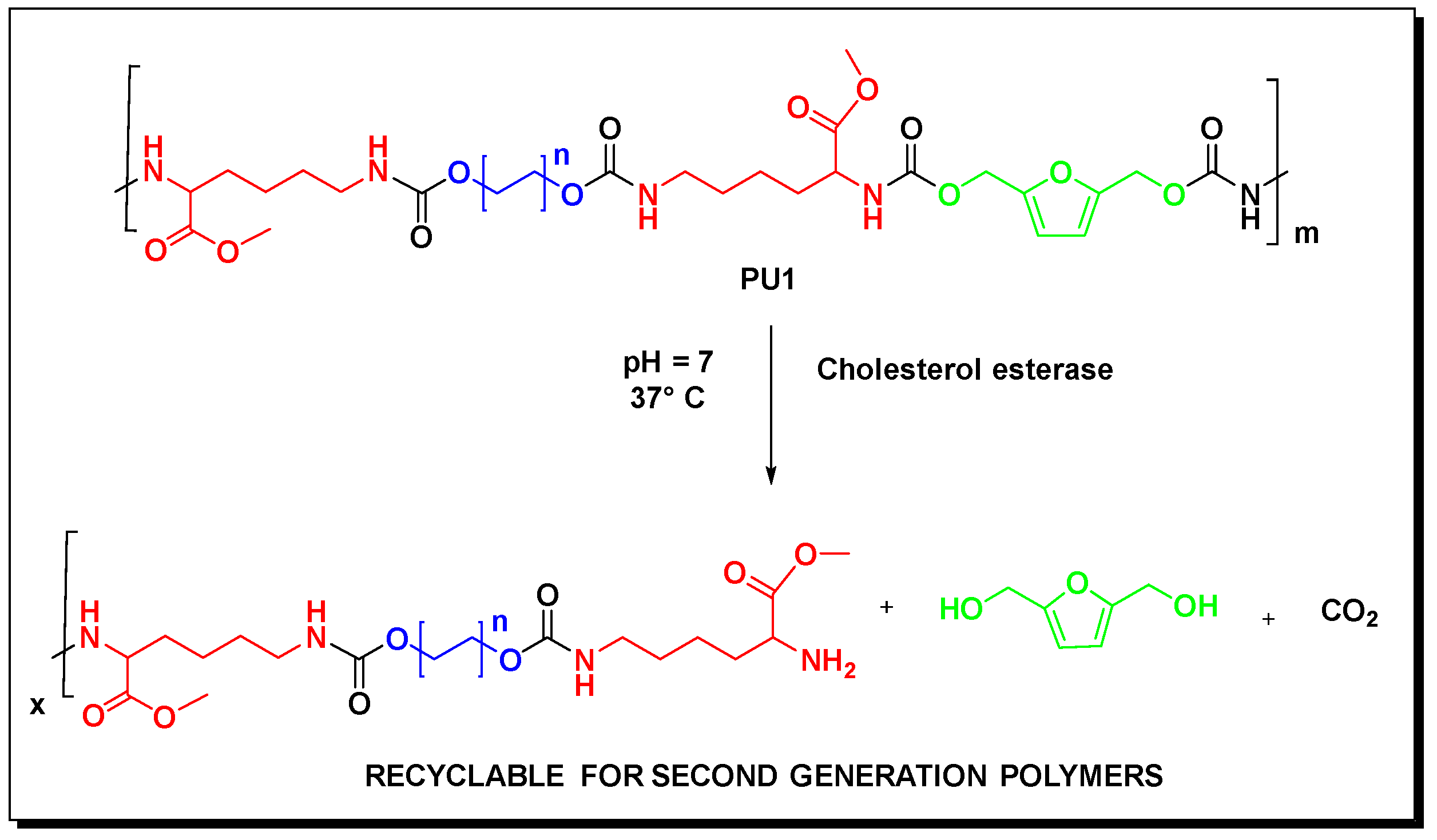
3.1. Synthesis of the Polyurethane PU1
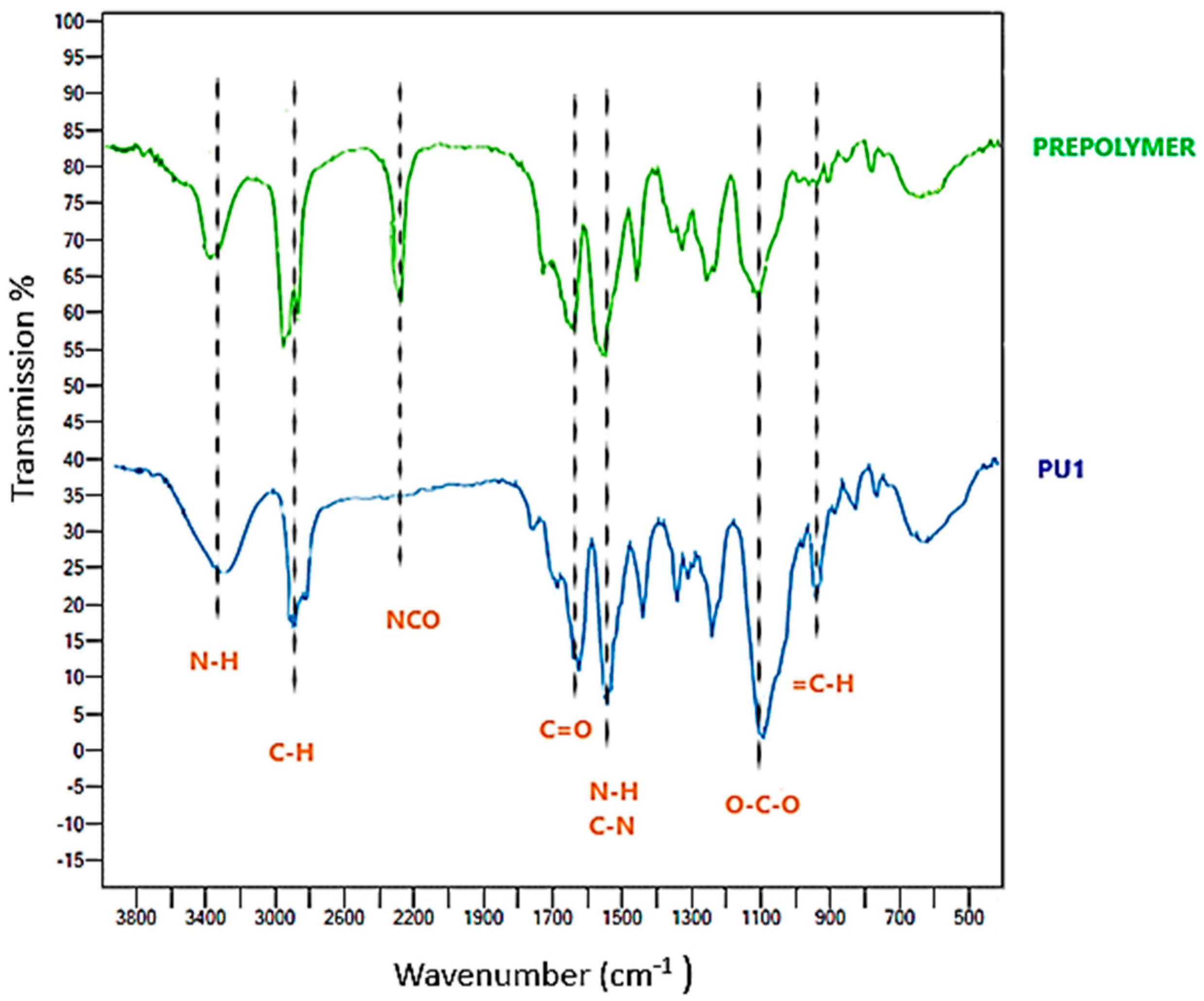
FT-IR Characterization
3.2. Biodegradability Tests
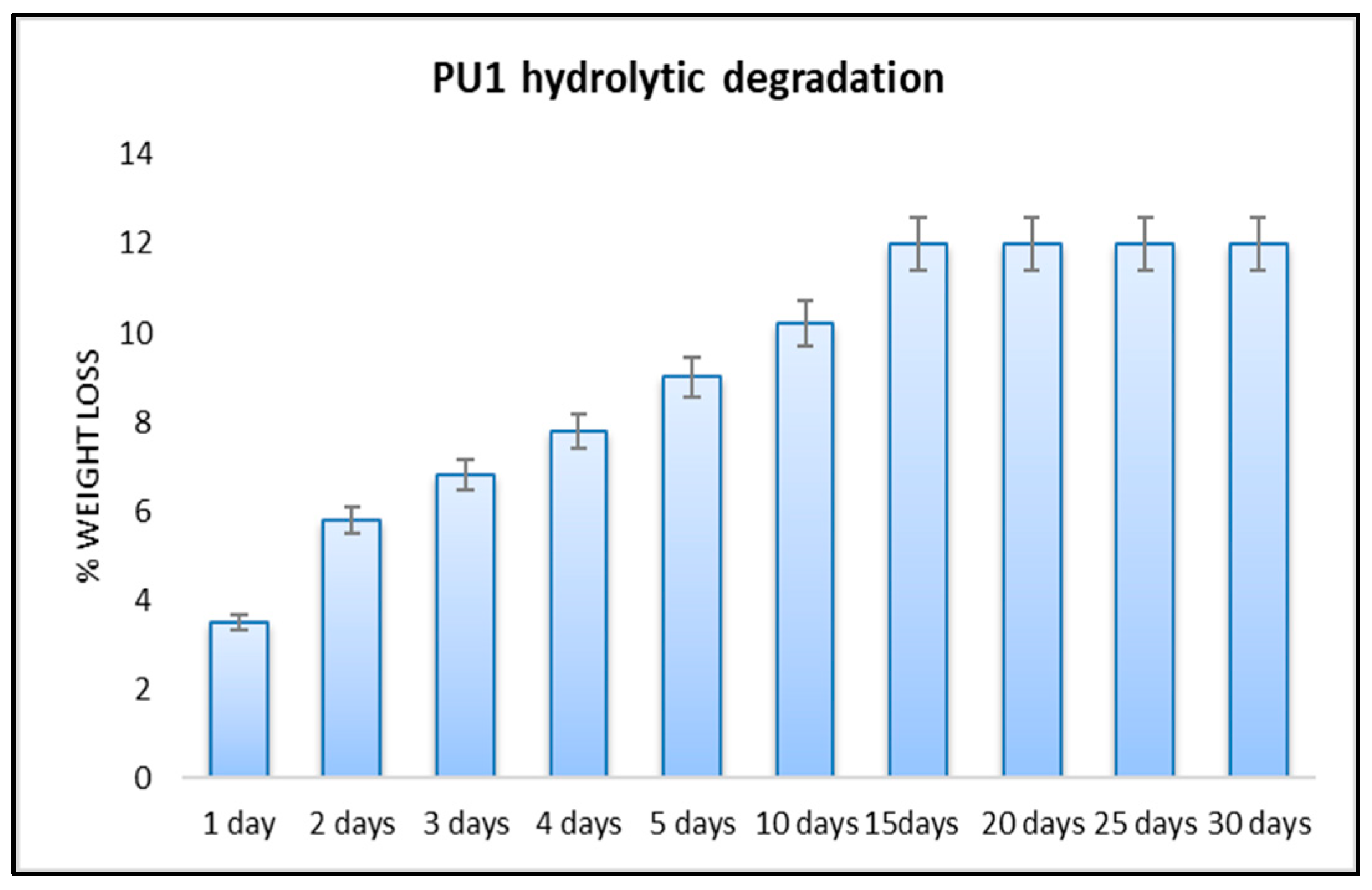
3.2.1. Hydrolytic Degradation
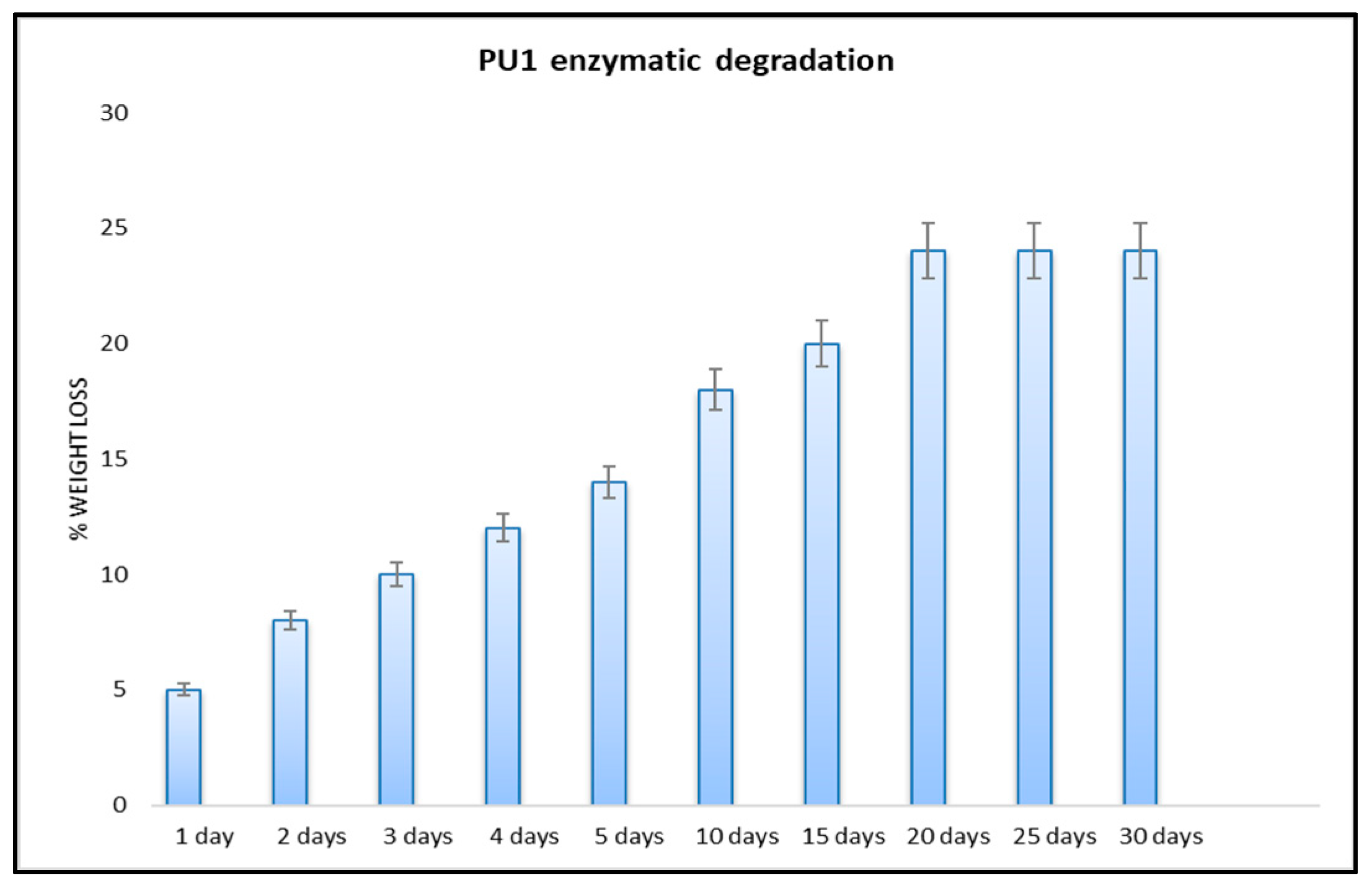
3.2.2. Enzymatic Degradation
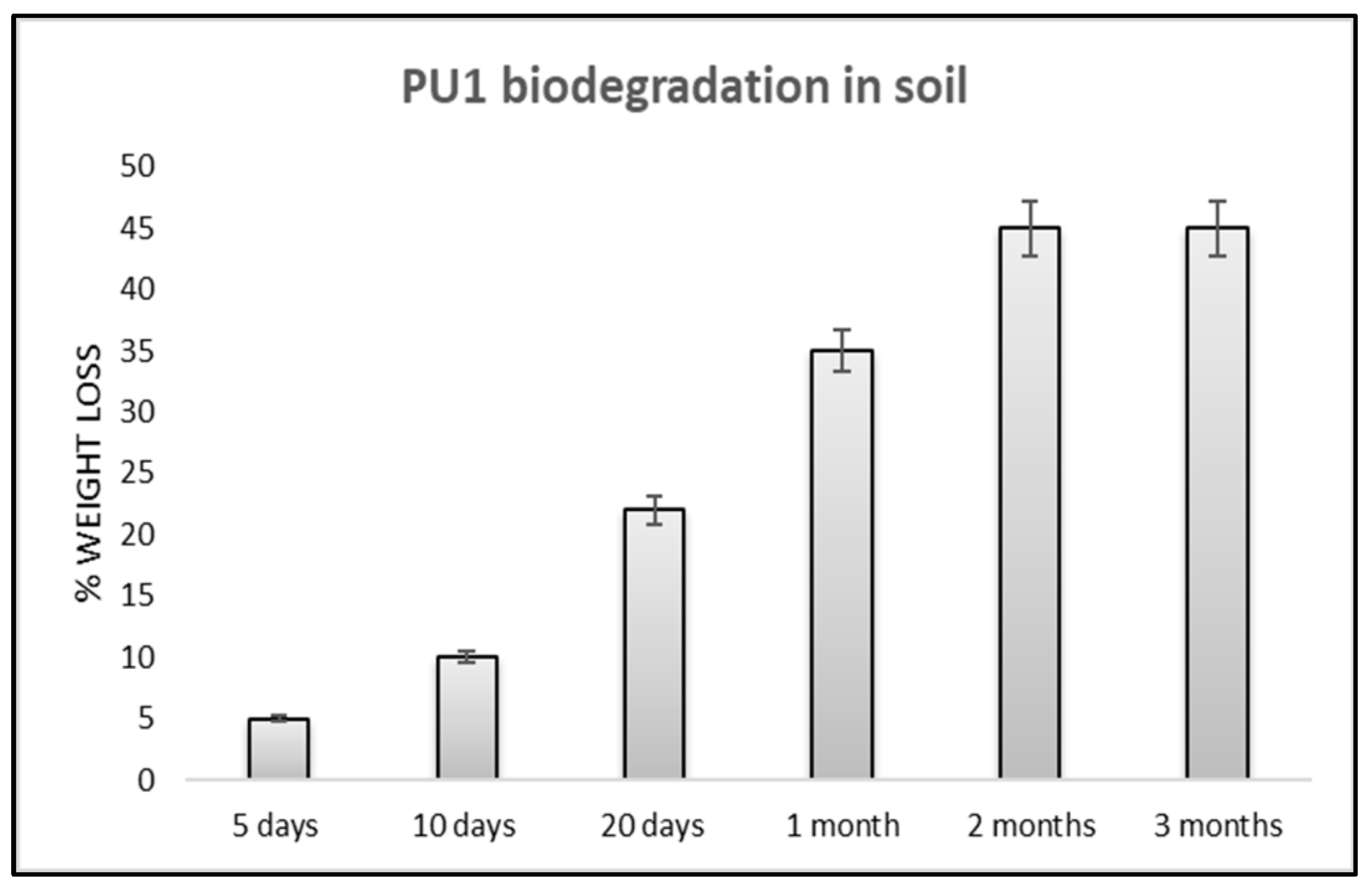
3.2.3. Soil Burial Degradation
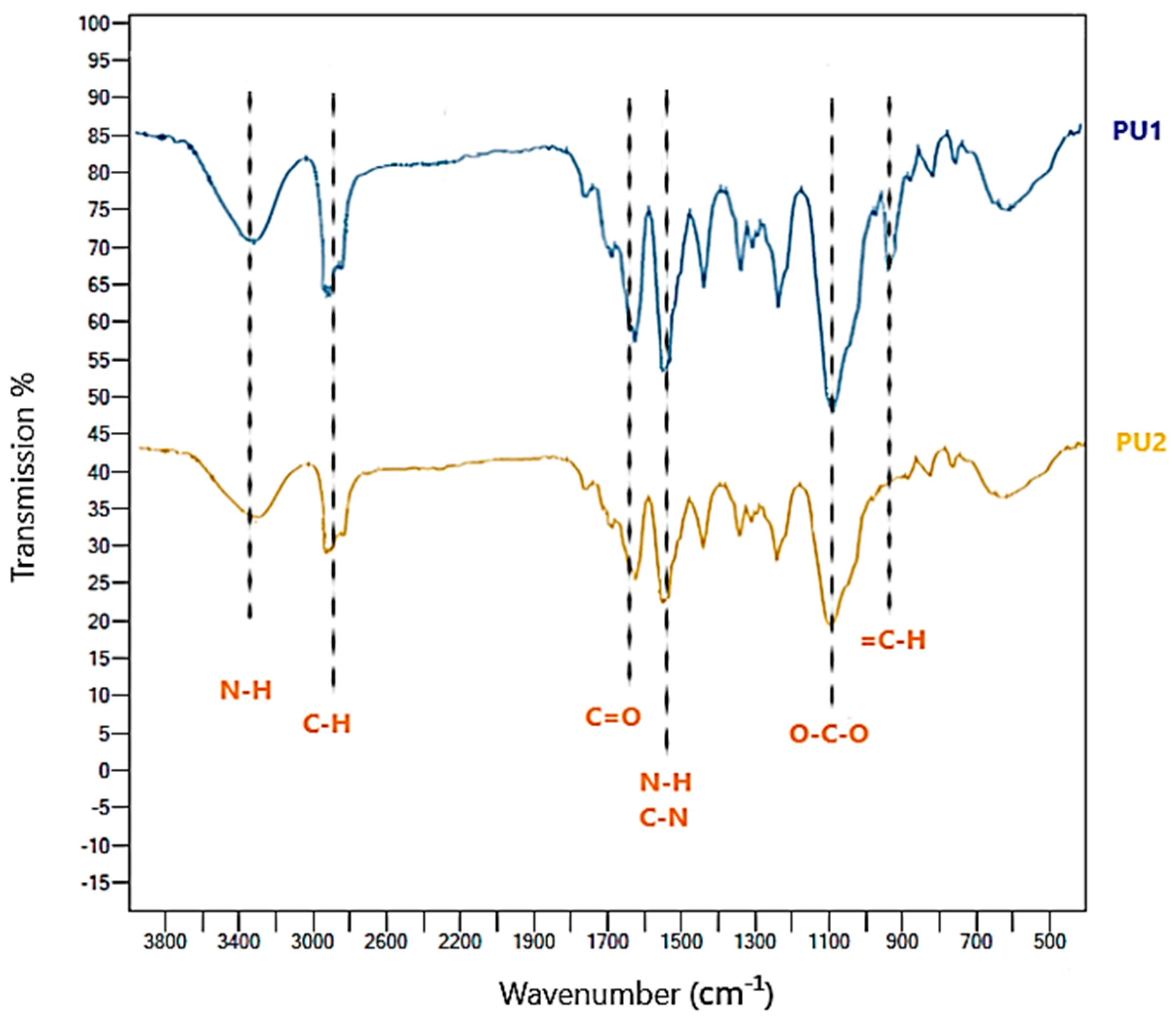
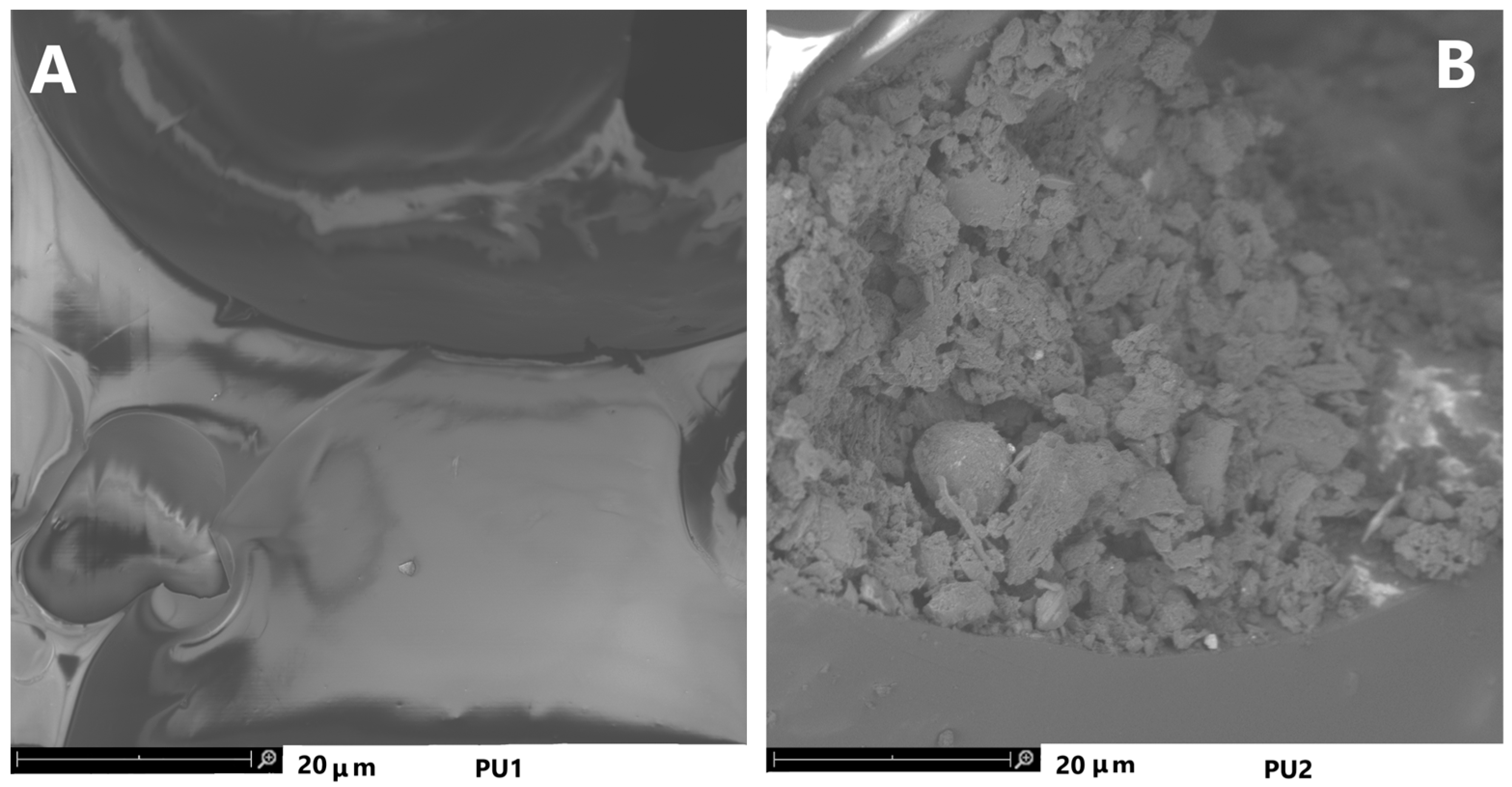
3.2.4. Properties of Polyurethane Foam before (PU1) and after (PU2) Biodegradation in Soil
FT-IR Spectra
SEM Images
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef] [Green Version]
- Bahramian, B.; Fathi, A.; Dehghani, F. A renewable and compostable polymer for reducing consumption of non-degradable plastics. Polym. Degrad. Stab. 2016, 133, 174–181. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhao, H.; Wen, X.; Lin, L.; Schlarb, A.K.; Shi, X. Optimization of 3D printing parameters for high-performance biodegradable materials. J. Appl. Polym. Sci. 2021, 138, e50782. [Google Scholar] [CrossRef]
- De Nino, A.; Olivito, F.; Algieri, V.; Costanzo, P.; Jiritano, A.; Tallarida, M.A.; Maiuolo, L. Efficient and Fast Removal of Oils from Water Surfaces via Highly Oleophilic Polyurethane Composites. Toxics 2021, 9, 186. [Google Scholar] [CrossRef]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopio, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Nardi, M.; Olivito, F.; Procopio, A. Simple and efficient Fmoc removal in ionic liquid. RSC Adv. 2017, 7, 36482–36491. [Google Scholar] [CrossRef] [Green Version]
