Molecular Mechanisms of Selenium Mitigating Lead Toxicity in Chickens via Mitochondrial Pathway: Selenoproteins, Oxidative Stress, HSPs, and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Sample Collection
2.3. The Detection of Oxidative Stress Indexes
2.4. Microstructural Observation
2.5. Ultrastructural Observation
2.6. The Detection of Genes at the Transcriptional Level
2.6.1. Primers
2.6.2. Total RNA Extraction and cDNA Synthesis
2.6.3. PCR Amplification
2.7. The Calculation of Integrated Biomarker Response Value
2.8. Multivariate Correlation Analysis and Principal Component Analysis
2.9. Statistical Analyses
3. Results
3.1. Morphological Structural Changes
3.2. H2O2 Content and Antioxidant Enzyme Activities
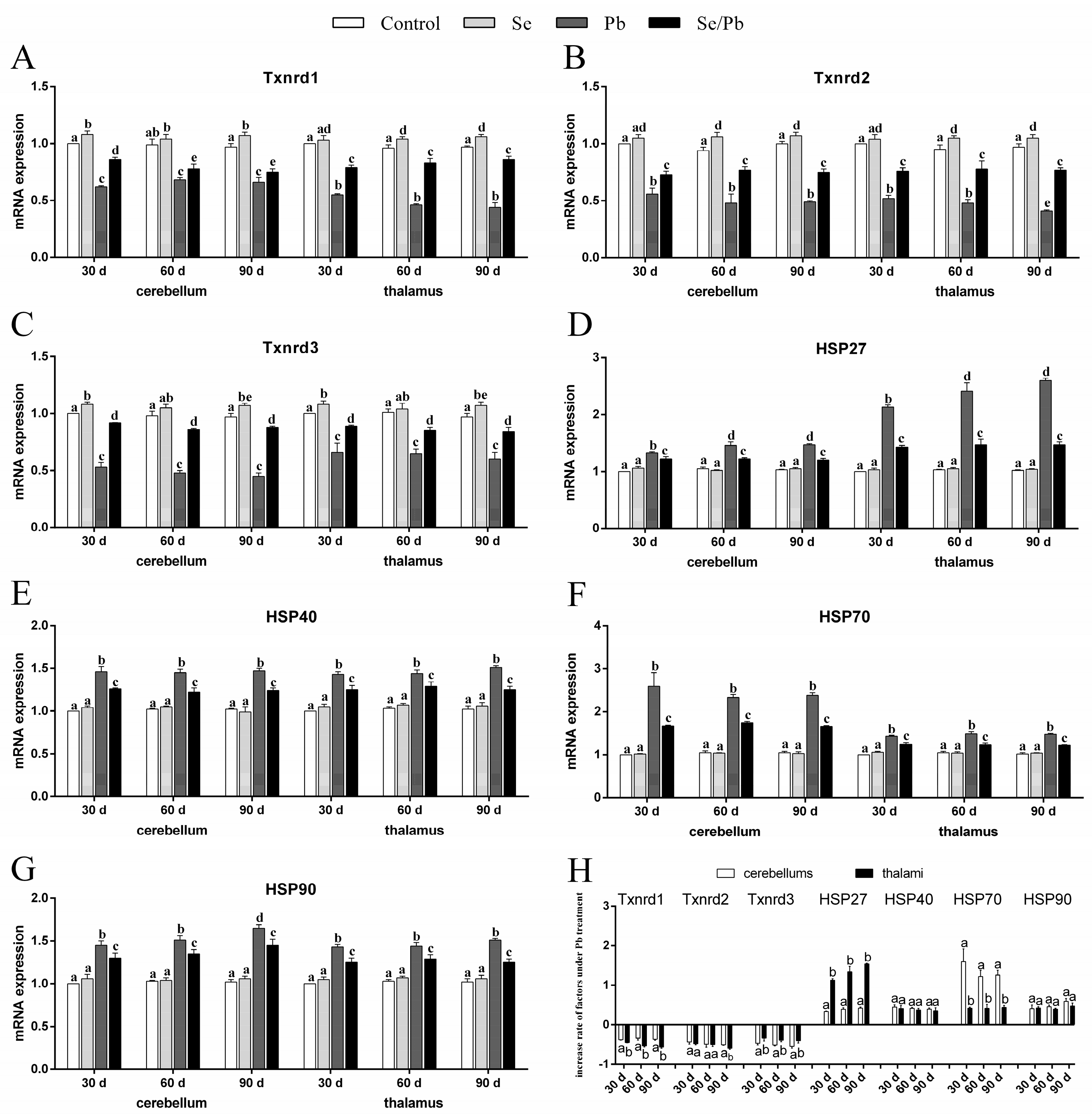
3.3. mRNA Levels of Txnrds and HSPs
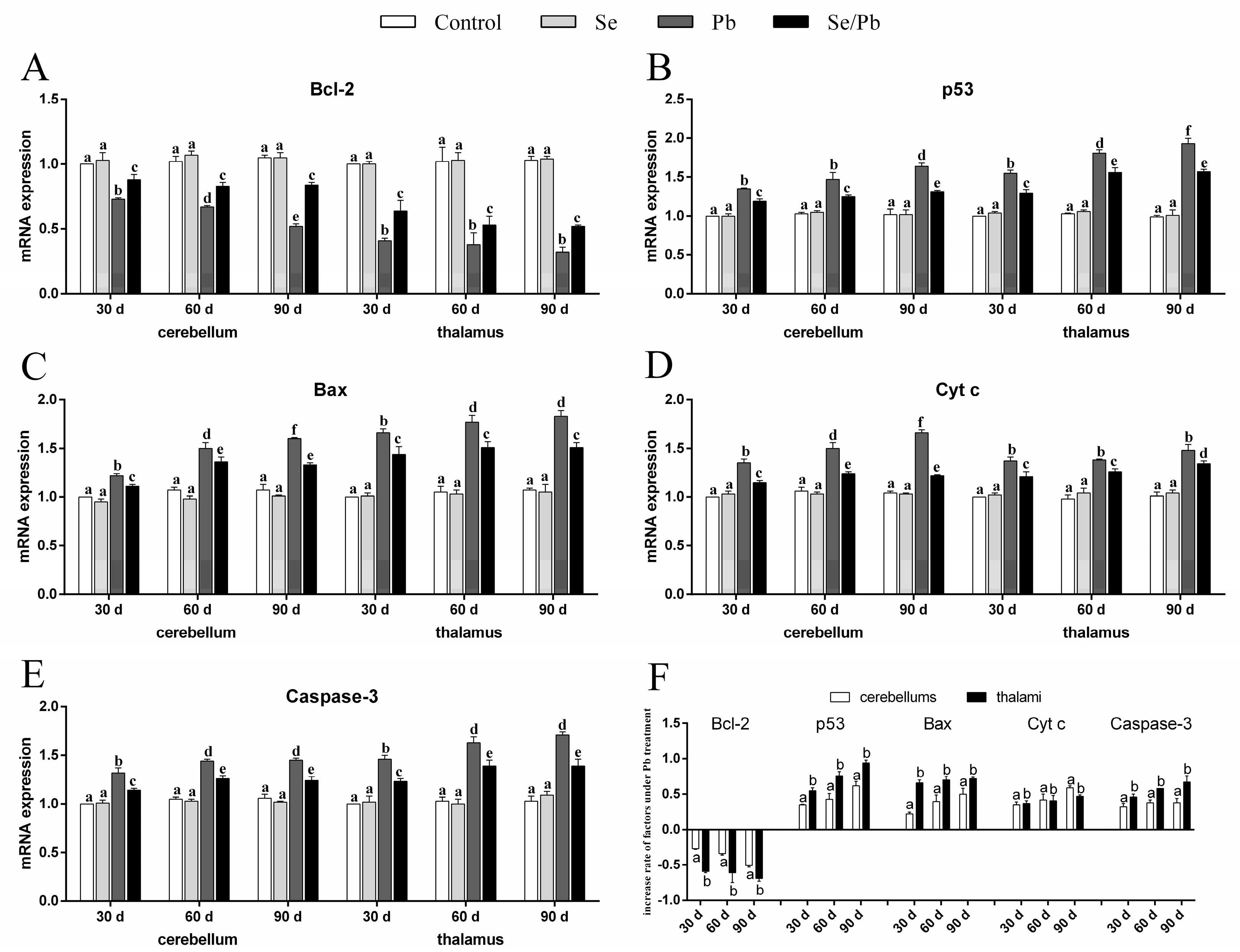
3.4. mRNA Levels of Apoptosis-Related Genes
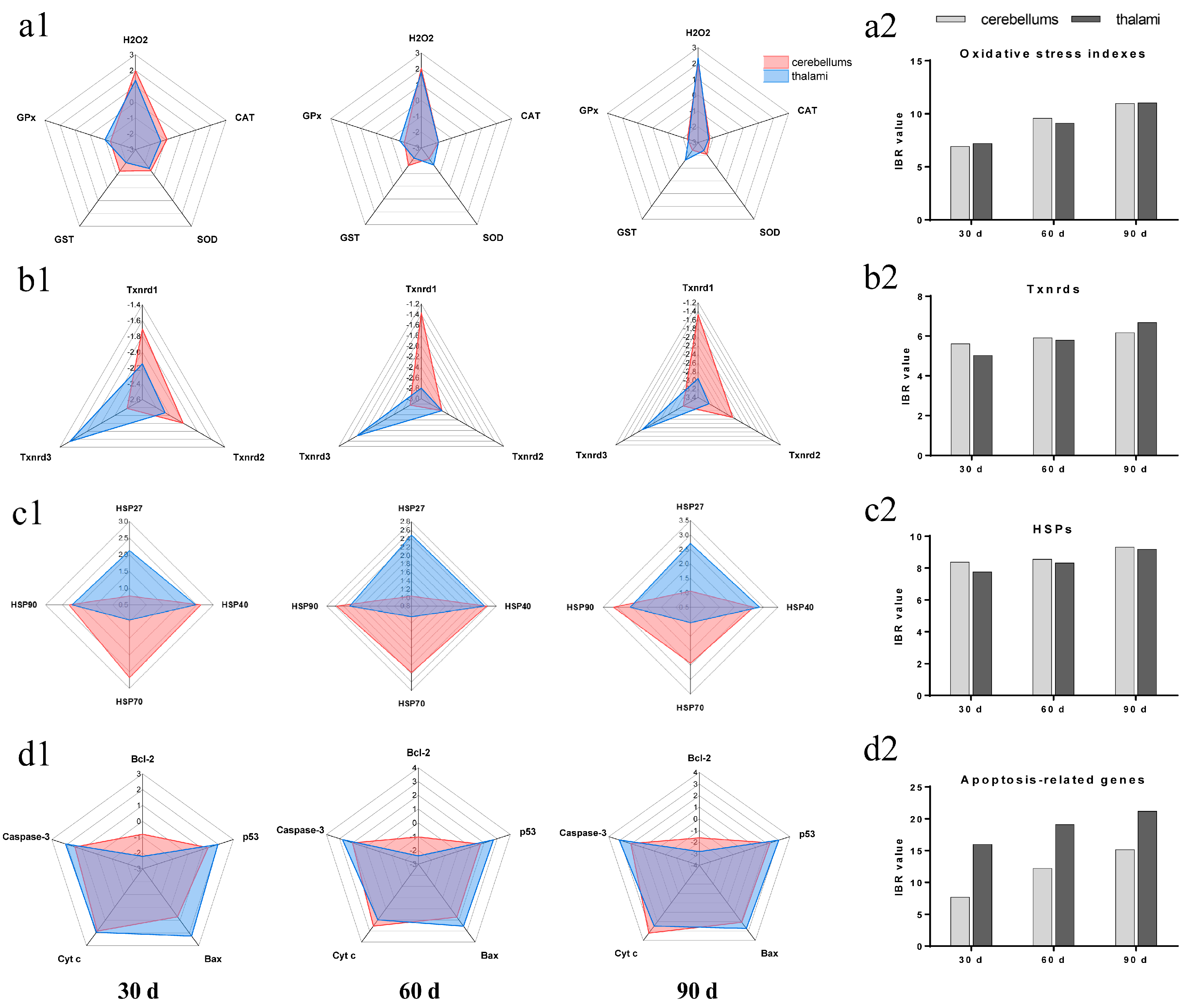
3.5. IBR Values for Txnrds, Oxidative Stress Indexes, HSPs, and Apoptosis-Related Genes
3.6. Correlation Matrix of All Measured Factors
3.7. PCA Results of the Seventeen Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kaneko, M.; Kazatani, T.; Shikata, H. Occupational Lead Poisoning in a Patient with Acute Abdomen and Normocytic Anemia. Intern. Med. 2020, 59, 1565–1570. [Google Scholar] [CrossRef]
- Ansari, J.A.; Mahdi, A.A.; Malik, P.S.; Jafar, T. Blood Lead Levels in Children Living Near an Informal Lead Battery Recycling Workshop in Patna, Bihar. J. Health Pollut. 2020, 10, 200308. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, E.; Camarero, P.R.; Sánchez-Barbudo, I.S.; Martinez-Haro, M.; Ortiz-Santaliestra, M.E.; Moreno-Opo, R.; Mateo, R. Integrating active and passive monitoring to assess sublethal effects and mortality from lead poisoning in birds of prey. Sci. Total. Environ. 2020, 750, 142260. [Google Scholar] [CrossRef]
- Yazdanparast, T.; Strezov, V.; Wieland, P.; Lai, Y.-J.; Jacob, D.E.; Taylor, M.P. Lead poisoning of backyard chickens: Implications for urban gardening and food production. Environ. Pollut. 2022, 310, 119798. [Google Scholar] [CrossRef] [PubMed]
- Leão, L.K.R.; Bittencourt, L.O.; Oliveira, A.C.; Nascimento, P.C.; Miranda, G.H.N.; Ferreira, R.O.; Nabiça, M.; Dantas, K.; Dionizio, A.; Cartágenes, S.; et al. Long-Term Lead Exposure Since Adolescence Causes Proteomic and Morphological Alterations in the Cerebellum Associated with Motor Deficits in Adult Rats. Int. J. Mol. Sci. 2020, 21, 3571. [Google Scholar] [CrossRef] [PubMed]