- Olivito, F.; Amodio, N.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Juli, G.; Tassone, P.; Procopio, A. Synthesis and preliminary evaluation of the anti-cancer activity on A549 lung cancer cells of a series of unsaturated disulfides. Med. Chem. Commun. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Stahl, R.G. Novel methodology for identification and quantification of microplastics in biological samples. Environ. Toxicol. Chem. 2020, 39, 2095–2096. [Google Scholar] [CrossRef]
- Skariyachan, S.A.; Patil, A.; Shankar, A.; Manjunath, M.; Bachappanavar, N.; Kiran, S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018, 149, 52–69. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Hossein Hamidian, A.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Pischedda, A.; Tosin, M.F. Degli-Innocenti, Biodegradation of plastics in soil: The effect of temperature. Polym. Degrad. Stab. 2019, 170, 109017. [Google Scholar] [CrossRef]
- Scarfato, P.; Di Maio, L.; Incarnato, L. Recent advances and migration issues in biodegradable polymers from renewable sources for food packaging. J. Appl. Polym. Sci. 2015, 132, 42597. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Magaziotis, A.; Nerantzaki, M.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and characterization of novel poly(ethylene furanoate-co-adipate) random copolyesters with enhanced biodegradability. Polym. Degrad. Stab. 2018, 156, 32–42. [Google Scholar] [CrossRef]
- Sang, T.; Wallis, C.J.; Hill, G.; Britovsek, G.J.P. Polyethylene terephthalate degradation under natural and accelerated weathering conditions. Eur. Polym. J. 2020, 136, 109873. [Google Scholar] [CrossRef]
- Okoro, C.; Mohammed, Z.; Jeelani, S.; Rangari, V. Plasticizing effect of biodegradable dipropylene glycol bibenzoate and epoxidized linseed oil on diglycidyl ether of bisphenol A based epoxy resin. J. Appl. Polym. Sci. 2021, 138, e50661. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Boonluksiri, Y.; Prapagdee, B.; Sombatsompop, N. Promotion of polylactic acid biodegradation by a combined addition of PLA-degrading bacterium and nitrogen source under submerged and soil burial conditions. Polym. Degrad. Stab. 2021, 188, 109562. [Google Scholar] [CrossRef]
- Patil, P.B.; Sarkar, D.; Poddar, K.; Sarkar, A. Synthesis and characterization of polyhydroxyalkanoates from soil bacterium Bacillus sp. PhNs9. J. Chem. Technol. Biotechnol. 2022, 98, 615–624. [Google Scholar] [CrossRef]
- Fernandes, M.; Salvador, A.M.; Alves, M.; Vicente, A.A. Factors affecting polyhydroxyalkanoates biodegradation in soil. Polym. Degrad. Stab. 2020, 182, 109408. [Google Scholar] [CrossRef]
- Wang, G.-X.; Huang, D.; Ji, J.-H.; Völker, C.; Wurm, F.R. Seawater-Degradable Polymers—Fighting the Marine Plastic Pollution. Adv. Sci. 2021, 8, 2001121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, J.; Wang, J.; Chu, F.; Xu, Z.; Hu, W.; Hu, Y. Rationally designed functionalized black phosphorus nanosheets as new fire hazard suppression material for polylactic acid. Polym. Degrad. Stab. 2020, 178, 109194. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, H.; Li, R.; Gao, S.; Wang, Q.; Wang, G.; Ouyang, X.; Wei, H. High-damping polyurethane/hollow glass microspheres sound insulation materials: Preparation and characterization. J. Appl. Polym. Sci. 2021, 138, e49970. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y.; Wang, J.; Wang, S.; Liu, X. A novel cationic waterborne polyurethane coating modified by chitosan biguanide hydrochloride with application potential in medical catheters. J. Appl. Polym. Sci. 2021, 138, e50290. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [Green Version]