- Albores-Garcia, D.; McGlothan, J.L.; Bursac, Z.; Guilarte, T.R. Chronic developmental lead exposure increases μ-opiate receptor levels in the adolescent rat brain. NeuroToxicology 2021, 82, 119–129. [Google Scholar] [CrossRef]
- Huang, H.; Wang, M.; Hou, L.; Lin, X.; Pan, S.; Zheng, P.; Zhao, Q. A potential mechanism associated with lead-induced spermatogonia and Leydig cell toxicity and mitigative effect of selenium in chicken. Ecotoxicol. Environ. Saf. 2020, 209, 111671. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiao, X.; An, Y.; Li, S.; Teng, X. Selenium against lead-induced apoptosis in chicken nervous tissues via mitochondrial pathway. Oncotarget 2017, 8, 108130–108145. [Google Scholar] [CrossRef]
- Fan, R.-F.; Liu, J.-X.; Yan, Y.-X.; Wang, L.; Wang, Z.-Y. Selenium relieves oxidative stress, inflammation, and apoptosis within spleen of chicken exposed to mercuric chloride. Poult. Sci. 2020, 99, 5430–5439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, H.; Hao, D.; Wang, J.; Zhu, R.; Liu, W.; Liu, C. Selenium regulates the mitogen-activated protein kinase pathway to protect broilers from hexavalent chromium-induced kidney dysfunction and apoptosis. Ecotoxicol. Environ. Saf. 2022, 239, 113629. [Google Scholar] [CrossRef] [PubMed]
- Elwej, A.; Ghorbel, I.; Chaabane, M.; Soudani, N.; Mnif, H.; Boudawara, T.; Zeghal, N.; Sefi, M. Zinc and selenium modulate barium-induced oxidative stress, cellular injury and membrane-bound ATPase in the cerebellum of adult rats and their offspring during late pregnancy and early postnatal periods. Arch. Physiol. Biochem. 2017, 124, 237–246. [Google Scholar] [CrossRef]
- Abubakar, K.; Muhammad Mailafiya, M.; Danmaigoro, A.; Musa Chiroma, S.; Abdul Rahim, E.B.; Abu Bakar Zakaria, M.Z. Curcumin attenuates lead-induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress. Biomolecules 2019, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cabungcal, J.-H.; Cuenod, M.; Uliana, D.L.; Do, K.Q.; Grace, A.A. Thalamic reticular nucleus impairments and abnormal prefrontal control of dopamine system in a developmental model of schizophrenia: Prevention by N-acetylcysteine. Mol. Psychiatry 2021, 26, 7679–7689. [Google Scholar] [CrossRef]
- Wang, S.; Hou, L.; Wang, M.; Feng, R.; Lin, X.; Pan, S.; Zhao, Q.; Huang, H. Selenium-Alleviated Testicular Toxicity by Modulating Inflammation, Heat Shock Response, and Autophagy Under Oxidative Stress in Lead-Treated Chickens. Biol. Trace Element Res. 2021, 199, 4700–4712. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Selective Evaluation of Thioredoxin Reductase Enzymatic Activities. Methods Mol. Biol. 2017, 1661, 301–309. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Xu, W.; Zhang, Y.; Sun, Q.; Li, Z.; Geng, L.; Teng, X. Transcriptome analysis revealed the mechanism of Luciobarbus capito (L. capito) adapting high salinity: Antioxidant capacity, heat shock proteins, immunity. Mar. Pollut. Bull. 2023, 192, 115017. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, K.; Bao, R.; Li, J.; Tang, Y.; Teng, X. Th1/Th2 imbalance and heat shock protein mediated inflammatory damage triggered by manganese via activating NF-κB pathway in chicken nervous system in vivo and in vitro. Environ. Sci. Pollut. Res. 2021, 28, 44361–44373. [Google Scholar] [CrossRef]
- Tan, S.; Chi, Q.; Liu, T.; Sun, Z.; Min, Y.; Zhang, Z.; Li, S. Alleviation Mechanisms of Selenium on Cadmium-Spiked Neutrophil Injury to Chicken. Biol. Trace Element Res. 2017, 178, 301–309. [Google Scholar] [CrossRef]
- Vengris, V.E.; Maré, C.J. Lead poisoning in chickens and the effect of lead on interferon and antibody production. Can. J. Comp. Med. Rev. Can. Med. Comp. 1974, 38, 328–335. [Google Scholar]
- Liu, Y.; Lin, X.; Hao, Z.; Yu, M.; Tang, Y.; Teng, X.; Sun, W.; Kang, L. Cadmium exposure caused cardiotoxicity in common carps (Cyprinus carpio L.): miR-9-5p, oxidative stress, energetic impairment, mitochondrial division/fusion imbalance, inflammation, and autophagy. Fish Shellfish. Immunol. 2023, 138, 108853. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Miao, Z.; Teng, X.; Xu, S. Melatonin alleviates lead-induced intestinal epithelial cell pyroptosis in the common carps (Cyprinus carpio) via miR-17-5p/TXNIP axis. Fish Shellfish. Immunol. 2022, 131, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Hao, Z.; Zhou, Q.; Qiu, M.; Liu, Y.; Liu, Y.; Teng, X.; Kang, L. Chlorpyrifos induced autophagy and mitophagy in common carp livers through AMPK pathway activated by energy metabolism disorder. Ecotoxicol. Environ. Saf. 2023, 258, 114983. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, Y.; Hao, Z.; Liu, Y.; Qiu, M.; Kang, L.; Teng, X.; Tang, Y. Cadmium induced time-dependent kidney injury in common carp via mitochondrial pathway: Impaired mitochondrial energy metabolism and mitochondrion-dependent apoptosis. Aquat. Toxicol. 2023, 261, 106570. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.P.; Teng, X.H.; Fu, J. Effects of Dietary Selenium Against Lead Toxicity Are Related to the Ion Profile in Chicken Muscle. Biol. Trace Element Res. 2016, 172, 496–503. [Google Scholar] [CrossRef]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish. Immunol. 2018, 86, 239–245. [Google Scholar] [CrossRef]