- Datta, J.; Kopczyńska, P.; Simón, D.; Rodríguez, J.F. Thermo-Chemical Decomposition Study of Polyurethane Elastomer Through Glycerolysis Route with Using Crude and Refined Glycerine as a Transesterification Agent. J. Polym. Environ. 2018, 26, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Godinho, B.; Gama, N.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane wastes using different carboxylic acids via acidolysis to produce wood adhesives. J. Polym. Sci. 2021, 59, 697–705. [Google Scholar] [CrossRef]
- Rutkowska, M.; Krasowska, K.; Heimowska, A.; Steinka, I.; Janik, H. Degradation of polyurethanes in sea water. Polym. Degrad. Stab. 2002, 76, 233–239. [Google Scholar] [CrossRef]
- Sardon, H.; Mecerreyes, D.; Basterretxea, A.; Avérous, L.; Jehanno, C. From Lab to Market: Current Strategies for the Production of Biobased Polyols. ACS Sustain. Chem. Eng. 2021, 9, 10664. [Google Scholar] [CrossRef]
- Mosayebi, M.; Sadeghi, G.M.M.; Jamjah, R. Synthesis of waterborne polyurethane nanocomposite adhesives of bio-based polyol from rapeseed cake residual and cellulose nanowhisker. J. Appl. Polym. Sci. 2022, 139, e51954. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Optimization of preparation process to produce polyurethane foam made by oilseed rape straw- based polyol. Polym. Degrad. Stab. 2019, 166, 31–39. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Avérous, L. Chapter Five-Characterization of biodegradation of plastics in insect larvae. In Methods in Enzymology; Weber, G.U., Bornscheuer, T., Wei, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 648, pp. 95–120. [Google Scholar]