- Shi, X.; Xu, W.; Che, X.; Cui, J.; Shang, X.; Teng, X.; Jia, Z. Effect of arsenic stress on the intestinal structural integrity and intestinal flora abundance of Cyprinus carpio. Front. Microbiol. 2023, 14, 1179397. [Google Scholar] [CrossRef]
- Zhao, C.; Teng, X.; Yue, W.; Suo, A.; Zhou, W.; Ding, D. The effect of acute toxicity from tributyltin on Liza haematocheila liver: Energy metabolic disturbance, oxidative stress, and apoptosis. Aquat. Toxicol. 2023, 258, 106506. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wang, Y.-L.; Luo, Z.; Zheng, C.; Yu, G.; Wu, S.; Zheng, F.; Li, H. NOX2 activation contributes to cobalt nanoparticles-induced inflammatory responses and Tau phosphorylation in mice and microglia. Ecotoxicol. Environ. Saf. 2021, 225, 112725. [Google Scholar] [CrossRef]
- Cui, J.; Qiu, M.; Liu, Y.; Liu, Y.; Tang, Y.; Teng, X.; Li, S. Nano-selenium protects grass carp hepatocytes against 4-tert-butylphenol-induced mitochondrial apoptosis and necroptosis via suppressing ROS-PARP1 axis. Fish Shellfish. Immunol. 2023, 135, 108682. [Google Scholar] [CrossRef]
- Wang, L.; Jing, L.; Zhang, Q.; Li, S.; Wang, Y.; Zhao, H. Lead induced thymic immunosuppression in Japanese quail (Coturnix japonica) via oxidative stress-based T cell receptor pathway signaling inhibition. J. Inorg. Biochem. 2022, 235, 111950. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zong, C.; Cheng, K. Chicken thalamic injury induced by copper (II) or/and arsenite exposure involves oxidative stress and inflammation-induced apoptosis. Ecotoxicol. Environ. Saf. 2020, 197, 110554. [Google Scholar] [CrossRef] [PubMed]
- Gholamigeravand, B.; Shahidi, S.; Amiri, I.; Samzadeh-Kermani, A.; Abbasalipourkabir, R.; Asl, S.S. Administration of Selenium Nanoparticles Reverses Streptozotocin-Induced Neurotoxicity in the male rats. Metab. Brain Dis. 2021, 36, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-X.; Chu, J.-H.; Chen, X.-W.; Gao, P.-C.; Wang, Z.-Y.; Liu, C.; Fan, R.-F. Selenium ameliorates mercuric chloride-induced brain damage through activating BDNF/TrKB/PI3K/AKT and inhibiting NF-κB signaling pathways. J. Inorg. Biochem. 2022, 229, 111716. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, A.B.; Eren, B.; Sağir, D.; Yilmaz, B.D. Inhibition of acrolein-induced apoptosis by the antioxidant selenium. Toxicol. Ind. Health 2020, 36, 84–92. [Google Scholar] [CrossRef]
- Fannami, I.M.; Garba, S.H.; Chiroma, S.M. Adansonia digitata L. fruit shell extract alleviates lead-induced neurotoxicity in mice via modulation of oxidative stress and a possible chelating activity. J. Trace Elements Med. Biol. 2022, 74, 127074. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.-W.; Talukder, M.; Guo, K.; Li, Y.-H.; Li, J.-L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef]
- Guo, H.; Yin, H.; Zuo, Z.; Yang, Z.; Yang, Y.; Wei, L.; Cui, H.; Deng, H.; Chen, X.; Chen, J.; et al. Oxidative stress-mediated apoptosis and autophagy involved in Ni-induced nephrotoxicity in the mice. Ecotoxicol. Environ. Saf. 2021, 228, 112954. [Google Scholar] [CrossRef]
- Saxena, G.; Pathak, U.; Flora, S. Beneficial role of monoesters of meso-2,3-dimercaptosuccinic acid in the mobilization of lead and recovery of tissue oxidative injury in rats. Toxicology 2005, 214, 39–56. [Google Scholar] [CrossRef]
- Liu, S.; Wu, W.; Chen, Q.; Zheng, Z.; Jiang, X.; Xue, Y.; Lin, D. TXNRD1: A Key Regulator Involved in the Ferroptosis of CML Cells Induced by Cysteine Depletion In Vitro. Oxidative Med. Cell. Longev. 2021, 2021, 7674565. [Google Scholar] [CrossRef]
- Bu, L.; Li, W.; Ming, Z.; Shi, J.; Fang, P.; Yang, S. Inhibition of TrxR2 suppressed NSCLC cell proliferation, metabolism and induced cell apoptosis through decreasing antioxidant activity. Life Sci. 2017, 178, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, W.; Liu, Q.; Liu, P.; Qiao, S.; Xu, L.; Sun, Y.; Gai, X.; Zhang, Z. Decreased thioredoxin reductase 3 expression promotes nickel-induced damage to cardiac tissue via activating oxidative stress-induced apoptosis and inflammation. Environ. Toxicol. 2023, 38, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Yan, D.; Liu, S.; Gao, N.; Ma, Z.; Shi, Z.; Dong, N.; Shan, A. Host Defense Peptides in Nutrition and Diseases: A Contributor of Immunology Modulation. J. Agric. Food Chem. 2023, 71, 3125–3140. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhu, J.; Li, S.; Tang, C.; Zhao, Q.; Zhang, J. Selenium supplementation protects against oxidative stress-induced cardiomyocyte cell cycle arrest through activation of PI3K/AKT. Metallomics 2020, 12, 1965–1978. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, L.; Zhang, R. Effects of Se and Cd Co-treatment on the Morphology, Oxidative Stress, and Ion Concentrations in the Ovaries of Laying Hens. Biol. Trace Element Res. 2017, 183, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Zhang, H.; Yuan, L.; Zhao, L.; Liu, R. Molecular mechanisms of lead-induced changes of selenium status in mice livers through interacting with selenoprotein P. Ecotoxicol. Environ. Saf. 2019, 175, 282–288. [Google Scholar] [CrossRef]