- Ravey, M.; Pearce, E.M. Flexible polyurethane foam. I. Thermal decomposition of a polyether-based, water-blown commercial type of flexible polyurethane foam. J. Appl. Polym. Sci. 1997, 63, 47–74. [Google Scholar] [CrossRef]
- Olivito, F.; Algieri, V.; Tallarida, M.A.; Jiritano, A.; Costanzo, P.; Maiuolo, L.; De Nino, A. High-yield synthesis of HMF from glucose and fructose by selective catalysis with water-tolerant rare earth metal triflates assisted by choline chloride. Green Chem. 2023, 25, 1679. [Google Scholar] [CrossRef]
- Trapasso, G.; Mazzi, G.; Chícharo, B.; Annatelli, M.; Dalla Torre, D.; Aricò, F. Multigram Synthesis of Pure HMF and BHMF. Org. Process Res. Dev. 2022, 10, 2830. [Google Scholar] [CrossRef]
- Maiuolo, L.; Olivito, F.; Ponte, F.; Algieri, V.; Tallarida, M.A.; Tursi, A.; Chidichimo, G.; Sicilia, E.; De Nino, A. A novel catalytic two-step process for the preparation of rigid polyurethane foams: Synthesis, mechanism and computational studies. React. Chem. Eng. 2021, 6, 1238. [Google Scholar] [CrossRef]
- Maiuolo, L.; Olivito, F.; Algieri, V.; Costanzo, P.; Jiritano, A.; Tallarida, M.A.; Tursi, A.; Sposato, C.; Feo, A.; De Nino, A. Synthesis, Characterization and Mechanical Properties of Novel Bio-Based Polyurethane Foams Using Cellulose-Derived Polyol for Chain Extension and Cellulose Citrate as a Thickener Additive. Polymers 2021, 13, 2802. [Google Scholar] [CrossRef] [PubMed]
- Acik, O.; Karabulut, H.R.F.; Altinkok, C.; Karatavuk, A.O. Synthesis and characterization of biodegradable polyurethanes made from cholic acid and l-lysine diisocyanate ethyl ester. Polym. Degrad. Stab. 2019, 165, 43–48. [Google Scholar] [CrossRef]
- Han, J.; Cao, R.W.; Chen, B.; Ye, L.; Zhang, A.Y.; Zhang, J.; Feng, Z.G. Electrospinning and biocompatibility evaluation of biodegradable polyurethanes based on llysine diisocyanate and l-lysine chain extender. J. Biomed. Mater. Res. A 2011, 96, 705–714. [Google Scholar] [CrossRef]
- Olivito, F.; Algieri, V.; Jiritano, A.; Tallarida, M.A.; Costanzo, P.; Maiuolo, L.; De Nino, A. Bio-Based Polyurethane Foams for the Removal of Petroleum-Derived Pollutants: Sorption in Batch and in Continuous-Flow. Polymers 2023, 15, 1785. [Google Scholar] [CrossRef]
- Chidichimo, F.; De Biase, M.; Tursi, A.; Maiolo, M.; Straface, S.; Baratta, M.; Olivito, F.; De Filpo, G. A model for the adsorption process of water dissolved elements flowing into reactive porous media: Characterization and sizing of water mining/filtering systems. J. Hazard. Mater. 2023, 445, 130554. [Google Scholar] [CrossRef] [PubMed]
- Olivito, F.; Algieri, V.; Jiritano, A.; Tallarida, M.A.; Tursi, A.; Costanzo, P.; Maiuolo, L.; De Nino, A. Cellulose citrate: A convenient and reusable bio-adsorbent for effective removal of methylene blue dye from artificially contaminated water. RSC Adv. 2021, 11, 34309. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues da Silva, G.; da Silva-Cunha, A.; Behar-Cohen, F.; Ayres, E.; Oréfice, R.L. Biodegradation of polyurethanes and nanocomposites to non-cytotoxic degradation products. Polym. Degrad. Stab. 2010, 95, 491–499. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zavargo, Z.; Flyn, J.H.; Macknight, W.J. Thermal degradation of segmented polyurethanes. J. Appl. Polym. Sci. 1994, 51, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Magnin, A.; Hoornaert, L.; Pollet, E.; Laurichesse, S.; Phalip, V.; Avérous, L. Isolation and characterization of different promising fungi for biological waste management of polyurethanes. Microb. Biotechnol. 2019, 12, 544–555. [Google Scholar] [CrossRef]