- He, B.; Xu, Z.; Hao, W.; Wang, S. Antagonistic action of organic selenium on lead poisoning. Wei Sheng Yan Jiu 1998, 27, 229–232. [Google Scholar]
- Dong, F.; Xiao, P.; Li, X.; Chang, P.; Zhang, W.; Wang, L. Cadmium triggers oxidative stress and mitochondrial injury mediated apoptosis in human extravillous trophoblast HTR-8/SVneo cells. Reprod. Toxicol. 2021, 101, 18–27. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, F.; Zhang, Z.; Xu, M.; Xu, Y.; Liu, Y.; Xu, H.; Sun, X.; Sang, M.; Luo, H. P53 inhibitor pifithrin-α inhibits ropivacaine-induced neuronal apoptosis via the mitochondrial apoptosis pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22822. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, C.; Xie, T.; Wang, D.; Chen, X.; Ma, L.; Zhang, A. Role of inhibiting Chk1-p53 pathway in hepatotoxicity caused by chronic arsenic exposure from coal-burning. Hum. Exp. Toxicol. 2021, 40, 1141–1152. [Google Scholar] [CrossRef]
- Wang, H.; Wang, A.; Wang, X.; Zeng, X.; Xing, H. AMPK/PPAR-γ/NF-κB axis participates in ROS-mediated apoptosis and autophagy caused by cadmium in pig liver. Environ. Pollut. 2022, 294, 118659. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-O.; Lee, J.-C.; Hitron, J.A.; Pan, J.; Zhang, Z.; Shi, X. Cadmium Induces Intracellular Ca2+- and H2O2-Dependent Apoptosis through JNK- and p53-Mediated Pathways in Skin Epidermal Cell line. Toxicol. Sci. 2010, 113, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; D’ascola, A.; Vicchio, T.M.; Campo, S.; Gianì, F.; Giovinazzo, S.; Frasca, F.; Cannavò, S.; Campennì, A.; Trimarchi, F. Selenium exerts protective effects against oxidative stress and cell damage in human thyrocytes and fibroblasts. Endocrine 2019, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, K.; Roshan-Milani, S.; Asgharzadeh, F.; Pourjabali, M.; Fard, A.A. In Vitro and In Vivo Pretreatment with Selenium Mitigates Tetrahydrocannabinol-Induced Testicular Cell Apoptosis: The Role of AKT and p53 Pathways. Biol. Trace Element Res. 2020, 199, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xu, Z.; Shi, Y.; Pei, F.; Yang, W.; Ma, N.; Kimatu, B.M.; Liu, K.; Qiu, W.; Hu, Q. Protection mechanism of Se-containing protein hydrolysates from Se-enriched rice on Pb2+-induced apoptosis in PC12 and RAW264.7 cells. Food Chem. 2017, 219, 391–398. [Google Scholar] [CrossRef]
- Nie, X.; Wang, Y.; Zhao, H.; Guo, M.; Liu, Y.; Xing, M. As3+ or/and Cu2+ exposure triggers oxidative stress imbalance, induces inflammatory response and apoptosis in chicken brain. Ecotoxicol. Environ. Saf. 2020, 203, 110993. [Google Scholar] [CrossRef]
- Bianchi, A.; Moulin, D.; Hupont, S.; Koufany, M.; Netter, P.; Reboul, P.; Jouzeau, J.-Y. Oxidative stress-induced expression of HSP70 contributes to the inhibitory effect of 15d-PGJ2 on inducible prostaglandin pathway in chondrocytes. Free. Radic. Biol. Med. 2014, 76, 114–126. [Google Scholar] [CrossRef]
- Profumo, E.; Buttari, B.; Tinaburri, L.; D’arcangelo, D.; Sorice, M.; Capozzi, A.; Garofalo, T.; Facchiano, A.; Businaro, R.; Kumar, P.; et al. Oxidative Stress Induces HSP90 Upregulation on the Surface of Primary Human Endothelial Cells: Role of the Antioxidant 7,8-Dihydroxy-4-methylcoumarin in Preventing HSP90 Exposure to the Immune System. Oxidative Med. Cell. Longev. 2018, 2018, 2373167. [Google Scholar] [CrossRef]
- Zhong, X.; Li, W.; Huang, X.; Zhang, L.; Yimamu, M.; Raiput, N.; Zhou, Y.; Wang, T. Impairment of cellular immunity is associated with overexpression of heat shock protein 70 in neonatal pigs with intrauterine growth retardation. Cell Stress Chaperones 2012, 17, 495–505. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, Y.; Xing, H.; Xu, S. Ameliorative Effect of Selenomethionine on Cadmium-Induced Hepatocyte Apoptosis via Regulating PI3K/AKT Pathway in Chickens. Biol. Trace Element Res. 2020, 195, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, M.; Cui, J.; Du, Y.; Teng, X.; Zhang, Z. Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-κB pathway in excess manganese-treated chicken livers. Ecotoxicol. Environ. Saf. 2021, 226, 112833. [Google Scholar] [CrossRef] [PubMed]

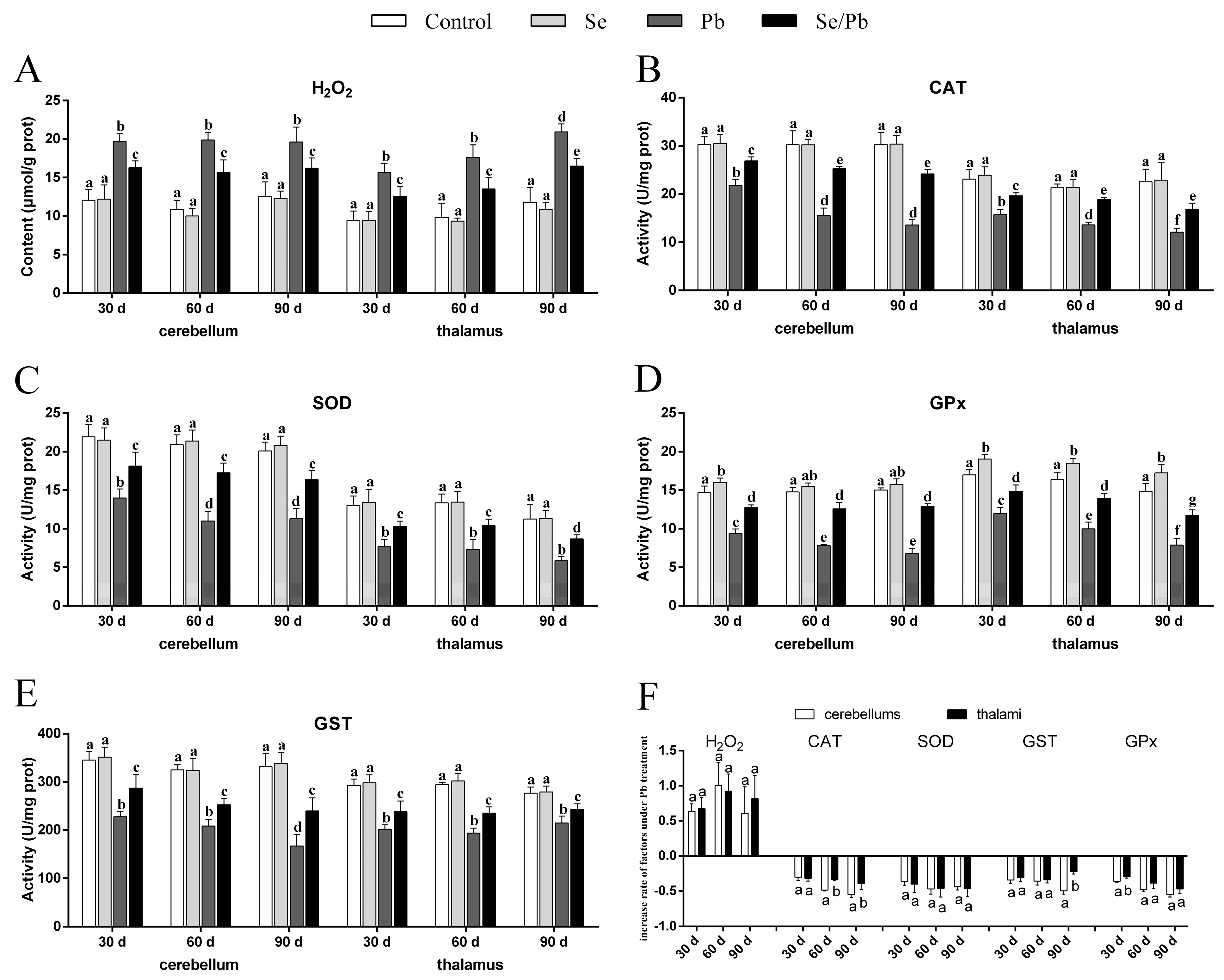
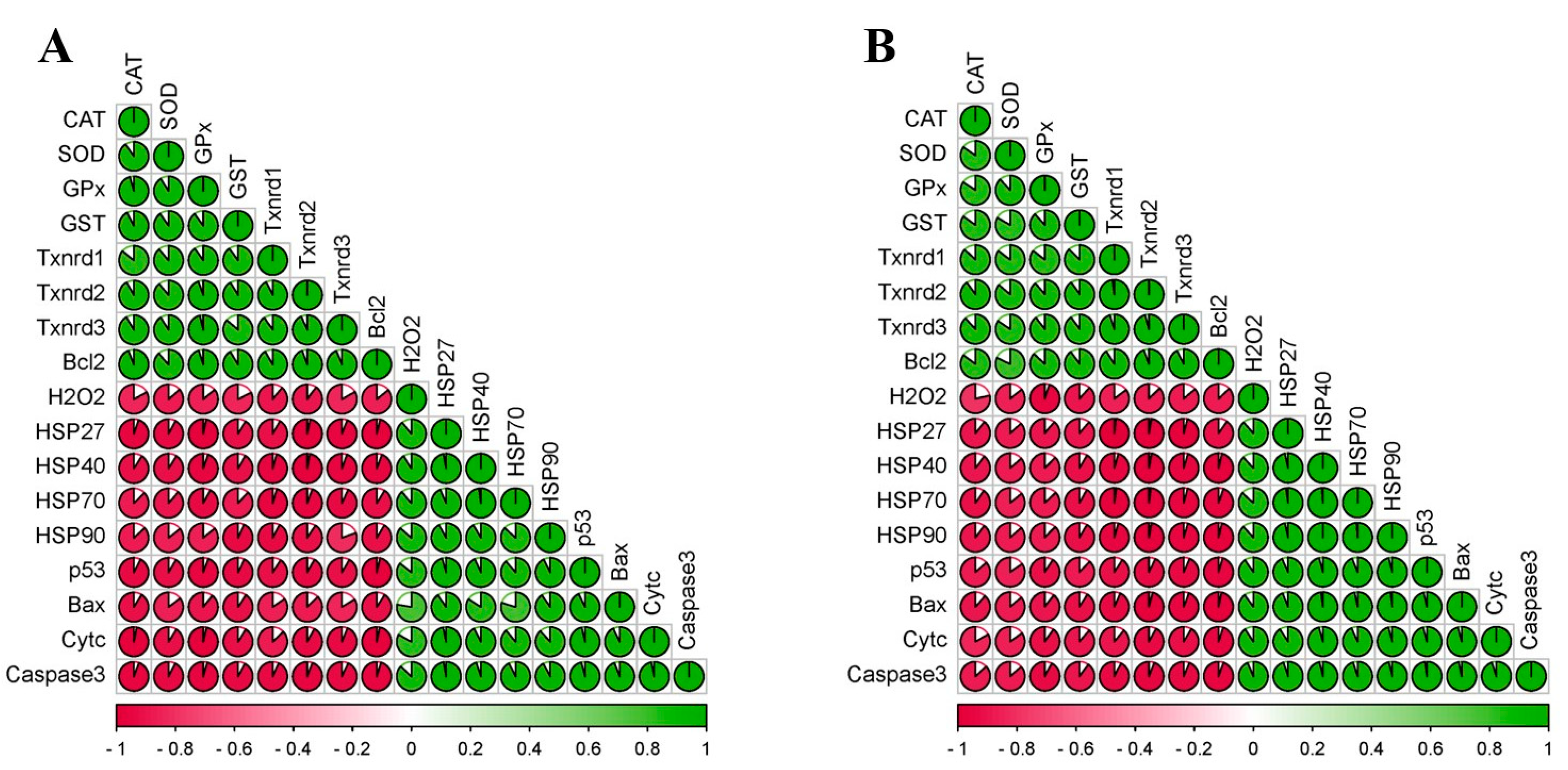
 ” represents the impact of Pb on the detected factors, and “
” represents the impact of Pb on the detected factors, and “ ” represents the impact of Se supplementation on the detected factors.
” represents the impact of Se supplementation on the detected factors.
 ” represents the impact of Pb on the detected factors, and “
” represents the impact of Pb on the detected factors, and “ ” represents the impact of Se supplementation on the detected factors.