- Jung, H.; Carberry, T.P.; Weck, M. Synthesis of First- and Second-Generation Poly(amide)-Dendronized Polymers via Ring-Opening Metathesis Polymerization. Macromolecules 2011, 44, 9075–9083. [Google Scholar] [CrossRef]
- Shen, X.; Dai, J.; Liu, Y.; Liu, X.; Zhu, J. Synthesis of high performance polybenzoxazine networks from bio-based furfurylamine: Furan vs benzene ring. Polymers 2017, 122, 258–269. [Google Scholar] [CrossRef]
- Boraldi, F.; Moscarelli, P.; Demetrio Lofaro, F.; Sabia, C.; Quaglino, D. The mineralization process of insoluble elastin fibrillar structures: Ionic environment vs degradation. Int. J. Biol. Macromol. 2020, 149, 693–706. [Google Scholar] [CrossRef]
- Veskova, J.; Sbordone, F.; Frisch, H. Trends in Polymer Degradation Across All Scales. Macromol. Chem. Phys. 2022, 223, 2100472. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Trajano, H.L.; Posarac, D.; Duf, S.J.B. Enzyme Recycling by Adsorption during Hydrolysis of Oxygen-Delignified Wheat Straw. ACS Sustain. Chem. Eng. 2017, 5, 9701. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyauchi, S. Poly(l-lactide): VI Effects of crystallinity on enzymatic hydrolysis of poly(l-lactide) without free amorphous region. Polym. Degrad. Stab. 2001, 71, 415–424. [Google Scholar] [CrossRef]
- Donelli, I.; Freddi, G.; Nierstrasz, V.A.; Taddei, P. Surface structure and properties of poly-(ethylene terephthalate) hydrolyzed by alkali and cutinase. Polym. Degrad. Stab. 2010, 95, 1542–1550. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, J.; Yao, M.; Jiang, Z.; Ma, Y. Hydrolysis-resistant polyurethane elastomers synthesized from hydrophobic bio-based polyfarnesene diol. J. Appl. Polym. Sci. 2019, 136, 47673. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Biodegradation and thermal stability of bacterial cellulose as biomaterial: The relevance in biomedical applications. Polym. Degrad. Stab. 2020, 179, 109232. [Google Scholar] [CrossRef]
- Pantani, R.; Sorrentino, A. Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions. Polym. Degrad. Stab. 2013, 98, 1089–1096. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Pitucha, M. Application of FTIR method for the assessment of immobilization of active substances in the matrix of biomedical materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, B.; Stutzman, J.; Xie, A. A vibrational spectral maker for probing the hydrogen-bonding status of protonated asp and glu residues. Biophys. J. 2005, 88, 2833–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivito, F.; Jagdale, P.; Oza, G. Synthesis and Biodegradation Test of a New Polyether Polyurethane Foam Produced from PEG 400, L-Lysine Ethyl Ester Diisocyanate (L-LDI) and Bis-hydroxymethyl Furan (BHMF). Toxics 2023, 11, 698. https://doi.org/10.3390/toxics11080698
Olivito F, Jagdale P, Oza G. Synthesis and Biodegradation Test of a New Polyether Polyurethane Foam Produced from PEG 400, L-Lysine Ethyl Ester Diisocyanate (L-LDI) and Bis-hydroxymethyl Furan (BHMF). Toxics. 2023; 11(8):698. https://doi.org/10.3390/toxics11080698
Chicago/Turabian StyleOlivito, Fabrizio, Pravin Jagdale, and Goldie Oza. 2023. "Synthesis and Biodegradation Test of a New Polyether Polyurethane Foam Produced from PEG 400, L-Lysine Ethyl Ester Diisocyanate (L-LDI) and Bis-hydroxymethyl Furan (BHMF)" Toxics 11, no. 8: 698. https://doi.org/10.3390/toxics11080698