” represents the impact of Se supplementation on the detected factors.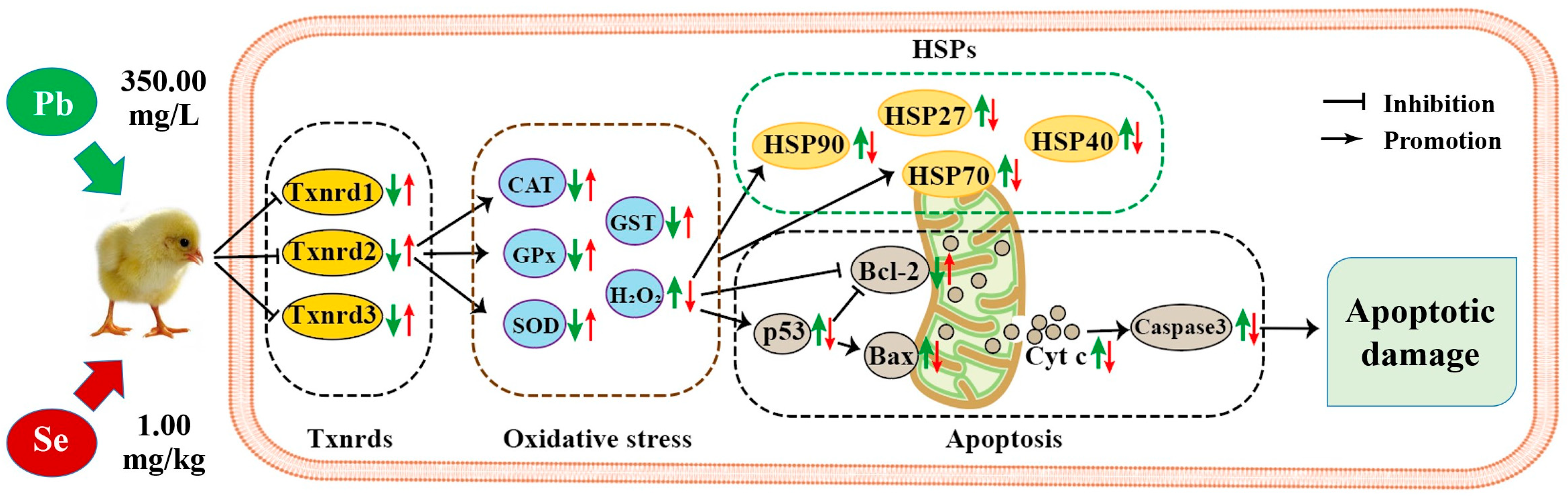
| Organs | Principal Component | Initial Eigenvalues | Extraction Sums of Squared Loadings | ||||
|---|---|---|---|---|---|---|---|
| Total | % of Variance | Cumulative % | Total | % of Variance | Cumulative % | ||
| cerebellum | 1 | 14.644 | 91.523 | 91.523 | 14.644 | 91.523 | 91.523 |
| 2 | 0.365 | 2.283 | 93.805 | ||||
| 3 | 0.302 | 1.886 | 95.692 | ||||
| 4 | 0.152 | 0.948 | 96.639 | ||||
| 5 | 0.143 | 0.892 | 97.532 | ||||
| 6 | 0.100 | 0.624 | 98.155 | ||||
| 7 | 0.074 | 0.464 | 98.619 | ||||
| 8 | 0.055 | 0.345 | 98.963 | ||||
| 9 | 0.051 | 0.317 | 99.280 | ||||
| 10 | 0.032 | 0.199 | 99.479 | ||||
| 11 | 0.031 | 0.191 | 99.671 | ||||
| 12 | 0.014 | 0.090 | 99.761 | ||||
| 13 | 0.013 | 0.083 | 99.844 | ||||
| 14 | 0.012 | 0.075 | 99.919 | ||||
| 15 | 0.008 | 0.051 | 99.970 | ||||
| 16 | 0.005 | 0.030 | 100.000 | ||||
| thalamus | 1 | 14.542 | 90.884 | 90.884 | 14.542 | 90.884 | 90.884 |
| 2 | 0.409 | 2.558 | 93.442 | ||||
| 3 | 0.312 | 1.950 | 95.392 | ||||
| 4 | 0.160 | 0.997 | 96.390 | ||||
| 5 | 0.149 | 0.932 | 97.322 | ||||
| 6 | 0.117 | 0.729 | 98.051 | ||||
| 7 | 0.093 | 0.583 | 98.634 | ||||
| 8 | 0.066 | 0.415 | 99.049 | ||||
| 9 | 0.047 | 0.296 | 99.344 | ||||
| 10 | 0.033 | 0.204 | 99.549 | ||||
| 11 | 0.025 | 0.158 | 99.707 | ||||
| 12 | 0.019 | 0.118 | 99.825 | ||||
| 13 | 0.017 | 0.104 | 99.929 | ||||
| 14 | 0.006 | 0.040 | 99.968 | ||||
| 15 | 0.004 | 0.026 | 99.995 | ||||
| 16 | 0.001 | 0.005 | 100.000 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, W.; Liu, Y.; Liang, J.; Jiang, C.; Yu, M.; Sun, W.; Huang, B.; Dong, N.; Kang, L.; Tang, Y. Molecular Mechanisms of Selenium Mitigating Lead Toxicity in Chickens via Mitochondrial Pathway: Selenoproteins, Oxidative Stress, HSPs, and Apoptosis. Toxics 2023, 11, 734. https://doi.org/10.3390/toxics11090734
Hong W, Liu Y, Liang J, Jiang C, Yu M, Sun W, Huang B, Dong N, Kang L, Tang Y. Molecular Mechanisms of Selenium Mitigating Lead Toxicity in Chickens via Mitochondrial Pathway: Selenoproteins, Oxidative Stress, HSPs, and Apoptosis. Toxics. 2023; 11(9):734. https://doi.org/10.3390/toxics11090734
Chicago/Turabian StyleHong, Weichen, Yuhao Liu, Jiatian Liang, Chunyu Jiang, Meijin Yu, Wei Sun, Bin Huang, Na Dong, Lu Kang, and You Tang. 2023. "Molecular Mechanisms of Selenium Mitigating Lead Toxicity in Chickens via Mitochondrial Pathway: Selenoproteins, Oxidative Stress, HSPs, and Apoptosis" Toxics 11, no. 9: 734. https://doi.org/10.3390/toxics11090734







